ABSTRACT
Persistence of human papillomavirus (HPV) and cervical disease in the context of HIV co-infection can be influenced by introduction of antiretroviral therapy (ART) and sustained immune activation despite ART. We conducted a cross-sectional study in order to evaluate immune activation/exhaustion in ART-suppressed HIV+ women with or without high-risk (HR) HPV-related cervical intraepithelial neoplasia (CIN). 55 South African women were recruited in three groups: HR (-) (n = 16) and HR (+) (n = 15) HPV with negative cervical histopathology, and HR (+) HPV with CIN grade 1/2/3 (n = 24). Sampling included endocervical brushing (HPV DNA genotyping), Pap smear (cytology), colposcopic punch biopsy (histopathology, histochemical evaluation of immune cells), and peripheral blood (clinical assessment, flow cytometry-based immune subset characterization). Statistics were done using R2.5.1. Irrespective of the presence of CIN, HR (+) HPV women had higher circulating levels of T cells expressing markers of activation/exhaustion (CD38, PD1, CTLA-4, BTLA, CD160), Tregs, and myeloid subsets expressing corresponding ligands (PDL1, PDL2, CD86, CD40, HVEM) than HR (-) HPV women. A decrease in circulating NK cells was associated with CIN grade. CD4+ T cell count associated negatively with T cell exhaustion and expression of negative regulators on myeloid cells. Women with CIN when compared to HR (-) HPV women, had higher cervical cell density in stroma and epithelium for CD4+, CD68+, and CD11c+ cells, and only in stroma for CD8+ cells. We conclude that in ART-suppressed HIV-infected women with HPV co-infection the levels of T and myeloid cell activation/exhaustion are associated with the presence of HR HPV genotypes.
KEYWORDS:
Abbreviations
| APC | = | Allophycocyanin |
| ART | = | antiretroviral therapy |
| BD | = | Becton Dickinson |
| BTLA | = | B and T lymphocyte attenuator |
| CIN | = | Cervical Intraepithelial Neoplasia |
| CSIL | = | Cervical Squamous Intraepithelial Lesions |
| CTLA-4 | = | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| DAB | = | 3,3'-Diaminobenzidine |
| DC | = | dendritic cells |
| FITC | = | fluorescein isothiocyanate |
| HIV-1 | = | Human immunodeficiency virus 1 |
| HPV | = | Human papillomavirus |
| HR | = | high risk |
| HVEM | = | Herpes virus entry mediator |
| LR | = | low risk |
| MDC | = | myeloid dendritic cells |
| MFI | = | mean fluorescent intensity |
| NK | = | natural killer |
| PCR | = | polymerase chain reaction |
| PDC | = | plasmacytoid dendritic cells |
| PDL1 | = | Programmed death-ligand 1 |
| PDL2 | = | Programmed death-ligand 2 |
| PD1 | = | Programmed cell death 1 |
| PE | = | phycoerythrin |
| PerCP-Cy5.5 | = | Peridinin chlorophyll Cy5.5 |
| % | = | percent positive |
| RT | = | room temperature |
| Tregs | = | regulatory T cells. |
Introduction
HPV is the cause of several skin and mucosal pathologies, including genital warts and cancer.Citation1-8 Among the more than 202 HPV genotypes (types) that have been identified, approximately 40 infect the human genital tract. These are characterized on the strength of their association with cervical cancer, as oncogenic (high risk, HR) and non-oncogenic (low risk, LR) types.Citation9-12 While LR HPV types 6 and 11 are responsible for more than 90% of genital warts, HR types 16 and 18 account for >70% of cervical cancer cases, followed by 31, 33, 35, 45, 52 and 58.Citation9,10,Citation13 Persistence of HR HPV infection may be associated with high levels of HR HPV DNA replication and/or Cervical Squamous Intraepithelial Iesions (SIL, cytological assessment, low or high)Citation14-16 or CIN (histological assessment, grades 1–3).
Human immunodeficiency virus 1 (HIV-1) infection alters the course of HPV-associated oncogenesis. Low-grade lesions are more likely to persist, and progress more rapidly to high-grade cervical dysplasia. They are refractory to treatment, with frequent recurrences. Introduction of ART results in partial restoration of the adaptive and innate immune compartment.Citation17,18 The impact of ART-mediated immune recovery on HPV-associated lesions remains controversial. While some studies show reduction of lesion severity and of rate of progression, as well as increased regression following ART,Citation19-21 others have shown that cervical dysplasia can persistCitation22-24 despite ART-mediated CD4+ naive and memory T cells recovery.Citation19,22,Citation25,26 A model proposed by PalefskyCitation15 stresses that early stages of CIN (SIL) may respond to the initiation of ART in subjects with limited HIV-associated immunosuppression, due to restoration of partially preserved HPV-specific responses. However, the beneficial effect of ART may be less pronounced in individuals with CIN 2-3 (High-grade SIL), especially if the CD4+ T cell count is <200 cells/mm3.Citation23
Despite the beneficial effect of ART in HIV infection, a large set of data suggest that long-term ART does not completely reverse the damage inflicted by chronic HIV replication. This is exemplified by the persistence of T cell activation and inflammationCitation27-30 and the lasting depletion and/or retained dysfunction of innate immune subsets [i.e., plasmacytoid dendritic cells (PDC)Citation31,32 and monocytesCitation33]. Increased levels of persistent immune activation and immune exhaustion [i.e., Programmed cell death-1 (PD1) expression]Citation34-37 are associated with limited CD4+ T cell recovery. Residual T cell and myeloid activation in ART-suppressed subjects may depend on multiple factors, including “leakage” of lipopolysaccharide and other bacterial products providing stimulation through pattern recognition receptors (i.e., Toll-like receptors). The relationship between persistent immune activation and HPV infection on ART-mediated immune reconstitution remains unclear. Recent findingsCitation38 support a role for chronic viral co-infections in contributing to cellular activation and impaired immune recovery. In addition, high levels of immune activation,Citation39,40 particularly T cell activation (i.e., CD38, HLA-DR), are found in HCV/HIV co-infected subjects, and can be observed even in the presence of suppressive ART.Citation41,42
Taken together, immune reconstitution on ART does not result in clearance of HPV related-disease. To address the relationship between persistent HPV infection, oncogenic type infection and immune activation/exhaustion, we evaluated a cohort of South African women receiving suppressive ART and with CD4+ T cell count recovery >200 cells/mm3 with or without HR HPV infection.
Results
Study subjects demographics, HPV infection, and cervical cell density-associated profile
Study subject characteristics are shown in . All participating women were on suppressive ART (viral load <50 copies/mL); CD4+ T cell count at recruitment was similar across the three groups {median 568.5 for group 1 [HR (-) HPV with negative cervical histopathology, n = 16], 517 for group 2 [HR (+) HPV with negative cervical histopathology, n = 15], and 435.5 for group 3 [HR (+) HPV with CIN1 or CIN2/3, n = 24]}. HPV 16 was the most prevalent type in group 2 [53.3 percent positive (%)] and the second type (33.3%) in group 3 after HPV 52 (37.5%), while the frequency of HPV 18 was 13.3% and 12.5% in groups 2 and 3, respectively. No statistical difference was observed among study groups for any of the clinical or demographic parameters shown in .
Table 1. Patients characteristics.
Histochemical evaluation of immune cells in the cervix was performed in order to confirm group assignment and cervical histopathology. Representative stainings for women from the three study groups are shown in . No difference was detected between group 1 and 2 despite differential of HR HPVs presence. Group 3 had higher total cell density per square millimeter for CD4+ (p <0.001), CD8+ (p <0.001), CD68+ (p <0.001), and CD11c+ cells (p <0.001) than group 1 (). Cuzick’s trend analysisCitation43 of total cell density per square millimeter in HR (+) HPV groups showed an increase in CD4+ (z = 2.45, p = 0.01), CD68+ (z = 2.66, p = 0.008) and CD11c+ cells (z = 2.91, p = 0.004) concurrent with an increased degree of cervical dysplasia by histology. Interestingly, the cell density per square millimeter for CD4+, CD68+, and CD11c+ cells was higher in both stroma and epithelium, whereas CD8+ cells were only increased in the stroma (). Segregation between CIN1 (n = 9) and CIN2/3 (n = 15) (Table S1) showed similar results and suggested that the difference between group 3 and group 1 could be attributed to change in both CIN1 and CIN2/3. Whereas no difference was detected overall between groups 2 and 3 (), higher levels of CD68+ and CD11c+ cells were detected in women with CIN2/3 when compared with group 2 (Table S1). Taken together, these results suggest that presence of increasing cervical dysplasia is associated with a local increase in immune cells density.
Figure 1. Association between cervical histopathology status and increased cervical cell density per square millimeter in ART-treated HIV+/HPV+ women. Immunohistochemical staining for CD4+, CD8+, CD68 and CD11c is shown in epithelium and stroma from cervical biopsies of representative women in each group: HR (-) HPV with negative cervical histopathology (group 1, study subject TJA1019081, top panel), HR (+) HPV with negative cervical histopathology (group 2, study subject TJA1040434, middle panel), and HR (+) HPV with CIN1 or CIN2/3 (group 3, study subject TJA1104317, bottom panel).
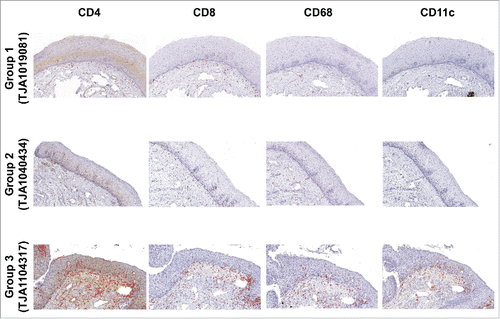
Table 2. Differences in cervical cell density per square millimeter amongst study groups.
HPV infection with HR genotypes rather than local dysplasia is associated with increases in regulatory T cells frequency and in the expression of activation and exhaustion markers on T and myeloid cells
To assess whether HPV types are associated with systemic immune activation/exhaustion or alteration of the regulatory T cell (Tregs) compartment, we compared the expression of markers of systemic immune activation and exhaustion in adaptive and innate immune effectors between HR (-) HPV (group 1) and HR (+) HPV groups with different cervical histopathology status (groups 2 and 3). Comparisons between HR (+) HPV groups with different cervical histopathology status were also performed to assess whether these markers were associated with cervical histopathology status. As summarized in , Table S2, and , the presence of HR HPV, but not the grade of dysplasia, was associated with distinct circulating cell subsets. Briefly, comparison of group 1 versus group 2 or 3 showed clear differences with women with HR HPV (groups 2 or 3) having significantly higher levels than women in group 1 of CD8- T cell activation [i.e., mean fluorescent intensity (MFI) of CD38 on CD3+CD8- T cells (group 1 vs. group 2 p = 0.02, group 1 vs. group 3 p = 0.02] and higher frequency of cell subsets associated with exhaustion [e.g., CD4+CD25hiFoxP3+ % of lymphocytes (Tregs, group 1 vs. group 2 p = 0.003, group 1 versus group 3 p = 0.02), CD3+CD8+PD1+ % of lymphocytes, (group 1 vs. group 2 p = 0.003, group 1 versus group 3 p <0.001)]. On the other hand, no detectable differences of T cell activation or exhaustion marker expression in circulating cells were observed between HR HPV groups, despite differences in grades of dysplasia and cervical cell infiltrates levels (, Table S2, and ). In conjunction with the T cell-related changes reported, regulation of myeloid ligands for immune exhaustion molecules was also increased in the HR HPV groups (, Table S2, and ). Specifically, expression of Programmed death-ligand 1 (PDL1), Programmed death-ligand 2 (PDL2), Herpes virus entry mediator (HVEM), and CD86 were higher in HR HPV women, suggesting a greater ligand interaction with PD1, CD160, and Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) on lymphocytes, respectively.
Figure 2. Association between HR HPV and systemic T cell activation and Tregs cell subsets. CD3+CD8+CD38+ percentages (%) of lymphocytes (top panel) and CD4+CD25+FoxP3+ (Tregs) % of lymphocytes (bottom panel) are shown for women in groups 1, 2 and 3. Data are shown as interquartile box plots with median and outliers for each group, and significant (<0.05) p values.
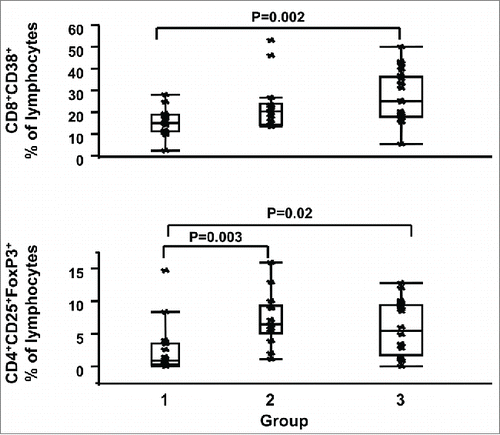
Figure 3. Association between HR HPV and systemic immune exhaustion. Left panel shows CD3+CD8+PD1+ (top), CD3+CD8+PD1-CTLA-4+ (middle), and CD3+CD8+BTLA+CD160+ (bottom) % of lymphocytes, while right panel shows Lin2-HLA-DR+CD11c+PDL1+PDL2+ (top), Lin2-HLA-DR+CD11c+CD86+CD40+ (middle), and Lin2-HLA-DR+CD11c+HVEM+ (bottom) % of live cells for women in groups 1, 2 and 3. Data are shown as interquartile box plots with median and outliers for each group, and significant (<0.05) p values.
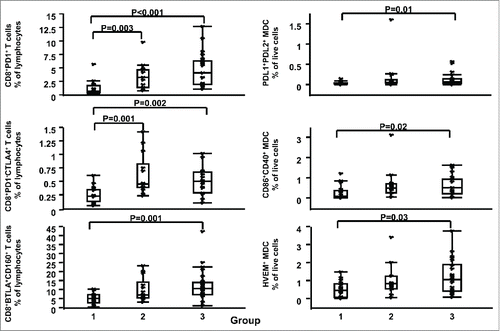
Table 3. Significant differences in immune variables amongst study groups.
To address the impact of immune reconstitution on markers of immune activation/exhaustion, we assessed the correlation between these markers and CD4+ T cell count. Irrespective of HPV type, CD38 expression on T cells was positively correlated with CD4+ T cell count, whereas in groups with HR HPV infection, immune exhaustion markers on myeloid and CD8+ T cells were negatively associated with CD4+ T cell count (). No correlation between CD4+ T cell count and Tregs frequency was detected. The shared negative association of exhaustion markers on lymphocytes and myeloid cells from HR HPV groups suggests an HPV type-dependent association between CD4+ T cell count and systemic immune activation/exhaustion in ART-suppressed HIV+/HPV+ women.
Table 4. Significant correlations with CD4+ T cell count.
Cervical dysplasia rather than HPV types is associated with a decrease in natural killer cell subsets
Analysis of natural killer (NK) cells showed that a decrease in their frequency is more closely aligned with cervical dysplasia status as opposed to presence or absence of HR HPV. Briefly, the frequency of NK cell subsets bearing molecules associated with activation/inhibition (i.e., CD94) was higher in group 2 as compared to group 1 (e.g., CD3-CD56brightCD94+ % of lymphocytes, p = 0.04) (). Interestingly, a lower frequency of CD3-CD56bright % of lymphocytes (p = 0.009) or CD3-CD56brightCD94+ % of lymphocytes (p = 0.006) in group 3 were the only variables that were different between groups 2 and 3 (, Table S2). To further explore differences between groups 2 and 3, Cuzick’s trend analysisCitation43 was completed for all NK variables detecting a negative association between cervical histopathology status and frequency of NK cell subsets (i.e. CD3-CD56bright % of lymphocytes, z = −2.18, p = 0.03; CD3-CD56brightCD94+ % of lymphocytes, z = −2.32, p = 0.02; CD3-CD56brightCD16-CD94+ % of lymphocytes, z = −2.11, p = 0.03), suggesting that the frequency of some NK cell subsets may decrease with lesion progression (group 2 to 3). Finally, to address the impact of immune reconstitution on NK subsets, we assessed the correlation between CD4+ T cell count and NK cell subset frequencies. This analysis showed that CD4+ T cell count was negatively associated with the frequency of CD56dimCD16- (immature) NK cell subsets in women with HR types and with the frequency of CD56bright in women without HR types ().
Irrespective of HPV type, CD8+ T cell activation is positively associated with expression of exhaustion markers, but not NK changes
A positive correlation between CD8+ T cell activation (CD8+CD38+ % of lymphocytes or CD38 MFI on CD8+) and T or myeloid cells immune exhaustion variables [PD1 or B and T lymphocyte attenuator (BTLA) expression on CD8+ subsets; HVEM in myeloid dendritic cells (MDC) or PDC subsets, ] was observed, irrespective of HPV genotype (). A negative association between CD8+ T cell activation and frequency of CD56dim or CD56bright NK subsets was detected across all groups. These data indicate that in ART-suppressed HIV+/HPV+ women systemic CD8+ T cell activation is associated with systemic immune activation/exhaustion and NK frequency, independently of HPV genotype.
Figure 4. Association between systemic CD8+ T cell activation and systemic immune activation/exhaustion. (A) Correlation between MFI of CD38 on CD3+CD8+ T cells and systemic immune activation/exhaustion [MFI HVEM on Lin2-HLA-DR+CD11c+ (top left), and MFI HVEM on Lin2-HLA-DR+CD123hi (top right)] in women with HR HPV (group 2 + 3); (B) Correlation between CD3+CD8+CD38+ % of lymphocytes and systemic immune activation/exhaustion [CD3+CD8+PD1+ (bottom left), and CD3+CD8+BTLA+ (bottom right) % of lymphocytes] in women with HR HPV (group 2+3). Data are shown as regression lines, with number of subjects (n), correlation and p values.
![Figure 4. Association between systemic CD8+ T cell activation and systemic immune activation/exhaustion. (A) Correlation between MFI of CD38 on CD3+CD8+ T cells and systemic immune activation/exhaustion [MFI HVEM on Lin2-HLA-DR+CD11c+ (top left), and MFI HVEM on Lin2-HLA-DR+CD123hi (top right)] in women with HR HPV (group 2 + 3); (B) Correlation between CD3+CD8+CD38+ % of lymphocytes and systemic immune activation/exhaustion [CD3+CD8+PD1+ (bottom left), and CD3+CD8+BTLA+ (bottom right) % of lymphocytes] in women with HR HPV (group 2+3). Data are shown as regression lines, with number of subjects (n), correlation and p values.](/cms/asset/75d50638-d827-44ac-acb6-bf2d3bf042a4/koni_a_1128612_f0004_b.gif)
Table 5. Significant correlations with CD8+ T cell activation.
Discussion
The present study is, to our knowledge, the first to evaluate an association between T cell and myeloid immune activation/exhaustion in blood and the presence of HR HPV types with or without dysplasia in HIV-infected women on suppressive ART. Our data support the hypothesis that HR HPV infection associates with increased immune activation/exhaustion and increased Tregs frequency in blood, independently of cervical dysplasia. Furthermore, they show that in HR HPV infection cervical dysplasia is associated with a local increase in immune cells density.
The association of elevated immune activation and exhaustion with HR HPV infection is consistent with HPV acting to impair or benefiting from already impaired adaptive immune responses not able to clear local HPV infections.Citation44-47 Persistence of HIV-dependent immune exhaustion on ART may limit immune clearance of HPV, in combination with other mechanisms such as: (a) the downregulation of Interferon-α inducible gene expressionCitation48,49 by HR HPV viruses (via direct interaction of HPV 16 E6 and E7 oncoproteins with components of the IFN signaling pathways),Citation50,51 (b) the lack of activation of Langerhans cells by HPV capsids resulting in delay of antigen presentation, (c) the HPV-mediated downregulation of keratinocyte cytokine secretion, (d) the absence of inflammation,Citation52 and (e) the decreased NK activity against HPV-harboring targets.Citation53 Despite these local mechanisms, the immune competent host is able to mount a response that leads to lesion regression in approximately 70–90% of HIV- women. Persistent HPV infection has been causally linked to development of CIN,Citation54 with a small proportion of women progressing to infiltrating cervical cancer. Our data linking a higher rate of T and myeloid cells immune exhaustion with higher Tregs in HIV-infected ART-suppressed women bearing HR HPV types suggest a mechanism of immune modulation that may contribute to higher prevalence and more aggressive course of HPV lesions in HIV co-infection. Our study supports a direct association between dysregulation of adaptive and innate subsets in HR HPV infection consistent with reports of decreased myeloid cell function and an increase in myeloid negative regulation against anti-HPV T cell function. Observations of an increase in Tregs and CTLA-4 expressing lymphocytes with HR HPV infection are also consistent with reports of these cell subsets being associated with cancer progression.Citation55-62
Because of its cross-sectional nature, our study does not address the question of whether sustained systemic immune activation/exhaustion is the cause of increased susceptibility to infection with HR HPV, or rather is the result of such infection. Future longitudinal studies in HIV-uninfected and in ART-suppressed HIV-infected women with HR (+) HPV co-infection, inclusive of follow-up after lesion excision, may help elucidate the causal relationship between HR HPV infection and immune activation noted here. Importantly, our data suggest that perturbations in the subset distribution of systemic immune cells are associated with persistent cervical HR HPV. Studies by Yang et al.,Citation63 based on cervical sampling in women with or without HR HPV and with different grades of cervical dysplasia, showed that in cervical cells PD1/PDL1 was associated with HR (+) HPV and increased cervical dysplasia, whereas these markers were similar in HR (-) HPV and HR (+) HPV women with negative histopathology. Our work extends these observations by showing that differences can be detected in the blood in association with HR HPV infection, even in the absence of detectable cervical dysplasia.
While we report the outcomes of correlations with CD4+ T cell count, it should be noted that all women in our cohort experienced CD4+ T cell recovery after ART initiation. In women with HR HPV infection, we found high levels of activation/exhaustion and shared negative association of exhaustion markers on lymphocytes and myeloid cells with CD4+ T cell count. In addition, these women showed a decrease in frequency of CD56bright NK with cervical dysplasia together with a negative association of CD4+ T cell count and CD8+ T cell activation with NK subsets. Taken together, these data suggest that a greater amount of immune exhaustion phenotypes are present in association with HR HPV infection and with lower CD4+ T cell count on ART. They also suggest an increased role for innate surveillance in controlling cervical dysplasia. In women without HR HPV infection, the observed lower immune activation/exhaustion suggests the potential for a local immune response irrespective of CD4+ T cell count as long as lower levels of T cell activation and exhaustion are maintained.
Our study has several limitations. First, the immunophenotypic characterization of cell subsets associated with immune activation/exhaustion was performed at a single time-point on peripheral blood mononuclear cells, restricting our ability to comment on longitudinal prospective changes. Second, while our data focus on cell subset frequencies and expression of markers on cells, our analysis both in blood and in the cervix did not address functional innate or adaptive anti-HPV responses nor in situ tissue determinations of activation and/or proliferation. Finally, due to the limited number of subjects with CIN1 (n = 9), all subjects with cervical dysplasia (group 3: CIN1/2/3 n = 24) were analyzed together [Tables S1 and S2 segregate data from group 3 into CIN1 (n = 9) and CIN2/3 (n = 15)]. Future studies should address the limitations of this study.
Determining whether chronic immune activation in ART-suppressed HIV+/HPV+ women can affect the persistence of HPV infection and its progression to cervical cancer remains a research priority. Conversely, determining whether or not HPV infection can support ongoing immune activation and HIV persistence in ART-suppressed subjects is also important for both clinical outcomes and HIV-1 eradication strategies. Overall, our data suggest an association between HR HPV and sustained immune activation/exhaustion in blood, independently of cervical dysplasia, in the presence of suppressive ART, that may hinder immune reconstitution, impair anti-HPV immune responses, and lead to initiation and/or progression of cervical dysplasia. Furthermore, they show an association in HR HPV infection between cervical dysplasia and cervical density of immune cells. Additional studies are needed to determine if the group of variables described here could serve as (i) potential biomarkers for the likely presence of HR HPV and and/or grade of dysplasia, or (ii) aid in identifying the source for the dysregulated state in HIV infection in spite of ART and whether this is dependent on HR HPV infection.
Subjects, Materials and Methods
Study population, enrollment and study related procedures
We conducted a study of 55 ART-treated HIV+/HPV+ women divided into the following three groups: (a) negative for HR HPV [HR (-) HPV] with negative cervical histopathology (group 1, n = 16), (b) positive for HR HPV [HR (+) HPV] with negative cervical histopathology (group 2, n = 15), and (c) positive for HR HPV [HR (+) HPV] with CIN1 or CIN2/3 (group 3, n = 24). All study women were negative for pregnancy and sexually transmitted infections tests at screening, with no clinical evidence of an inflammatory disease, and confirmed CD4+ T cell count >200 cells/μL and HIV-1 viral load <50 copies for >6 mo and at screening.
Eligible women were initially identified from populations of patients of the Themba Lethu clinic and Clinical HIV research Unit at the Helen Joseph hospital in South Africa. Women were already identified to be HIV+, on ART, with negative, low- or high-grade SIL cytology results from routine gynecological follow-up, and with CD4+ T cell count >200 cells/μL and HIV-1 viral load <50 copies within 24 weeks of review from clinical follow-up. These women were invited for a screening visit consisting of study education, written consent form sign-up, medical history information related interview, and collection of the following specimens: (a) urine for pregnancy test, (b) cervical swab for sexually transmitted infections testing [Chlamydia, gonorrhea and herpes simplex virus], (c) venous blood for clinical parameter testing [complete blood count differential, CD4+ count and HIV viral load confirmation], (d) endocervical brush for HPV DNA genotyping, (e) pap smear (cytology) for diagnosis (only if available cytology record was older than 12 weeks), and (f) colposcopic punch biopsy for histopathology diagnosis and immunohistochemistry. The HPV type, CD4+ count, viral load and pathology results from screening were used to determine group assignment.
Group-assigned study participants (n = 55) were called back within 6 weeks after the screening visit for a second visit consisting of collection of a urine sample for pregnancy test and a venous blood sample for same day clinical assessment (i.e., complete blood count differential, CD4+ count and HIV viral load confirmation) and immune subset characterization. In the case of CIN2/3 lesions, the second visit was scheduled before a planned follow-up LEEP, or cone biopsy (not uncommon to be done within 12 weeks of diagnosis). In no case was a woman’s treatment delayed on account of participation on this study. The study protocol was approved by the Institutional Review Boards of the authors' institutions.
HPV DNA PCR laboratory testing
A cytobrush sample was collected by inserting a Digene DNA Collection Device (brush) (Digene Corporation, MD) within the cervical os, rotating it 360° and immediately placing it in Digene specimen transport media. DNA was subsequently extracted from the cytobrush sample using the Qiagen blood and body fluid DNA extraction kit (Qiagen Inc. CA), and HPV genotyping was performed by the qualitative Roche Linear Array HPV genotyping test (Roche Molecular Systems, Inc., NJ) according to the manufacturer’s instructions. Briefly, HPV DNA and the human ß-globin gene (quality control of the specimen to confirm the presence of human DNA) were amplified by polymerase chain reaction (PCR) with a pool of biotinylated primers, and the amplified biotinylated PCR products were hybridized to oligonucleotide probes on a genotyping strip. The probe-bound amplified products were detected by colorimetric determination with a streptavidin-horseradish peroxidase conjugate and a substrate solution containing hydrogen peroxide and 3,3',5,5'-tetramethylbenzidine. Cross contamination of specimens with amplicons was controlled by usage of deoxyuridine triphosphate in addition to deoxythymidine triphosphate in the Master Mix reagent. Amplicons that contain deoxyuridine were destroyed by uracil-N-glycosylase, contained in the master mix, while the uracil-N-glycosylase was inactivated during the PCR. This qualitative test allowed for the detection of 37 HPV types. Individual HPV types were divided into 14 HR types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 23 LR types: HPV 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 and CP6108.Citation9-11,Citation64
Histochemical evaluation of immune cells in cervical biopsies
One colposcopic punch biopsy was used for histopathology diagnosis and preparation of slides for histochemical evaluation of immune cells in the cervix. Manual immunohistochemistry was performed according to standard protocols. Briefly, tissue sections (3–4 micron) were cut from the relevant paraffin-wax-embedded tissue blocks onto adhesive coated slides. Sections were exposed to an antigen retrieval buffer (Dako, Carpinteria, CA) for 20 min using the Dako PT Link Instrument, and subsequently incubated in a 1XPBS buffer for 10 min, and a Dual Endogenous Enzyme Block (3% hydrogen peroxidase, Dako) for 5 min at room temperature (RT). After rinsing with 1XPBS buffer, sections were incubated for 20 min with the following primary antibodies from Novocastra (Leica Microsystems Inc., Buffalo Grove, IL): (a) CD4+ (clone IF6, dilution 1/40), (b) CD8+ (clone 4B11, dilution 1/100), (c) CD68 (clone PG-M1, dilution 1/50), and (d) CD11c (clone 5D11, dilution 1/100). After rinsing with 1XPBS buffer, sections were incubated with the Real Envision detection system (Dako) for 20 min, rinsed with 1XPBS buffer, and stained with 3,3'-Diaminobenzidine (DAB, Dako) for 10 min. After rinsing with tap water, sections were lightly counterstained with Mayer’s haematoxylin (Dako) for 10 sec, blued in running tap water for 10 min, dehydrated in alcohol, cleared in xylene and mounted with entellan. Each batch of immunohistochemistry for a specific antibody was accompanied by known positive and negative control sections.
Whole stained sections were scanned using a whole slide digital scanner (Aperio Scanscope CS at 20X magnification, Aperio, Leica Microsystems Inc., Buffalo Grove, IL), and Spectrum imaging software (version 11.0.0.725, Aperio). One to two representative still images were captured at 10X power for each of the four immunohistochemical stains. The area of epithelium and stroma was manually marked in each subject image for each stain. Each image’s epithelium and stromal areas were then analyzed for the number of DAB-positive cells per pixel area and data expressed reported for the stroma, epithelium and total (stroma + epithelium) regions (representative image illustrating approach shown in Fig. S1) by using the cell counting algorithm (inForm software version 2.0, Perkin Elmer, Waltham, MA).
Real time whole blood-based phenotype assessment by flow cytometry
Immunophenotypic characterization of adaptive and innate cell subsets associated with immune activation/exhaustion was performed by same day whole blood five-color staining as previously describedCitation65 by using the following combinations of directly fluorochrome-conjugated anti-human cell surface monoclonal antibodies: (a) PD1-fluorescein isothiocyanate (FITC), CTLA-4-phycoerythrin (PE), CD3-Peridinin chlorophyll Cy5.5 (PerCP-Cy5.5), CD38-Allophycocyanin (APC), CD8-V450, (b) HLA-DR-FITC, BTLA-PE, CD3-PerCP-Cy5.5, CD160-APC, CD8-V450, (c) CD16-FITC, CD94-PE, CD3-PerCP-Cy5.5, CD56-APC, (d) Lin2-FITC, PDL1-PE, PDL2-PE, CD123-PerCP-Cy5.5, HVEM-APC, HLA-DR-V450, (e) Lin2-FITC, PDL1-PE, PDL2-PE, CD11c-PerCP-Cy5.5, HVEM-APC, HLA-DR-V450, (f) Lin2-FITC, CD86-PE, CD11c-PerCP-Cy5.5, CD40-APC, HLA-DR-V450. Stainings a–b allowed for the identification of activation/exhaustion markers (i.e. CD38, HLA-DR, PD1, CTLA-4, BTLA, CD160) on T cells (CD3+CD8+, CD3+CD8-), staining c allowed for the assessment of markers of activation/inhibition (i.e., CD94) on NK cell subsets (identified as CD3-CD56bright, CD56dim ± CD16), and stainings d–f allowed for the identification of exhaustion markers (i.e. PDL1, PDL2, HVEM, CD86, CD40) on DC [identified as Lin2-HLA-DR+CD123hi (PDC), and Lin2-HLA-DR+CD11c+ (MDC)]. The following isotypes were used: IgG1k-FITC, IgG1k-PE, IgG1k-PerCP-Cy5.5, IgG1k-APC, IgG1k-V450. All antibodies were from Becton Dickinson (BD) Biosciences (San Diego, CA). In addition, Tregs staining was performed by using the BD Biosciences FoxP3 staining kit (Tregs defined as CD4+CD25hiFoxP3+).
Briefly, 200 μL of whole blood were incubated for 15 min at RT with the appropriate Ab combinations, lysed for 10 min at RT with 3 mL of FACS Lysis solution (BD), washed once with 3 mL of FACS washing buffer at 1200 rpm for 5 min and re-suspended in 200 μL of FACS washing buffer. Tregs staining was performed according to the manufacturer’s instructions. Cells were analyzed on LSRII cytometer (BD) by collecting >100,000 events appropriately gated on lymphocytes (for NK, T, Tregs) or on all live cells (for DC) defined by size and granularity in forward scatter and side scatter. The samples were then analyzed after duplet exclusion using FloJo software (Tree Star, San Carlos, CA). Thresholds were set by isotype-matched negative controls and unstained cells. Results were expressed as MFI and %.
Statistical analysis
Real time whole blood-based phenotypes and data derived from histochemical evaluation of cervical immune cells from colposcopic-derived punch biopsy samples were described as medians, 25th and 75th percentiles. Comparisons among study groups were done by Kruskall–Wallis test, to test for equality of medians across the groups. Post-hoc pairwise comparisons between groups for variables with a significant difference (p values that were less than 0.05) in medians across groups were identified using the Mann–Whitney test. A multiple testing adjustment based on the Bonferroni approach was performed; adjusted p values that were less than 0.05 were considered statistically significant. Spearman correlations were used to evaluate associations between real time whole blood-based phenotypes and CD4+ T cell levels or T cell activation, with p values less than 0.05 being considered statistically significant. Cuzick’s trend analysis was performed for whole blood-based phenotypes and immunohistochemical data in HPV HR patients. p values that were less than 0.05 together with direction of the z score were used for the interpretation of the results with a positive or a negative z interpreted as an increasing or decreasing trend respectively in relation to an increasing degree of cervical dysplasia from absent dysplasia to CIN1 and CIN2/3. Analysis was done by using R version 2.5.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Materials.zip
Download Zip (393.8 KB)Acknowledgments
We would like to thank the subjects who participated in the study and their providers. We would like to thank Bruce Allan for HPV genotyping.
Funding
This work was partially supported by NIH/NIAID grant UO1AI51986 to LJ Montaner. Additional support was provided by The Philadelphia Foundation (Robert I. Jacobs Fund), The Stengel-Miller family, AIDS funds from the Commonwealth of Pennsylvania and from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health, as well as by a Cancer Center Grant (P30 CA10815). Nurses and doctors were supported by a USAID grant (USAID PEPFAR 674-A-00-08-00007-00). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content of this publication does not necessarily reflect the views or policies of NIAID, nor does mention of trade names, commercial projects, or organizations imply endorsement by the US Government.
References
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995; 87:796–802; PMID:7791229; http://dx.doi.org/10.1093/jnci/87.11.796.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–8; PMID:9459645; http://dx.doi.org/10.1056/NEJM199802123380703.
- Maiman M, Fruchter RG, Clark M, Arrastia CD, Matthews R, Gates EJ. Cervical cancer as an AIDS-defining illness. Obstet Gynecol 1997; 89:76–80; PMID:8990442; http://dx.doi.org/10.1016/S0029-7844(96)00378-X.
- Nonnenmacher B, Hubbert NL, Kirnbauer R, Shah KV, Munoz N, Bosch FX, de Sanjosé S, Viscidi R, Lowy DR, Schiller JT. Serologic response to human papillomavirus type 16 (HPV-16) virus-like particles in HPV-16 DNA-positive invasive cervical cancer and cervical intraepithelial neoplasia grade III patients and controls from Colombia and Spain. J Infect Dis 1995; 172:19–24; PMID:7797910; http://dx.doi.org/10.1093/infdis/172.1.19.
- Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. Aids 1998; 12:495–503; PMID:9543448; http://dx.doi.org/10.1097/00002030-199805000-00011.
- Petter A, Heim K, Guger M, Ciresa-Ko Nig A, Christensen N, Sarcletti M, Wieland U, Pfister H, Zangerle R, Höpfl R. Specific serum IgG, IgM and IgA antibodies to human papillomavirus types 6, 11, 16, 18 and 31 virus-like particles in human immunodeficiency virus-seropositive women. J Gen Virol 2000; 81:701–8; PMID:10675407; http://dx.doi.org/10.1099/0022-1317-81-3-701.
- Steele JC, Mann CH, Rookes S, Rollason T, Murphy D, Freeth MG, Gallimore PH, Roberts S. T-cell responses to human papillomavirus type 16 among women with different grades of cervical neoplasia. Br J Cancer 2005; 93:248–59; PMID:15986031; http://dx.doi.org/10.1038/sj.bjc.6602679.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–9; PMID:10451482; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F.
- Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. Virology 2015; 476:341–4; PMID:25577151; http://dx.doi.org/10.1016/j.virol.2014.12.028.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56; PMID:20952254; http://dx.doi.org/10.1016/S1470-2045(10)70230-8.
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27; PMID:12571259; http://dx.doi.org/10.1056/NEJMoa021641.
- Schiffman MH. Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst 1992; 84:394–8; PMID:1311392; http://dx.doi.org/10.1093/jnci/84.6.394.
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121:621–32; PMID:17405118; http://dx.doi.org/10.1002/ijc.22527.
- Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, He W, Romney SL, Johnson A, Angeletti R et al. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev 2002; 11:483–8; PMID:12010863.
- Palefsky J. Human papillomavirus-related tumors in HIV. Curr Opin Oncol 2006; 18:463–8; PMID:16894294; http://dx.doi.org/10.1097/01.cco.0000239885.13537.36.
- Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 Consensus Guidelines for the Management of Women with Cervical Cytological Abnormalities. J Low Genit Tract Dis 2002; 6:127–43; PMID:17051012; http://dx.doi.org/10.1097/00128360-200204000-00012.
- Connors M, Kovacs JA, Krevat S, Gea-Banacloche JC, Sneller MC, Flanigan M, Metcalf JA, Walker RE, Falloon J, Baseler M et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med 1997; 3:533–40; PMID:9142122; http://dx.doi.org/10.1038/nm0597-533.
- Grant M, Pardoe I, Whaley M, Montaner JS, Harrigan PR. The T cell receptor V β repertoire shows little change during treatment interruption-related viral rebound in chronic HIV infection. Aids 2002; 16:287–90; PMID:11807314; http://dx.doi.org/10.1097/00002030-200201250-00019.
- Heard I, Schmitz V, Costagliola D, Orth G, Kazatchkine MD. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. Aids 1998; 12:1459–64; PMID:9727566; http://dx.doi.org/10.1097/00002030-199812000-00007.
- Heard I, Tassie JM, Kazatchkine MD, Orth G. Highly active antiretroviral therapy enhances regression of cervical intraepithelial neoplasia in HIV-seropositive women. Aids 2002; 16:1799–802; PMID:12218392; http://dx.doi.org/10.1097/00002030-200209060-00013.
- Minkoff H, Ahdieh L, Massad LS, Anastos K, Watts DH, Melnick S, Muderspach L, Burk R, Palefsky J. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. Aids 2001; 15:2157–64; PMID:11684935; http://dx.doi.org/10.1097/00002030-200111090-00011.
- Lillo FB, Ferrari D, Veglia F, Origoni M, Grasso MA, Lodini S, Mastrorilli E, Taccagni G, Lazzarin A, Uberti-Foppa C. Human papillomavirus infection and associated cervical disease in human immunodeficiency virus-infected women: effect of highly active antiretroviral therapy. J Infect Dis 2001; 184:547–51; PMID:11494160; http://dx.doi.org/10.1086/322856.
- Schuman P, Ohmit SE, Klein RS, Duerr A, Cu-Uvin S, Jamieson DJ, Anderson J, Shah KV; HIV Epidemiology Research Study (HERS) Group. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis 2003; 188:128–36; PMID:12825181; http://dx.doi.org/10.1086/375783.
- Ahdieh-Grant L, Li R, Levine AM, Massad LS, Strickler HD, Minkoff H, Moxley M, Palefsky J, Sacks H, Burk RD et al. Highly active antiretroviral therapy and cervical squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Natl Cancer Inst 2004; 96:1070–6; PMID:15265968; http://dx.doi.org/10.1093/jnci/djh192.
- Conley LJ, Ellerbrock TV, Bush TJ, Chiasson MA, Sawo D, Wright TC. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet 2002; 359:108–13; PMID:11809252; http://dx.doi.org/10.1016/S0140-6736(02)07368-3.
- Palefsky JM, Holly EA, Ralston ML, Da Costa M, Bonner H, Jay N, Berry JM, Darragh TM. Effect of highly active antiretroviral therapy on the natural history of anal squamous intraepithelial lesions and anal human papillomavirus infection. J Acquir Immune Defic Syndr 2001; 28:422–8; PMID:11744829; http://dx.doi.org/10.1097/00042560-200112150-00003.
- French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009; 200:1212–5; PMID:19728788; http://dx.doi.org/10.1086/605890.
- Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, Gripshover B, Salata RA, Taege A, Lisgaris M et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26; PMID:21917895; http://dx.doi.org/10.1093/infdis/jir507.
- Valdez H, Connick E, Smith KY, Lederman MM, Bosch RJ, Kim RS, St Clair M, Kuritzkes DR, Kessler H, Fox L et al. Limited immune restoration after 3 years' suppression of HIV-1 replication in patients with moderately advanced disease. AIDS 2002; 16:1859–66; PMID:12351945; http://dx.doi.org/10.1097/00002030-200209270-00002.
- Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, Kembabazi A, Neilands TB, Bangsberg DR, Deeks SG et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011; 25:2123–31; PMID:21881481; http://dx.doi.org/10.1097/QAD.0b013e32834c4ac1.
- Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol 2002; 168:4796–801; PMID:11971031; http://dx.doi.org/10.4049/jimmunol.168.9.4796.
- Sachdeva N, Asthana V, Brewer TH, Garcia D, Asthana D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-α but not proinflammatory cytokines. J Immunol 2008; 181:2887–97; PMID:18684980; http://dx.doi.org/10.4049/jimmunol.181.4.2887.
- Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 2012; 26:843–53; PMID:22313961; http://dx.doi.org/10.1097/QAD.0b013e328351f756.
- Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol 2006; 120:163–70; PMID:16765088; http://dx.doi.org/10.1016/j.clim.2006.04.570.
- Goicoechea M, Smith DM, Liu L, May S, Tenorio AR, Ignacio CC, Landay A, Haubrich R. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis 2006; 194:29–37; PMID:16741879; http://dx.doi.org/10.1086/504718.
- Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43; PMID:12721933; http://dx.doi.org/10.1086/374786.
- Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, Nabatanzi R, Kamya MR, Cao H. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis 2011; 11:43; PMID:21299909; http://dx.doi.org/10.1186/1471-2334-11-43.
- Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, Deeks SG. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83; PMID:21502083; http://dx.doi.org/10.1093/infdis/jir060.
- Kottilil S, Yan MY, Reitano KN, Zhang X, Lempicki R, Roby G, Daucher M, Yang J, Cortez KJ, Ghany M et al. Human immunodeficiency virus and hepatitis C infections induce distinct immunologic imprints in peripheral mononuclear cells. Hepatology 2009; 50:34–45; PMID:19551908; http://dx.doi.org/10.1002/hep.23055.
- Rempel H, Sun B, Calosing C, Abadjian L, Monto A, Pulliam L. Monocyte activation in HIV/HCV coinfection correlates with cognitive impairment. PLoS One 2013; 8:e55776; PMID:23437063; http://dx.doi.org/10.1371/journal.pone.0055776.
- Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis 2008; 197:1402–7; PMID:18444798; http://dx.doi.org/10.1086/587696.
- Gonzalez VD, Falconer K, Blom KG, Reichard O, Morn B, Laursen AL, Weis N, Alaeus A, Sandberg JK. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by α interferon and ribavirin treatment. J Virol 2009; 83:11407–11; PMID:19710147; http://dx.doi.org/10.1128/JVI.01211-09.
- Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985; 4:87–90; PMID:3992076; http://dx.doi.org/10.1002/sim.4780040112.
- Bergot AS, Ford N, Leggatt GR, Wells JW, Frazer IH, Grimbaldeston MA. HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5- mediated recruitment of mast cells. PLoS Pathog 2014; 10:e1004466; PMID:25340820; http://dx.doi.org/10.1371/journal.ppat.1004466.
- Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, Levine AM, Darragh TM, Weinberg V, Smith-McCune KK. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res 2004; 64:6766–74; PMID:15374995; http://dx.doi.org/10.1158/0008-5472.CAN-04-1091.
- Mhatre M, McAndrew T, Carpenter C, Burk RD, Einstein MH, Herold BC. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex Transm Dis 2012; 39:591–7; PMID:22801340; http://dx.doi.org/10.1097/OLQ.0b013e318255aeef.
- Prata TT, Bonin CM, Ferreira AM, Padovani CT, Fernandes CE, Machado AP, Tozetti IA. Local immunosuppression induced by high viral load of human papillomavirus: characterization of cellular phenotypes producing interleukin-10 in cervical neoplastic lesions. Immunology 2015; 146:113–21; PMID:26059395; http://dx.doi.org/10.1111/imm.12487.
- Chang YE, Laimins LA. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J Virol 2000; 74:4174–82; PMID:10756030; http://dx.doi.org/10.1128/JVI.74.9.4174-4182.2000.
- Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J Virol 2001; 75:4283–96; PMID:11287578; http://dx.doi.org/10.1128/JVI.75.9.4283-4296.2001.
- Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-α. Virology 1999; 259:305–13; PMID:10388655; http://dx.doi.org/10.1006/viro.1999.9771.
- Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev 1998; 12:2061–72; PMID:9649509; http://dx.doi.org/10.1101/gad.12.13.2061.
- Stanley M. Immune responses to human papillomavirus. Vaccine 2006; 24 Suppl 1:S16–22; PMID:16219398; http://dx.doi.org/10.1016/j.vaccine.2005.09.002.
- Malejczyk J, Malejczyk M, Majewski S, Orth G, Jablonska S. NK-cell activity in patients with HPV16-associated anogenital tumors: defective recognition of HPV16-harboring keratinocytes and restricted unresponsiveness to immunostimulatory cytokines. Int J Cancer 1993; 54:917–21; PMID:8392981; http://dx.doi.org/10.1002/ijc.2910540608.
- Mougin C, Mo L, Dalstein V. [Natural history of papillomavirus infections]. Rev Prat 2006; 56:1883–9; PMID:17243385.
- Visser J, Nijman HW, Hoogenboom BN, Jager P, van Baarle D, Schuuring E, Abdulahad W, Miedema F, van der Zee AG, Daemen T. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol 2007; 150:199–209; PMID:17937675; http://dx.doi.org/10.1111/j.1365-2249.2007.03468.x.
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8:765–72; PMID:8671665; http://dx.doi.org/10.1093/intimm/8.5.765.
- Hatam LJ, Devoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM, Bonagura VR. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res 2012; 18:1925–35; PMID:22322668; http://dx.doi.org/10.1158/1078-0432.CCR-11-2941.
- Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol 2008; 181:6738–46; PMID:18981091; http://dx.doi.org/10.4049/jimmunol.181.10.6738.
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65:1089–96; PMID:15705911.
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003; 100:4712–7; PMID:12682289; http://dx.doi.org/10.1073/pnas.0830997100.
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003; 100:8372–7; PMID:12826605; http://dx.doi.org/10.1073/pnas.1533209100.
- Su TH, Chang TY, Lee YJ, Chen CK, Liu HF, Chu CC, Lin M, Wang PT, Huang WC, Chen TC et al. CTLA-4 gene and susceptibility to human papillomavirus-16-associated cervical squamous cell carcinoma in Taiwanese women. Carcinogenesis 2007; 28:1237–40; PMID:17341658; http://dx.doi.org/10.1093/carcin/bgm043.
- Yang W, Song Y, Lu YL, Sun JZ, Wang HW. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 2013; 139:513–22; PMID:23521696; http://dx.doi.org/10.1111/imm.12101.
- Firnhaber C, Van Le H, Pettifor A, Schulze D, Michelow P, Sanne IM, Lewis DA, Williamson AL, Allan B, Williams S et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control 2010; 21:433–43; PMID:19949850; http://dx.doi.org/10.1007/s10552-009-9475-z.
- Papasavvas E, Ortiz GM, Gross R, Sun J, Moore EC, Heymann JJ, Moonis M, Sandberg JK, Drohan LA, Gallagher B et al. Enhancement of human immunodeficiency virus type 1-specific CD4 and CD8 T cell responses in chronically infected persons after temporary treatment interruption. J Infect Dis 2000; 182:766–75; PMID:10950770; http://dx.doi.org/10.1086/315748.
