ABSTRACT
The recurrence rates of Hepatocellular carcinoma (HCC) are high, necessitating novel and effective adjuvant therapies. Therefore, we conducted a phase II study of glypican-3 (GPC3) peptide vaccine as an adjuvant therapy for HCC patients. Forty-one patients with initial HCC who had undergone surgery or radiofrequency ablation (RFA) were analyzed in this phase II, open-label, single-arm trial. Ten vaccinations were performed for 1 y after curative treatment. We also investigated case-control subjects, where selected patients treated surgically during the same period were analyzed. The expression of GPC3 in the available primary tumors was determined by immunohistochemical analysis. Six patients received RFA therapy while 35 received surgery. The recurrence rate tended to be lower in the 35 patients treated with surgery plus vaccination compared to 33 patients who underwent surgery alone (28.6% vs. 54.3% and 39.4% vs. 54.5% at 1 and 2 y, respectively; p = 0.346, 0.983). Twenty-five patients treated with surgery and vaccination had GPC3-positive tumors; the recurrence rate in this group was significantly lower compared to that in 21 GPC3-positive patients who received surgery only (24% vs. 48% and 52.4% vs. 61.9% at 1 and 2 y, respectively; p = 0.047, 0.387). The GPC3 peptide vaccine improved the 1-y recurrence rate in patients with GPC3-positive tumors. This study demonstrated that GPC3 expression by the primary tumor may be used as a biomarker in a putative larger randomized clinical trial to determine the efficacy of the GPC3-derived peptide vaccine.
Abbreviations
| AFP | = | α-fetoprotein |
| CTL | = | cytotoxic T lymphocyte |
| GPC3 | = | glypican-3 |
| HCC | = | hepatocellular carcinoma |
| HLA | = | human leukocyte antigen |
| IFA | = | incomplete Freund's adjuvant |
| IFNγ | = | interferon-γ |
| MHC | = | major histocompatibility complex |
| PBMC | = | peripheral blood mononuclear cell |
| RFA | = | radiofrequency ablation |
Introduction
HCC is one of the most common forms of cancer. The prognosis for patients with HCC is very poor, and it is the third leading cause of cancer mortality worldwide.Citation1,2 The 5-y recurrence rate of HCC exceeds 70% following surgery or RFA due to a high risk of metastasis and the development of de novo tumors.Citation3-5 One of the major reasons for such a poor prognosis of HCC patients is the limited availability of treatment options to prevent recurrence. In recent years, clinical trials with sorafenib, such as the STORM trial, showed that this kinase inhibitor was not an effective intervention in the adjuvant setting for HCC following resection or ablationCitation6, although the recurrence-free survival rate could be improved by adjuvant therapy with vitamin K2Citation7, retinoidCitation8, or interferon.Citation9 However, immunotherapy using activated lymphocytes or tumor vaccines was shown to reduce the risk of cancer recurrence in randomized controlled trials.Citation10,11 As these data are derived from clinical trials, and no standard adjuvant therapy has been established, the development of an effective method of inhibiting the occurrence and recurrence of HCC remains a necessity.
The carcinoembryonic antigen GPC3 is an ideal target for antigen-specific immunotherapy against HCC, as it is specifically overexpressed in HCC.Citation12,13 Our previous study demonstrated that the tumor expression profile of GPC3 was not correlated with hepatic virus infection. Furthermore, the prognosis of patients with GPC3-positive HCC, as determined by immunohistochemical analysis, was found to be poor following initial hepatectomy.Citation14 We therefore anticipate that GPC3-targeted therapy will prevent hepatocarcinogenesis following surgery in patients with GPC3-positive HCC.
A phase I clinical trial of a GPC3-derived peptide vaccine for advanced HCC patients was conducted in Japan. This trial demonstrated that the vaccine was well tolerated and that the frequency of GPC3-derived, peptide-specific cytotoxic T lymphocytes (CTLs) in peripheral blood mononuclear cells (PBMCs) was correlated with overall survival.Citation15 The GPC3298–306 peptide (EYILSLEEL) was used in human leukocyte antigen (HLA)-A24-positive patients and the GPC3144–152 peptide (FVGEFFTDV) in HLA-A2-positive patients, as HLA-A2 and A24 are common HLA class I alleles within the Japanese population. Furthermore, we reported remarkable tumor lysis in advanced HCC patients immediately following GPC3-derived peptide vaccination Citation16, as well as significant clinical response of progressive recurrent ovarian clear cell carcinoma to GPC3-derived peptide vaccine.Citation17
RFA induced or enhanced T-cell responses specific for HCC-associated antigens in PBMCs derived from patients with HCC.Citation18,19 Similarly, we demonstrated that RFA induced GPC3-specific CTLs in HCC patients and tumor-bearing mice.Citation20 Therefore, we hypothesized that use of an adjuvant GPC3-peptide vaccine would benefit patients with initial HCC who had previously undergone surgery or RFA.
To test this hypothesis, we conducted a single-arm phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for HCC patients in an effort to provide a larger randomized clinical trial that would determine the efficacy of the GPC3-derived peptide vaccine.
Results
Patient characteristics
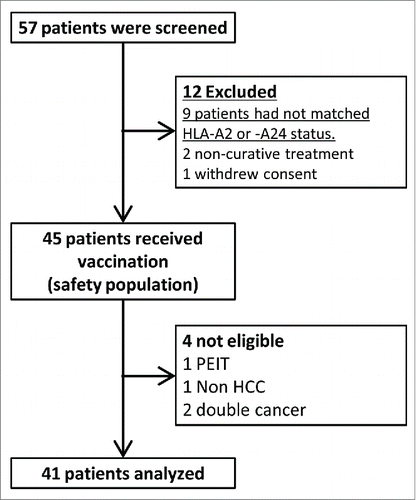
Fifty-seven patients were screened in this trial. Twelve patients were excluded from the study because of HLA-A status (nine patients), non-curative treatment (one patient), or withdrawal of consent (one patient). Forty-five patients received the vaccine; however, four discontinued injections due to protocol violation, including having double cancers (two patients), not having HCC (one patient), and undergoing percutaneous ethanol injection therapy (PEIT) (one patient). Forty-one patients were ultimately analyzed in this study (). None of the patients dropped out due to adverse events caused by peptide vaccination. Nine patients discontinued the regimen due to recurrence within 1 y, after receiving five or more vaccinations. Patient characteristics are presented in . The median age of the patients was 64.0 y; 30 of 41 were males. The performance status was one in a single patient and 0 in the remainder. All patients had Child-Pugh class A disease. Thirty-two patients (78%) had a hepatic virus infection, 11 with HBV, and 21 with HCV. Twenty-six patients received the HLA-A*24:02-restricted GPC3298–306 peptide, and 15 patients received the HLA-A2-restricted GPC3144–152 (FVGEFFTDV) peptide.
Table 1. Patient characteristics.
Toxicity
One patient received PEIT therapy before vaccination, and was dropped from the study after discontinuing vaccinations owing to protocol violations immediately after the first vaccination. This patient did not receive adequate follow-up to monitor toxicity. The remaining 44 patients received adequate follow-up.
All adverse events observed in this trial are listed in detail in . All patients experienced a grade 1 or 2 local skin reaction at the injection site, and six patients experienced transient fever. These immune-related adverse events were well-tolerated. The treatment did not affect the reactivation of viral hepatitis.
Table 2. Adverse events.
Other adverse effects are unlikely to be attributable to the treatment. Grade 3 laboratory adverse events were observed in eight patients; one patient experienced a central nervous system hemorrhage (grade 2), and two developed interstitial pneumonia 2 and 10 mo after the observation period, respectively.
Recurrence rate
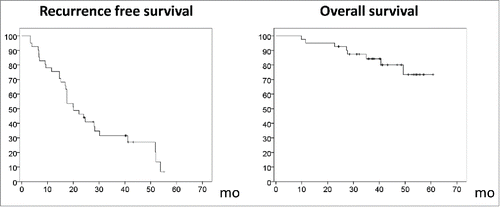
The median follow-up period was 40.4 mo (range 24–60 mo). All patients were followed for 2 y or more. The median overall survival rate was 20.1 mo (95% confidence interval, 14.7–25.5) (). HCC recurred in 31 patients, most commonly within the liver (28/31) during follow-up. Two metastases were detected in the lung, as were two in the lymph node and one in bone. The 1- and 2-y recurrence rates for the 41 patients who received the vaccine were 24.4% and 53.7%, respectively (); the expected corresponding recurrence rates were 20% and 45%, respectively, in this single-arm trial (p = 0.635, 0.436). The recurrence rates tended to be lower in the 41 patients treated with surgery or RFA together with vaccination compared to the 80 patients who received surgery only (24.4% and 53.7% vs. 42.5% and 66.2% at 1 and 2 y, respectively; p = 0.054, 0.198) (Fig. S1). The primary endpoint was not reached for 1- and 2-y recurrence rates.
Immunological analysis of PBMCs and recurrent tumor
To determine whether the GPC3 peptide vaccine could induce a specific immune response, PBMCs obtained from all patients before and after vaccination were subjected to an ex vivo interferonγ (IFNγ) ELISPOT assay as described previously.Citation21 Prior to the vaccination, the number of GPC3 peptide-specific CTLs in 5 × 105 PBMCs was below 10 in all patients. However, a number greater than 10 was achieved in 35 of the 41 patients (85.4%) after vaccination. Furthermore, we found that induction of GPC3-specific CTL tended to correlate with GPC3 expression in the primary tumor (p = 0.224, Student’s t-test; Fig. S2A). However, there was no statistically significant correlation between the maximum number of GPC3-peptide-specific CTLs and the recurrence-free or overall survival (p = 0.136 and p = 0.415 [Spearman’s test], respectively; Fig. S2B).
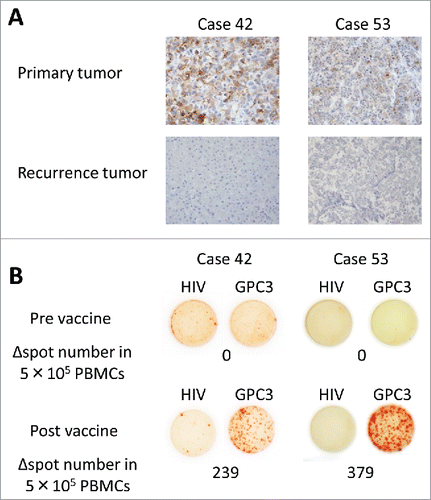
In 11 patients, GPC3 expression in recurrent tumors was subjected to immunohistochemical analysis. Two of the 11 cases appeared to lack GPC3 expression in the recurrent tumor, although GPC3 was expressed in the primary HCC tissue prior to GPC3-peptide vaccination (cases 42 and 53) (). In both these cases, the spot number of GPC3-specific CTLs after vaccination was high, as determined by an ex vivo IFNγ ELISPOT assay (Case 42: 239; Case 53: 379) (). These results indicate that GPC3-peptide-specific CTLs were present in these patients after having undergone vaccination. In the other nine patients whose recurrent tumors did express GPC3, the spot numbers of GPC3-specific CTLs tended to be lower than the aforementioned two patients (Case 4: 42, 11: 56, 13: 21, 21: 56, 38: 22, 45: 2, 50: 20, 51: 130, 52: 353; p = 0.059, Mann–Whitney U test). These results suggested that GPC3-peptide-specific CTLs induced by vaccination prevented the recurrence of GPC3 expressing tumors in patients 42 and 53. Although peptide-specific CTLs were induced by vaccination, the reduction of recurrence due to the peptide vaccine might be limited.
Figure 3. Cytotoxic T lymphocyte (CTL) analysis in peripheral blood mononuclear cells (PBMCs) and recurrent tumors. (A) Immunohistochemical staining for glypican-3 (GPC3) showed positivity in the primary tumor. The recurrent tumor appeared to lack GPC3 expression. Change in the expression of GPC3 in cases 42 and 53 after GPC3 peptide vaccination. Magnification = 200X. (B) Ex vivo interferonγ (IFNγ) ELISPOT assays for GPC3 in 5 × 105 peripheral blood mononuclear cells were performed before and after vaccination. Δ spot number indicates the number of GPC3-peptide-specific CTLs. The number of IFNγ-positive spots increased in the wells pre-incubated with GPC3 peptide.
We established GPC3-peptide-specific CTL lines from needle-biopsy specimens of the recurrent tumor of Case 52. These CTL lines recognized GPC3 peptide presented by target cells. In this patient, we were able to compare the expression of the inhibitory molecules PD-1 and Tim-3 in CD8-positive T-cells among PBMCs and in recurrent tumor tissues obtained by needle biopsy using flow cytometry. The expression of PD-1 was higher in CD8-positive T-cells in the recurrent tumor then in PBMCs (data not shown).
Discussion
The primary endpoint was not attained for 1- and 2-y recurrence rates in this study. However, there were significant differences in the clinical backgrounds of the 41 patients who received the vaccine compared to the historical control group of 80 patients who underwent surgery between 2001 and 2002 (Table S1). Hence, it was difficult to evaluate the reduction in the risk of post-operative recurrence by vaccination.
Therefore, we analyzed the case-control study, and selected those HCC patients who had undergone curative resection at National Cancer Center Hospital East during the same period as the vaccine trial and had provided written informed consent. The recurrence rate tended to be lower in the 35 patients treated with surgery plus vaccination compared to 33 patients who underwent surgery alone (28.6% vs. 54.3% and 39.4% vs. 54.5% at 1 and 2 y, respectively; p = 0.346, 0.983) (). There were no significant differences in the patients’ clinical backgrounds (Table S2). We evaluated GPC3 expression by immunohistochemical analysis of specimens from 31 of the 35 patients treated with surgery plus vaccination whose tissues were available; GPC3 expression was detected in 25 of these 31 patients. On the other hand, GPC3 expression was detected in 21 of the 33 patients in the surgery-only group. We previously reported that the prognosis of patients with GPC3-positive HCC was poor following initial hepatectomy.Citation14 We compared the 25 surgery plus vaccine-treated patients who were GPC3-positive in their primary tumors to the 21 GPC3-positive patients who underwent surgery only, and found that the recurrence rate was significantly lower in the former group than in the latter (24% vs. 48% and 52.4% vs. 61.9% at 1 and 2 y, respectively; p = 0.047, 0.387) (). There were no significant differences in the patients’ clinical backgrounds ().
Figure 4. Kaplan–Meier curves for recurrence-free and overall survival. (A) Thirty-five patients treated with surgery and vaccination did not have significantly longer recurrence-free survival or overall survival rates than the 33 patients who underwent surgery only. (B) Among patients with glypican-3 (GPC3)-positive tumors, the recurrence rate was significantly lower in the 25 patients treated with surgery and vaccination compared to the 21 patients who underwent surgery only (24% vs. 48% and 52.4% vs. 61.9% at 1 and 2 y, respectively; p = 0.047, 0.387). The 25 patients treated with surgery and vaccination tended to have longer recurrence-free and overall survival rates compared to the 21 patients who underwent surgery only.
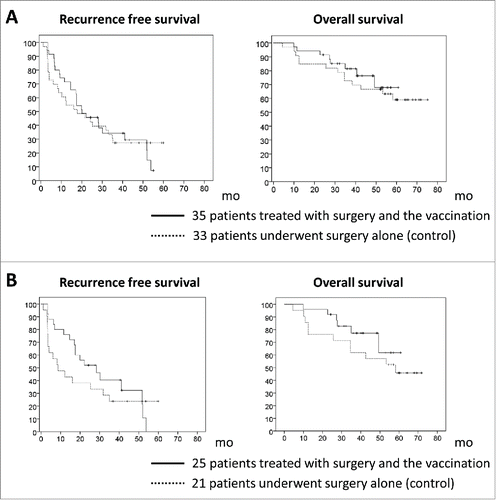
Table 3. Patient characteristics.
Several clinical trials have reported that immunotherapy improves the prognosis of postoperative HCC patients, and is thus a promising adjuvant therapy.Citation10,11,22 However, immunotherapy for HCC is still in the preclinical and clinical trial phases of development. Analysis of the correlation between clinical and immunological responses is required to improve the efficacy of immunotherapy.Citation23 To our knowledge, no clinical trial that included proper immunological analysis, especially for immune-effector cells, has been conducted for HCC in an adjuvant setting. In this trial, we analyzed vaccine-induced CTLs in PBMCs as well as in the recurrent tumors. Furthermore, we performed immunohistochemical analysis of the primary and recurrent tumors to evaluate the target cancer.
Early recurrence corresponds to intrahepatic metastasis undetected at the time of curative treatment.Citation24 In this study, the 1-y recurrence rate was lower in the vaccination group than in the control group. There was a limitation in our ability to evaluate the efficacy of the vaccine, as this was not a randomized-controlled study, but rather a case-controlled study. However, our phase II study, which included immunological analysis to examine the role of tumor-antigen-specific CTLs in immunotherapy against GPC3, was necessary prior to conducting a larger randomized clinical trial.
In this vaccination trial, marked antigen-specific immune responses were observed in PBMCs by ex vivo IFNγ ELISPOT assays. Prior to vaccination, the number of GPC3-peptide-specific CTLs in 5 × 105 PBMCs was below 10 in all patients. In contrast, the number of GPC3-peptide-specific CTLs before vaccination in a phase I trial for advanced HCC was greater than 10 in 5 of the 33 patients.Citation15 Differences in the presence of tumors may account for the varied spontaneous GPC3-peptide-specific CTL responses between the two vaccination trials. Although the GPC3-specific CTL frequency after vaccination was correlated with overall survival in the previous phase I trial, we found no correlation between GPC3-peptide-specific CTL frequency and recurrence-free survival in this study. In a clinical trial of MART-1 peptide vaccine for resected melanoma, recurrence-free survival was not correlated with an ELISPOT response after in vitro sensitization via cytokine release ELISA.Citation25 In a randomized trial of advanced melanoma treatment with 12 major histocompatibility complex (MHC) class I peptides alone or in combination with either a T-helper tetanus peptide or a mixture MHC class II peptide, an ELISPOT response after in vitro sensitization to MHC class II peptides was associated with clinical response and overall survival.Citation26 Further studies are necessary to determine whether an antigen-specific immune response in PBMCs was associated with the clinical outcome.
We demonstrated that GPC3-peptide-specific CTLs are present in PBMCs and recurrent tumors, which suggests that the GPC3-peptide-specific CTLs induced by the peptide vaccine had a limited effect on recurrence. Fourcade et al. reported that tumor-antigen-specific CTLs were detected ex vivo in PBMCs from patients immunized with incomplete Freund's adjuvant (IFA), CpG oligodeoxynucleotide, and HLA-A2-restricted analog NY-ESO-1 peptide and Pan-DR epitope peptides.Citation27 However, this trial did not demonstrate dramatic antitumor efficacy in patients with advanced melanoma. To explain the limitation of the cancer vaccine, the authors showed that the expansion of antigen-specific CTLs was associated with the upregulation of PD-1 and Tim-3 at the time of immunization, and that PD-1 and Tim-3 blockade further augmented the expansion of, and cytokine production by, CTLs. In a mouse model, expression of the death receptor was increased on the surface of CTLs at the vaccination site after peptide/IFA vaccination. Furthermore, peptide/IFA vaccination induced higher antigen-driven expression of inhibitory receptor PD-1, lymphocyte activating gene 3, CTLA-4, and Tim-3 in CTLs, suggesting partial exhaustion.Citation28 In our study, the expression of PD-1 among CD8-positive T-cells was higher in the recurrent tumor compared to PBMCs. These results suggest that the expression of inhibitory receptors in humans induced by vaccination is similar to that in the mouse model.
The previous study using mice showed that peptide/IFA vaccination primed tumor-specific CD8+ T-cells, which accumulated not in the tumors but rather at the persisting, antigen-rich vaccination site.Citation28 In contrast, T-cell recruitment to the site of injection with IFA alone was observed in a randomized clinical trial in advanced melanoma that aimed to assess the cellular and molecular events at the site of immunization with IFA alone or a peptide in IFA.Citation29 Further studies are necessary to determine whether the peptide vaccine induces antigen-specific T-cell recruitment to the site of vaccination in humans.
We found no GPC3 expression in the recurrent tumor following GPC3 peptide vaccination. In another GPC3 peptide vaccine trial for ovarian clear-cell carcinoma, one case demonstrated a partial response using the RECIST criteria, but later appeared to lack GPC3 expression in the resected right inguinal lymph node metastasis following vaccination.Citation17 These results suggest the activation of immune-escape mechanisms following GPC3 peptide vaccination, including the downregulation of cancer-specific antigens by tumor cells.
We clearly demonstrated the presence of GPC3-peptide-specific CTLs in peripheral blood, and that GPC3-peptide-specific CTLs had infiltrated into the recurring tumor following GPC3 peptide vaccination. Questions remain as to why many vaccinated patients show increased numbers of circulating tumor-specific T-cells in the absence of a clinical response.Citation30 Our data suggest that vaccine-induced CTLs are partially exhausted. The challenge is to overcome T-cell exhaustion by developing new adjuvants, novel vaccine formulations, or combination therapies with immune-modulating antibodies.Citation31 Recently, we reported that a PD-1-blocking antibody augmented GPC3-specific CTL clones that degranulate against liver tumor cells evaded by the CTLs because of PDL1 expression in vitro.Citation32 This should be investigated further to enhance the antitumor effect of the GPC3 peptide vaccine. The immunological analysis in this study may lead to improvement of the antitumor effect of GPC3 peptide vaccines.
In this case-control study, patients treated with surgery and vaccination did not have longer recurrence-free or overall survival than patients who underwent surgery only. However, GPC3 peptide vaccination in patients with GPC3-positive tumors did improve 1-y recurrence rates. This study demonstrated that GPC3 expression by the primary tumor could be a potential biomarker for a future clinical trial of a GPC3-derived peptide vaccine. We are planning another randomized clinical trial with eligibility criteria that include GPC3 expression in the primary tumor to determine the efficacy of a GPC3-derived peptide vaccine.
Materials and methods
Patient eligibility
This clinical trial was approved by the Ethics Committee of the National Cancer Center and was performed between September 2009 and August 2014. Patients with initial HCC who had undergone surgery or RFA were enrolled after providing written informed consent. The following eligibility criteria were employed: initial HCC, had undergone curative surgery or RFA (curability was evaluated by computed tomography [CT] or magnetic resonance imaging [MRI] before vaccination); HLA-A24- or HLA-A2-positive status, as determined by commercially available genomic DNA typing tests (Mitsubishi Chemical Medience, Tokyo, Japan); age between 20 and 85 y; an Eastern Cooperative Oncology Group performance status of 0–2; Child-Pugh liver function class A–B; and adequate organ function (total bilirubin ≤3.0 mg/dL, aspartate aminotransferase ≤200 IU/L, alanine aminotransferase ≤200 IU/L, and serum creatinine ≤1.5 mg/dL). The following exclusion criteria were applied: massive vascular invasion before curative surgery or RFA; extensive ascites; other active malignancy; serious infection; severe cardiac insufficiency; clinically serious comorbidity; pregnancy or lactation; and concurrent treatments unsuitable for the trial based on clinical judgment.
Study design and endpoints
This study was an open-label, single-arm, phase II clinical trial of the GPC3 peptide vaccine as an adjuvant therapy for initial HCC patients. The HLA-A*24:02-restricted GPC3298–306 peptide (EYILSLEEL) (American Peptide Company, Sunnyvale, CA) was used in HLA-A24-positive patients, and the HLA-A2-restricted GPC3144–152 peptide (FVGEFFTDV) (American Peptide Company) was used in HLA-A2-positive patients. GPC3 peptide (3.0 mg) was emulsified with IFA (Montanide ISA-51VG, SEPPIC) and administered by intradermal injection. Peptides and IFA were synthesized according to Good Manufacturing Practice guidelines. The first vaccine was administered within 4 weeks after curative surgery or RFA. Ten vaccinations were performed for 1 y after curative treatment; initially six times every 2 weeks followed by four times every 2 mo. Vaccine treatment was discontinued in cases in which patients experienced recurrence within 1 y following curative treatment. Primary endpoints were 1- and 2-y recurrence rates; secondary endpoints were safety and immunological responses. In this single-arm trial, the expected 1- and 2-y recurrence rates were 20% and 45%, respectively. We estimated that we would require the enrollments of 40 patients to justify proceeding with a follow-up randomized controlled trial. We noted that the 1- and 2- y recurrence rates of 80 patients who had undergone surgery between 2001 and 2002 were 42.5% and 66.2%, respectively (the estimated rates were 40.5% and 58.7%, respectively) at the National Cancer Center Hospital East. The study would have at least 80% power to detect a relative reduction in the risk of recurrence compared to a historical control, using a two-sided α level of 0.05. This study was approved by the Ethics Committee of the National Cancer Center, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The trial has been registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR number, 000002614).
Evaluation of toxicity and recurrence-free survival
Patients were examined for signs of toxicity during and after vaccination. Adverse events were graded according to the Common Terminology Criteria for Adverse Events v3.0. Hematological examinations were conducted prior to each vaccination. Contrast-enhanced CT was performed every 8–12 weeks. Contrast-enhanced MRI was performed, as needed, to diagnose suspicious recurrent lesions. We defined recurrence as the appearance of new lesions with radiological features typical of HCC. All time estimates were recorded with the date of curative treatment as the baseline. All patients received follow-up for at least 2 y, or until death.
Measurement of immunological response
PBMCs and ex vivo IFNγ enzyme-linked immunospot (ELISPOT) assay
An ex vivo IFNγ ELISPOT assay was performed to evaluate the antigen-specific CTL response, as described previously.Citation21 Peripheral blood (30 mL) was obtained for 2 y; initially every 2 weeks before each vaccination during the initial 3 mo, then every month for 9 mo (pre-vaccination), and finally every 2 mo during the second year. Samples were centrifuged with a Ficoll-Paque gradient and PBMCs were frozen prior to immunological analysis. All PBMCs obtained from an individual patient were incubated in the same plate and analyzed by an ex vivo IFNγ ELISPOT assay at the same time. Non-cultured PBMCs (5 × 105/well) were added to plates in the presence of peptide antigens (10 µg/mL) and incubated for 20 h at 37°C in 5% CO2. The antigen for GPC3 was either the HLA-A2-restricted GPC3144–152(FVGEFFTDV) peptide or the HLA-A*24:02-restricted GPC3298–306 peptide (EYILSLEEL). PBMCs plus HLA-A2-restricted HIV19–27 (TLNAWVKVV) peptide (ProImmune) or HLA-A*24:02-restricted HIV583–591 (RYLKDQQLL) (ProImmune) were used as negative controls. All analyses were performed in duplicate.
Immunohistochemical analysis
Biopsy or resected specimens were taken from some of the vaccinated patients, each of whom provided informed consent. Specimens were stained with hematoxylin and eosin or monoclonal antibodies against GPC3 (clone 1G12; dilution 1:300; BioMosaics), CD8 (clone 1A5; dilution 1:80; Novocastra), and HLA class I (clone EMR8/5; dilution 1:2500; Hokudo), according to the manufacturers’ directions.
Statistical analysis
All statistical analyses were performed using the PASW Statistics software, version 18.0 (SPSS Inc..). Survival rates were analyzed by the Kaplan–Meier method. Comparisons of patient characteristics were done using the Pearson χ2, Student’s t, and Mann–Whitney U tests. Statistical significance was defined by a value of p < 0.05.
Disclosure of potential conflicts of interest
T. N. is a scientific advisor for Ono Pharmaceutical co, Ltd. T. N. and Y. S. are supported by a fundamental research funding from Ono Pharmaceutical co, Ltd. The other authors have no potential conflicts of interest to declare with regard to this study.
Supplemental_Materials.zip
Download Zip (155.7 KB)Acknowledgments
Y.S. would like to thank the Foundation for Promotion of Cancer Research (Japan) for the Third-Term Comprehensive Control Research for Cancer for the award of a research resident fellowship. We also thank Manami Shimomura, Kayoko Shoda, and Yukiko Kozaki for technical assistance.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557-76; PMID:17570226; http://dx.doi.org/10.1053/j.gastro.2007.04.061
- Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg 2000; 191:381-8; PMID:11030243; http://dx.doi.org/10.1016/S1072-7515(00)00700-6
- Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y et al. Radiofrequency Ablation for Hepatocellular Carcinoma: 10-Year Outcome and Prognostic Factors. Am J Gastroenterol 2012; 107:569-77; PMID:22158026; http://dx.doi.org/10.1038/ajg.2011.425
- Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology 1997; 25:87-92; PMID:8985270; http://dx.doi.org/10.1002/hep.510250116
- Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015; 16(13):1344-54; PMID:26361969; http://dx.doi.org/10.1016/S1470-2045(15)00198-9
- Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA 2004; 292:358-61; PMID:15265851; http://dx.doi.org/10.1001/jama.292.3.358
- Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med 1996; 334:1561-7; PMID:8628336; http://dx.doi.org/10.1056/NEJM199606133342402
- Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology 2000; 32:228-32; PMID:10915728; http://dx.doi.org/10.1053/jhep.2000.9409
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802-7; PMID:11022927; http://dx.doi.org/10.1016/S0140-6736(00)02654-4
- Kuang M, Peng BG, Lu MD, Liang LJ, Huang JF, He Q, Hua YP, Totsuka S, Liu SQ, Leong KW et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res 2004; 10:1574-9; PMID:15014006; http://dx.doi.org/10.1158/1078-0432.CCR-03-0071
- Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 2003; 306:16-25; PMID:12788060; http://dx.doi.org/10.1016/S0006-291X(03)00908-2
- Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003; 125:89-97; PMID:12851874; http://dx.doi.org/10.1016/S0016-5085(03)00689-9
- Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009; 100:1403-7; PMID:19496787; http://dx.doi.org/10.1111/j.1349-7006.2009.01206.x
- Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18:3686-96; PMID:22577059; http://dx.doi.org/10.1158/1078-0432.CCR-11-3044
- Sawada Y, Yoshikawa T, Fujii S, Mitsunaga S, Nobuoka D, Mizuno S, Takahashi M, Yamauchi C, Endo I, Nakatsura T. Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican-3-derived peptide vaccination: An autopsy case. Human Vaccines and Immunotherapeutics. 2013; 9(7):1228-1233; PMID:23466818; http://dx.doi.org/10.4161/hv.24179
- Suzuki S, Shibata K, Kikkawa F, Nakatsura T. Significant clinical response of progressive recurrent ovarian clear cell carcinoma to glypican-3-derived peptide vaccine therapy: two case reports. Hum Vaccin Immunother 2013; 19:10(2):338–343; PMID: 24252799; http://dx.doi.org/10.4161/hv.27217
- Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 2006; 66:1139-46; PMID:16424051; http://dx.doi.org/10.1158/0008-5472.CAN-05-2244
- Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Yamashita T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma Hepatology 2013; 57(4):1448-57; PMID:23174905; http://dx.doi.org/10.1002/hep.26153
- Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, Ishii H, Furuse J, Gotohda N et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol 2012; 40:63–70; PMID:21922136; http://dx.doi.org/10.3892/ijo.2011.1202
- Yoshikawa T, Nakatsugawa M, Suzuki S, Shirakawa H, Nobuoka D, Sakemura N, Motomura Y, Tanaka Y, Hayashi S, Nakatsura T. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci 2011; 102:918-25; PMID:21281401; http://dx.doi.org/10.1111/j.1349-7006.2011.01896.x
- Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol 2013; 20:4305-11; PMID:23892527; http://dx.doi.org/10.1245/s10434-013-3144-x
- Sawada Y, Ofuji K, Sakai M, Nakatsura T. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. In: Reeves H, Manas DM, Lochan R, eds. editors Liver Tumors - Epidemiology, Diagnosis, Prevention and Treatment. Rijeka: Intech, 2013. 59-90 p; http://dx.doi.org/10.5772/54594
- Llovert JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. Journal of Hepatology 2008; 48:S20-S37; PMID:18304676; http://dx.doi.org/10.1016/j.jhep.2008.01.022
- Wang F, Bade E, Kuniyoshi C, Spears L, Jeffery G, Marty V, Groshen S, Weber J. Phase I trial of a MART-1 peptide vaccine with incomplete freund’s adjuvant for resected high-risk melanoma. Clin Cancer Res 1999; 5:2756-65; PMID:10537339
- Slingluff CL Jr, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, Whiteside TL, Leming PD, Kirkwood JM. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res 2013; 19:4228-38; PMID:23653149; http://dx.doi.org/10.1158/1078-0432.CCR-13-0002
- Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8+ T cellsinduced by melanoma vaccines. Cancer Res 2014; 74(4):1045-55; PMID:24343228; http://dx.doi.org/10.1158/0008-5472.CAN-13-2908
- Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med 2013; 19:465-72; PMID:23455713; http://dx.doi.org/10.1038/nm.3105
- Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL Jr. Activation, dissfunction and retention of T cell in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol Immunother 2013; 62:1149-59; PMID:23657629; http://dx.doi.org/10.1007/s00262-013-1435-5
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol 2005; 175:6169-76; PMID:16237114; http://dx.doi.org/10.4049/jimmunol.175.9.6169
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012; 62:309-335; PMID:22576456; http://dx.doi.org/10.3322/caac.20132
- Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol 2015; 46:28-36; PMID:25354479; http://dx.doi.org/10.3892/ijo.2014.2737