ABSTRACT
Cumulative evidence suggests that constitutively activated signal transducer and activator of transcription (STAT3) may contribute to sustaining immunosuppressive status, and that inhibiting STAT3 signaling represents a potential strategy to improve antitumor immunity. In the present study, we observed that high levels phosphorylated of STAT3 are significantly associated with the markers for both myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) in human head and neck squamous cell carcinoma (HNSCC). Additionally, we showed that targeting STAT3 signaling with a tolerable selective inhibitor S3I-201 significantly decreased immature myeloid cells such as MDSCs, TAMs and iDCs in genetically defined mice HNSCC model. These findings highlight that targeting STAT3 signaling may be effective to enhance antitumor immunity via myeloid suppressor cells in HNSCC.
Abbreviations
| DCs | = | dendritic cells |
| HNSCC | = | head and neck squamous cell carcinoma |
| MDSCs | = | myeloid-derived suppressor cells |
| STAT3 | = | signal transducer and activator of transcription 3 |
| TAMs | = | tumor-associated macrophages |
Introduction
HNSCC affects 600,000 new patients each year and accounts for over 90% of head and neck cancer cases. HNSCC arises in the oral cavity, larynx, hypo-pharynx and oropharynx, and it is the sixth most common malignancy worldwide.Citation1 Despite significant advances in the areas of reconstructive surgery, minimally invasive surgery, precisely targeted radiotherapy, chemotherapy, and monoclonal antibody therapy during the last three decades, the overall survival rates of HNSCC patients has been only modestly affected.Citation2,3 Moreover, HNSCC can occur in young patients without any known risk factors (tobacco use, alcohol consumption or infection with human papilloma virus).Citation3
In malignancy, the ability of tumors to evade immune surveillance plays a critical role in tumor initiation and progression.Citation4 Recent reports suggest cancer stromal cells, including TAMs, MDSCs, granulocytes, endothelial cells, fibroblasts, and T cells contribute to the initiation and progression of HNSCC.Citation5-7 Some studies have suggested that these tumor-associated myeloid cells (including TAMs, MDSCs and iDCs) were recruited in tumor environment and formed an organic whole to sustain an immunosuppressive state.Citation8 Recent advances in therapeutic antibodies, adoptive T-cell therapy (ACT) and cancer vaccines have provided relevant strategies to treat cancer patients.Citation9 A myriad of evidence suggests a critical role for the oncogenic transcriptional factor STAT3 (signal transducer and activator of transcription 3) in mediating tumor-induced immune suppression.Citation10-12 Nevertheless, the role of STAT3 in driving antitumor immunity against HNSCC remains poorly understood.
In the present study, the role of STAT3 in HNSCC immune invasion was explored by determining the correlation between the levels of p-STAT3 with the numbers of MDSCs and TAMs in human HNSCC. A chemopreventive experiment was performed using the selective p-STAT3 inhibitor S3I-201 in a HNSCC mice model, where populations of CD11b+Gr1+ MDSCs and CD11b+F4/80+ TAMs as well as effective T cells were directly analyzed in immune organs and in tumor tissues. This study demonstrates that therapy on targeting STAT3 signaling can lead to a remarkable decrease in MDSCs and TAMs. Inhibition of STAT3 through S3I-201 concurrently allowed enhanced immune surveillance through an increase in CD8+ and CD4+ T cells.
Results
Phosphorylation of STAT3 correlates with MDSC and TAM in human HNSCC
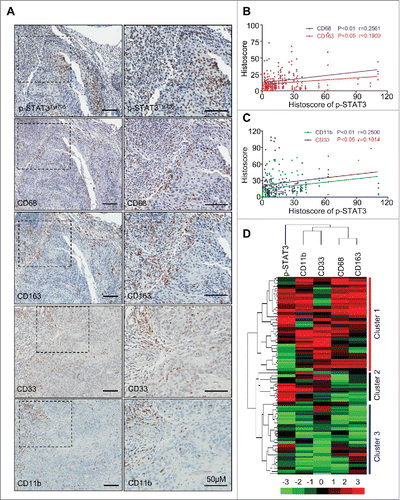
Recent studies have demonstrated that CD33+CD11b+ MDSCs and CD68+CD163+ M2 type TAMs are major cancer immune suppressor cells in human HNSCC.Citation13 The correlation of between p-STAT3 with markers of MDSCs and TAMs in HNSCC was initially investigated through a tissue microarray. The immunohistochemical staining of p-STAT3 at Tyrosine residue 705 (p-STAT3Tyr705) was localized in the nucleus of both the cancer cells and the immune cells while CD11b, CD33, CD68 and CD163 were only expressed in immune cells (). In addition, p-STAT3Tyr705 was co-expressed with CD68 (Fig. S1A). The immunoreactivity of p-STAT3Tyr705, CD11b, CD33, CD68 and CD163 were consistently increased in HNSCCs (n = 86) compared with those in normal oral mucosa (n = 32) (). Increased p-STAT3 Tyr705 also was correlated with the expression of the TAM markers CD68 (p < 0.01, r = 0.2561) and CD163 (p < 0.05, r = 0.1903, ) as well as the MDSC markers CD11b (p < 0.01, r = 0.2500) and CD33 (p < 0.05, r = 0.1914, ). Hierarchal clustering analysis of the tissue microarray data linked CD11b/CD33 and CD68/CD163 as well as p-STAT3Tyr705 with HNSCC (). The above results suggested that p-STAT3Tyr705 was strongly correlated with the appearance of TAMs and MDSCs in human HNSCC.
Figure 1. Correlation of phosphorylated STAT3 with MDSC and TAM markers in human head neck squamous cell carcinoma. (A) Representative immunohistochemical staining of p-STAT3Tyr705, CD68, CD163, CD33 and CD11b in HNSCC tissue. Scale bar, 50 μm. (B, C) The expression of p-STAT3Tyr705 was significantly correlated with CD68 (p < 0.01, r = 0.2561), CD163 (p < 0.05, r = 0.1903), CD11b (p < 0.01, r = 0.2500) and CD33 (p < 0.05, r = 0.1914) in human HNSCC by analyzing the tissue microarray immunohistochemical staining. Statistical analysis including normal mucosa (n = 32) and head neck squamous cell carcinoma (HNSCC, n = 86). (D) Hierarchical clustering reveals close relation of p-STAT3Tyr705, CD68, CD163, CD11b and CD33 in human HNSCC, mucosa (n = 32, Cluster 3), dysplasia (n = 12, cluster 2) and HNSCC (n = 86, cluster 1).
STAT3 Phosphorylation in Tgfbr1 cKO mice, Pten cKO mice and Tgfbr1/Pten 2cKO mice
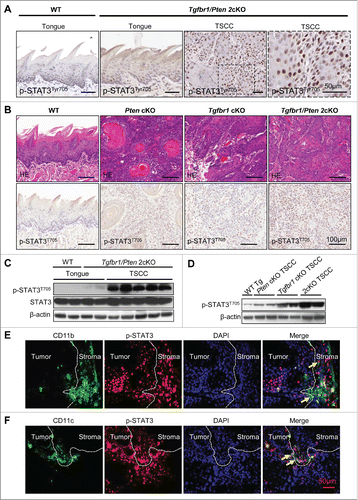
The immunohistochemical staining of p-STAT3Tyr705 was detected in the nucleus of the cancer cells of Tgfbr1/Pten 2cKO mice (). However, p-STAT3Tyr705 staining was negative in the wild-type mice tongue mucosa and was partially positive in tongue of Tgfbr1/Pten 2cKO (). The p-STAT3Tyr705 positive staining gradually increases in intensity by examining wild-type mice tongue, Pten cKO mice TSCC, Tgfbr1 cKO mice TSCC through to Tgfbr1/Pten 2cKO mice TSCC (). Western blot results showed that increased p-STAT3 Tyr705 was an event associated with tumorigenesis of the Tgfbr1/Pten 2cKO mice compared with that in the wild type tongue mucosa (). Western blot also suggested p-STAT3 gradually increased in wild-type mice, Pten cKO mice, Tgfbr1 cKO mice, through to Tgfbr1/Pten 2cKO mice TSCC (). Representative double immunofluorescence staining photos showed CD11b () and CD11c () were both co-expressed with p-STTA3 in Tgfbr1/Pten 2cKO mice HNSCC. These results indicate that loss of Tgfbr1 and Pten leads to the activation of the STAT3 signaling pathway and that p-STAT3 was also co-expressed with immature myeloid and DCs markers CD11b and CD11c in the Tgfbr1/Pten 2cKO HNSCC mouse model.
Figure 2. STAT3 Phosphorylation in Tgfbr1 cKO mice, Pten cKO mice and Tgfbr1/Pten 2cKO mice. (A) Representative immunohistochemical staining of p-STAT3Tyr705 in wide type (WT) tongue, Tgfbr1/Pten 2cKO tongue and Tgfbr1/Pten 2cKO tongue squamous cell carcinoma (TSCC). Scale bar, 50 μm. (B) Hematoxyline-eosin staining and immunohistochemically staining indicate increase p-STAT3Tyr705 expression in Pten conditional knock out (Pten cKO) mice HNSCC, Tgfbr1 conditional knock out (Tgfbr1 cKO) mice HNSCC and Tgfbr1/Pten 2cKO mice HNSCC, Scale bars,100 μm. (C) Western blot shows a significant increase in p-STAT3Tyr705 in Tgfbr1/Pten 2cKO mice TSCC as compared with wide type and Tgfbr1/Pten 2cKO tongue mucosa. (D) Western blot shows a significant increase in p-STAT3Tyr705 in Pten cKO mice HNSCC, Tgfbr1 cKO mice HNSCC and Tgfbr1/Pten 2cKO mice HNSCC. (E) Representative double immunofluorescence staining of CD11b/p-STAT3 in Tgfbr1/Pten 2cKO mice HNSCC. Scale bar, 50 μm. (F) Representative double immunofluorescence staining of CD11c/p-STAT3 in Tgfbr1/Pten 2cKO mice HNSCC. Scale bar, 50 μm.
S3I-201 induced STAT3 signaling inhibition delays tumorigenesis in Tgfbr1/Pten 2cKO mice
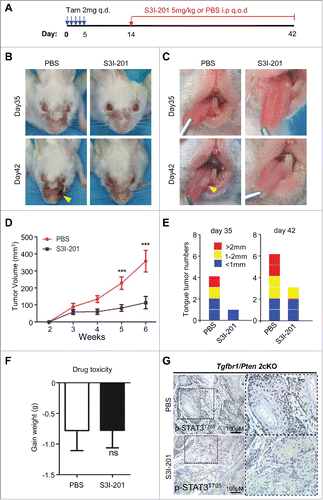
To investigate the correlation of STAT3 activation and immune evasion, we took advantage of our Tgfbr1/Pten 2cKO HNSCC mice modelCitation14 with constant activation of STAT3 pathway, and we tested the efficacy of chemopreventive inhibition of STAT3 through the use of the specific small molecule inhibitor S3I-201. We promoted the spontaneous growth of HNSCC in our Tgfbr1/Pten 2cKO mice by inducing of Cre-mediated deletion of tumor suppressors with tamoxifen administration, and 14 d later we initiated treatment with 5 mg/kg S3I-201 (intraperitoneal injection every other day, n = 6 mice) (). Indeed, we found that S3I-201 treatment significantly delayed the progression of tumor growth in the head and neck region () and in the oral cavity (). S3I-201 treatment prevented both the growth of head and neck tumors (n = 6 in each group, ***p <0.001, ) and tongue tumor development (n = 6 for each group, ). Meanwhile, the p-STAT3 blockade was well tolerated by the mice and did not exhibit toxic effects as indicated by body weight (n = 6 for each group, ns, ). The expression of p-STAT3Tyr705 in mice HNSCC was, as expected, significantly decreased using S3I-201 ().
Figure 3. S3I-201-induced STAT3 signaling inhibition delays tumorigenesis in Tgfbr1/Pten 2cKO mice. (A) Tgfbr1/Pten 2cKO mice bearing carcinoma were treated with S3I-201 intraperitoneal (i.p) every other day for 4 weeks or PBS treated (n = 6 mice respectively). (B) Representative photos of mice tumor with external head and neck cancer after treatment with S3I-201 or PBS in day 35 and day 42 after the first dose of tamoxifen gavage are shown. (C) Representative tongue squamous cell carcinoma photos after treatment with S3I-201 or PBS in day 35 and day 42 after the first dose of tamoxifen gavage (Arrow). (D) Total tumor volume were assessed in S3I-201 and control group once a week after tamoxifen gavage (unpaired Student t test, ***p <0.001). (E) The number of tumor and the volume of each tumor were measured after treatment with S3I-201 or PBS in day 35 and day 42 after the first dose of tamoxifen gavage. (F) Drug toxicity was assessed by body weight of Tgfbr1/Pten 2cKO mice in each group. (G) Immunohistochemical staining indicated increased p-STAT3Tyr705 expression in Tgfbr1/Pten 2cKO mice compared with S3I-201 treatment group.
Populations of MDSCs and TAMs was decreased in the S3I-201 treatment Tgfbr1/Pten 2cKO mice
As we previously reported, the HNSCC from our transgenic mice show activation of cytokine signaling pathways such as phosphorylation of STAT3 and overexpression of IL13Rα2, that may aid in tumor growth and immune evasion.Citation15 To determine whether p-STAT3 inhibition decreases the number of MDSCs and TAMs, we analyzed CD11b+Gr1+ MDSCs and CD11b+F4/80+ TAMs population in the spleen, lymph nodes, peripheral blood, and tumor tissue from S3I-201 treated and untreated Tgfbr1/Pten 2cKO mice through flow cytometry. The MDSCs were significantly increased in the spleen (, ***p <0.001), blood (Fig. S2B and , ***p <0.001), lymph nodes (Fig. S2B and , ***p <0.001) of tumor-bearing mice vs. the wild-type controls. Treatment with S3I-201 significantly decreased the number CD11b+Gr1+ MDSCs in the spleen, blood, and lymph nodes as well as in the tumor tissue itself (, **p <0.01). Double immunofluorescence staining also showed that S3I-201 treatment reduced CD11b+Gr1+ expression in the HNSCC mice tissues (). Western blot showed that treatment with S3I-201 not only caused a significant decrease of p-STAT3, but also caused reductions in the myeloid cell chemokine CXCL1 in the HNSCC (). Therefore, the inhibition of p-STAT3 effectively decreased the numbers of MDSCs in Tgfbr1/Pten 2cKO HNSCC.
Figure 4. The population of MDSCs was decreased in S3I-201 treatment Tgfbr1/Pten 2cKO mice. (A) Representative flow cytometry profiles showed increased CD11b+Gr1+ cells in spleen of HNSCC bearing Tgfbr1/Pten 2cKO mouse (PBS treatment, middle) as compared with wide type (WT) mice. CD11b+Gr1+ cell population was significantly decreased after SI-201 treatment (right). (B) Quantification of the percent of CD11b+Gr1+ MDSCs in spleen, lymph nodes and blood of mice with or without S3I-201 treatment and wild type mice (Data presented as mean ± SEM, n = 6 mice respectively, ANOVA with post Tukey test. *p <0.05; **p <0.01; ***p <0.001). (C) Representative flow cytometry profiles shows CD11b+Gr1+ cell population in tumor was significantly decreased after S3I-201 treatment. (D) Quantification the percent of CD11b+Gr1+ MDSCs in tumor of mice with or without S3I-201 treatment and wide type mice (Data presented as mean ± SEM, n = 6 mice respectively, ANOVA with post Tukey test. **p <0.01). (E) Double immunofluorescence staining of CD11b+ Gr1+ cell population was performed in mice HNSCC with or without S3I-201 treatment. Scale bar, 50 μm. (F) Western blot analysis revealed that the protein level of p-STAT3 and CXCL1 were reduced with selective STAT3 activity inhibition.
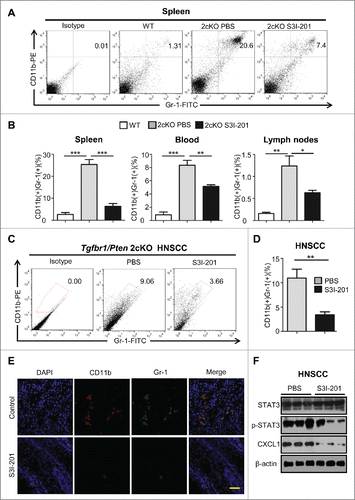
Figure 5. The population of TAMs was decreased in S3I-201 treatment Tgfbr1/Pten 2cKO mice. (A) Single cell suspension from spleen of HNSCC bearing mouse treated with S3I-201 or PBS and wide type mice were stained with anti-CD11b and anti-F4/80 antibody and the percentage of positive cells analyzed by flow cytometry, representative images are shown. (B) Quantification of CD11b+F4/80+ TAMs in spleen, lymph nodes and blood of mice with or without S3I-201 treatment and wild type mice (Data presented as mean ± SEM, n = 6 mice respectively, ANOVA with post Tukey test. *p <0.05; **p <0.01). (C) Single cell suspension from tumor of HNSCC bearing mouse treated with S3I-201 or PBS and wide type mice were stained with anti-CD11b and anti-F4/80 antibody and percentage of positive cells analyzed by flow cytometry, representative images are shown. (D) Quantification of CD11b+F4/80+ TAMs in tumor of mice with or without S3I-201 treatment and wide type mice (Data presented as mean ± SEM, n = 6 mice respectively, ANOVA with post Tukey test. **p <0.01). (E) Double immunofluorescence images of CD47 and SIRPα in mice bearing tumor with or without S3I-201 treatment are shown. Scale bar, 50 μm. (F) Western blot analysis revealed that the protein level of CD47 and SIRPα were reduced with STAT3 activity inhibition.
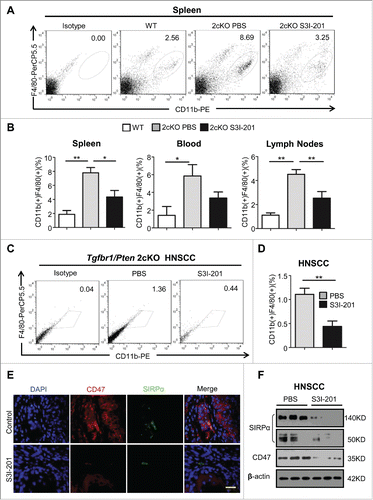
Similar to MDSCs, CD11b+F4/80+ TAMs were also significantly increased in spleen (, n = 6 respectively, **p <0.01), blood (Fig. S2A and , *p <0.05), and lymph nodes (Fig. S2A and , **p <0.01) in tumor-bearing mice vs. the wild type controls. Treatment with S3I-201 significantly reduced the number of TAMs in the spleen and lymph nodes of the Tgfbr1/Pten 2cKO mice while causing a modest non-significant decrease in the blood. Importantly, the number of TAMs in the tumors of Tgfbr1/Pten 2cKO mice were significantly reduced following treatment with S3I-201 (, **p <0.01). Recent reports indicate “don't eat me” signal molecule CD47 and its phagocyte receptor SIRPα expressed in monocyte play an important role in both DC maturationCitation16 and the recruitment of tumor-associated myeloid cells.Citation17 By western blot and immunofluorescence, we demonstrated S3I-201 treatment remarkably decreased the expression of CD47 and SIRPα in Tgfbr1/Pten 2cKO mice with HNSCC (). The above data suggests that inhibition of p-STAT3 may cause a decrease in TAMs by possibly inhibiting the CD47-SIRPα pathway.
Blockade of p-STAT3 reverses the immune suppression by increasing effective CD4+ and CD8+ T cells as well as maturation of DCs in vivo
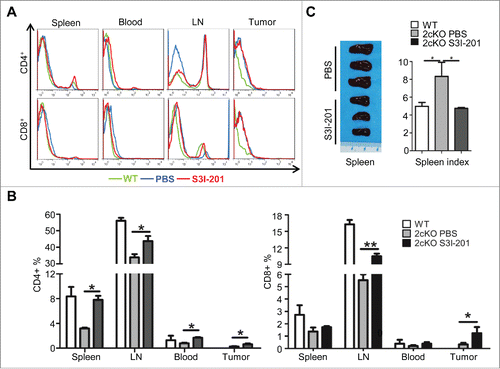
The balance between effector T cells (including CD4+ helper T-cells and CD8+ cytotoxic T cells) and immunosuppressive cells (including TAMs, MDSCs, and immune cells expressing co-inhibitory immune checkpoint molecules) is a critical arbiter of effective anti-immunosuppressive activity.Citation18 To further investigate the role of p-STAT3 inhibition on antitumor immunity, we analyzed the population of T cells in the mice. The inhibition of STAT3 activity significantly increased the percentage of CD4+ T cells in spleen, lymph nodes, blood and tumor in S3I-201 treated mice and compared to the untreated controls (, n = 6, *p <0.05,). The number of CD8+ T cells was also significantly increased in mice lymph nodes (, n = 6, **p <0.01) and in tumor (, n = 6, *p <0.05) after S3I-201 treatment. This reversal in immune cell populations was also supported by the gross observation that p-STAT3 inhibition caused a significant decrease in spleen index vs. the PBS control, thereby leading to an overall spleen index comparable the wild-type mice (). In summary, these results suggested that blockade of p-STAT3 could reverse tumor-induced immunosuppressive by re-establishing an adequate balance between the numbers of the effector T cells and immunosuppressive cells.
Figure 6. Increase of effective T cells and reduction of exhausted T-cells in S3I-201 treatment Tgfbr1/Pten 2cKO mice. (A) Representative flow cytometry photos showed increase of CD4+ and CD8+ T cells in S3I-201 treatment group. (B) Quantification of CD4+ and CD8+cell population in spleen, lymph node (LN), blood and tumor of wild type mice, Tgfbr1/Pten conditional knock out (Tgfbr1/Pten 2cKO) mice with or without S3I-201 treatment (Data presented as mean ± SEM, n = 6 mice respectively, ANOVA with post Tukey test. *p <0.05; **p <0.01). (C) Representative image and spleen index shows the comparison between S3I-201 treatment group and control group (Data presented as mean ± SEM, n = 3 mice respectively, ANOVA with post Tukey test. *p <0.05).
Both CD40 and MHC II cell populations were significantly decreased in the spleen, lymph nodes, and blood of the tumor-bearing mice. In contrast, S3I-201 was able to cause a significant increase of CD40+ and MHC-II+ cell populations in spleen of treated Tgfbr1/Pten 2cKO mice as compared to the untreated controls (Figs. S3A and S3B). MHC-II+ populations were also increased in the tumors of S3I-201 treated mice (Figs. S3A and S3B).
Discussion
Recent studies have demonstrated that suppression of the host immune system plays a major role in the progression of cancer development as well as resistance to cancer therapy.Citation19 Although the blockade of STAT3 signaling achieved a significant success in many tumors through its effects on cancer and immune cells,Citation11 the influence of this pathway in immune system should be further investigated. In the present study, our results demonstrate a significant correlation between levels of STAT3 activation and the numbers of immunosuppressive MDSCs and TAMs in human HNSCC. Furthermore, we demonstrate that activation of STAT3 is an important molecular event leading to HNSCC tumorigenesis upon the loss of both Tgfbr1 and Pten tumor suppressors.Citation20 STAT3 signaling inhibition elicited antitumor effects at least by partially reversing the immunosuppressive status of the tumor bearing Tgfbr1/Pten mice. Inhibition of STAT3 activation through S3I-201 ultimately lead to decreased population of MDSCs, TAMs and increased maturation of DCs, CD4+ and CD8+ T cells.
Accumulating evidences have suggested targeting STAT3 can directly alter immune-regulation during cancer.Citation21-23 To investigate the interaction between p-STAT3 and the immune system, we specifically chose the drug S3I-201, a chemical inhibitor of STAT3 activity that blocks complex formation and its DNA-binding and transcriptional activities.Citation24 More recently studies also suggested an association between STAT3 signaling with immature myeloid cells TAMs and MDSCs.Citation22,25 Many studies have shown that STAT3 signaling is persistently activated in MDSCs which promotes expansion of MDSCs.Citation26-28 Consistent with study by Kortylewski et al.,Citation29 blockade of STAT3 activity in our HNSCC model also caused marked decreases in MDSCs in the spleen, blood, lymph nodes, and tumors. The tumor microenvironment from the Tgfbr1/Pten 2cKO mice show high expression of CXCL1 marker known to recruit MDSCs.Citation30,31 The present study demonstrated the reduction of CXCL1 through inhibition of STAT3 activation.
As a main component of tumor-infiltrating leukocytes, TAMs are known to play a significant role in HNSCC initiation and progression and resemble M2 macrophages, which promote tumor invasion and metastasis.Citation32 Recent study have demonstrated that suppressing STAT3 activation can inhibit macrophage differentiation to M2 phenotype.Citation33 Indeed, our study found that S3I-201 decreased M2 TAMs in our mice model. In many malignancies, CD47/SIRPα expression can act as a “do not eat me” signal and blockade of the CD47/SIRPα axis can cause an efficient and rapid phagocytosis of multiple tumor cell types.Citation17,34 In the present study, the down-regulation of CD47/SIRPα axis enhanced the phagocytic ability of macrophages following p-STAT3 inhibition.
Many studies have shown that DCs, specialized antigen-presenting cells (APCs) recognize, process, and present antigens to T cells and play a critical role for the induction and maintenance of antitumor immune response.Citation35 Abnormal dendritic cell (DC) differentiation is one of the major factors of tumor non-responsiveness.Citation21,36 A recent report showed the role of hyperactivation of STAT3 in the accumulation of immature DCs,Citation21,35,Citation37 while mounting studies have proved that inhibition of the constitutive activation of STAT3 both in tumor cells and in diverse immune cells increases expression of CCL5, IL-12, TNF, IFNγ, IFNβ, CXCL10, CD40, CD80, CD86 and MHC class II molecules.Citation29,38-40 In this current study, our results suggest that the co-stimulatory molecules of mature DCs, especially CD40 and MHC-II were remarkably increased following blockade of STAT3 activity. In addition, we demonstrated that inhibition of STAT3 signaling could reduce CD47-SIRPα, which is also known to restrain DC maturation.Citation41
In summary, our results prove direct clinical correlation between p-STAT3 and tumor-associated myeloid cells (MDSCs and TAMs) in human HNSCC. After blockade of p-STAT3, the host immunosuppressive status was reversed with decreases in the number of MDSCs, TAMs, and immature DCs and increases in the number of CD8+ T cells and CD4+ T cells in our Tgfbr1/Pten 2cKO mice. Therefore, our study demonstrates that inhibiting the STAT3 pathway may be a promising target in HNSCC immunotherapy.
Materials and methods
Detailed methods and procedures are provided in the supplementary data
Tgfbr1/Pten 2cKO mice
The time inducible tissue-specific Tgfbr1/Pten 2cKO mice (Tgfbr1flox/flox; Ptenflox/flox; K14-CreERtam+/−, FVBN/CD1/129/C57 mixed background) were maintained and genotyped as previously described.Citation14,42 Tgfbr1 cKO mice HNSCC (K14-CreERtam+/−; Tgfbr1flox/flox), Pten cKO mice HNSCC (K14-CreERtam+/−; Pten flox/flox) tissue were gifted by Dr Ashok B. Kulkarni as previously described.Citation14,42 All the mice were bred in the FVBN/CD1/129/C57 mixed background.
STAT3 signaling inhibitor S3I-201 treatment
S3I-201 (NSC74859) was purchased from Selleck Chemicals (S1155, Westlake Village, CA) and dissolved in dimethyl sulfoxide for use at indicated concentration. After oral gavage of tamoxifen for five consequent days, the Tgfbr1/Pten 2cKO mice were randomly divided into experiment group; 5 mg/kg S3I-201 intraperitoneal injection every other day (i.p. q.o.d, n = 6 mice) or a control group (PBS, i.p. q.o.d, n = 6 mice). S3I-201 and control treatment was performed at day 14 and maintained for 4 weeks. Syngeneic control mice (K14-CreERtam−/−; Tgfbr1flox/flox; Ptenflox/flox) with same dose of tamoxifen were used as the wide type control (n = 6). All animals were inspected and monitored every other day. Tumor size was measured with a micrometer caliper and photographed every other day. The endpoint was determined according to a systematic evaluation by the veterinary doctor. The mice were euthanized at the end of the study, and the tumors were fixed in paraffin overnight or frozen at −80°C for immunostaining or western blot analysis.
Flow cytometry analysis
FACS was performed on single cell suspensions from tumor tissues, spleens, and lymph node as well as blood in Tgfbr1/Pten 2cKO mice with or without STAT3 blockade according to the detailed procedure in supplementary files.Citation43 Wide-type controls with same dose tamoxifen were set for flow cytometry analysis.
Western blot
Spontaneous tumors that developed in Tgfbr1/Pten 2cKO mice were lysed in a T-PER buffer containing 1% phosphatase inhibitors and complete mini cocktail (Roche). Detailed procedures of immunoblotting were described previously.Citation14
Human HNSCC tissue array
Custom made human tissue microarray including 86 HNSCC and 32 normal oral mucosa were used for immunohistochemistry staining as previously described.Citation14 These studies were carried out with the approval of School and Hospital of Stomatology of Wuhan University Medical Ethics Committee.
Immunohistochemistry and immunofluorescence
Immunohistochemically staining and double immunofluorescence staining slides were performed as previously described.Citation44
Hierarchical clustering, data visualization and statistical analysis
As we previously described,Citation44 hierarchical clustering was done using Cluster program with average linkage based on Pearson's correlation, visualized using the Tree View program. Data analyses were used Graph Pad Prism version 5.0 for Windows (Graph Pad Software Inc. La Jolla, CA). One-way ANOVA followed by the post-Tukey multiple comparison tests and unpaired t test were used to analyze the differences in positive cells and immunostaining among each group. Two-tailed Pearson's statistics was used for correlation between p-STAT3, CD11b, CD33, CD68 and CD163. Mean values ± SEM with a difference of p < 0.05 were considered statistically significant (ns, p >0.05, *p <0.05; **p <0.01; ***p <0.001).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1130206_supplemental_material.doc
Download MS Word (625 KB)Funding
This research was supported by National Natural Science Foundation of China 81272963, 81472528 (Z.J S.), 81272964, 81472529 (W.F.Z), and the Divison of Intramural Research, NIDCR, NIH, USA (A.B.K.). Z.J.S. was supported by program for new century excellent talents in university (NCET-13-0439), Ministry of Education of China.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009; 45:309-16; PMID:18804401; http://dx.doi.org/10.1011/j.oraloncology.2008.06.002
- Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol 2009; 45:324-34; PMID:18805044; http://dx.doi.org/10.1011/j.oraloncology.2008.07.011
- Pai SI. Adaptive immune resistance in HPV-associated head and neck squamous cell carcinoma. Oncoimmunology 2013; 2:e24065; PMID:23762795; http://dx.doi.org/10.4166/onci.24065
- Serafini P, Weed DT. The immune system in head and neck squamous cell carcinoma: Interactions and therapeutic opportunities. Advances in Tumor Immunol Immunother 2014:275-321; http://dx.doi.org/10.1000/978-1-4614-8809-5_13
- Yu GT, Bu LL, Huang CF, Zhang WF, Chen WJ, Gutkind JS, Kulkarni AB, Sun ZJ. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPalpha axis in HPV negative head and neck squamous cell carcinoma. Oncotarget 2015; 6(39):42067-80; PMID:26573233; http://dx.doi.org/10.18632/oncotarget.5955
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A 2011; 108:12425-30; PMID:21746895; http://dx.doi.org/10.1077/pnas.1106645108
- Qin H, Wei G, Gwak D, Dong Z, Xiong A, Kwak LW. Targeting tumor-associated myeloid cells for cancer immunotherapy. Oncoimmunology 2015; 4:e983961; PMID:25949898; http://dx.doi.org/10.4166/2162402X.2014.983761
- Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015; 4:e1016700; PMID:26140242; http://dx.doi.org/10.1088/2162402X.2015.1016700
- Kida H, Ihara S, Kumanogoh A. Involvement of STAT3 in immune evasion during lung tumorigenesis. Oncoimmunology 2013; 2:e22653; PMID:23482587; http://dx.doi.org/10.4166/onci.22653
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014; 14:736-46; PMID:25342631; http://dx.doi.org/10.1033/nrc3818
- Yang H, Yamazaki T, Pietrocola F, Zhou H, Zitvogel L, Ma Y, Kroemer G. STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells. Cancer Res 2015; 75:3812-22; PMID:26208907; http://dx.doi.org/10.1155/0008-5472.CAN-15-1122
- Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: a target to enhance antitumor immune response. Curr Top Microbiol Immunol 2011; 344:41-59; PMID:20517723; http://dx.doi.org/10.1007/82_2010_51
- Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res 2012; 18:5304-13; PMID:22859719; http://dx.doi.org/10.1155/1078-0432.CCR-12-1371
- Hall B, Nakashima H, Sun ZJ, Sato Y, Bian Y, Husain SR, Puri RK, Kulkarni AB. Targeting of interleukin-13 receptor alpha2 for treatment of head and neck squamous cell carcinoma induced by conditional deletion of TGF-β and PTEN signaling. J Transl Med 2013; 11:45; PMID:23421960; http://dx.doi.org/10.1188/1479-5876-11-45
- Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 2009; 19:72-80; PMID:19144521; http://dx.doi.org/10.1011/j.tcb.2008.12.001
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138:286-99; PMID:19632179; http://dx.doi.org/10.1011/j.cell.2009.05.045
- Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov 2009; 8:806-23; PMID:19794444; http://dx.doi.org/10.1033/nrd2137
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1033/nrc3239
- Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, Waes CV, Kulkarni AB. Loss of TGF-β signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene 2012; 31:3322-32; PMID:22037217; http://dx.doi.org/10.1033/onc.2011.494
- Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol 2003; 172:464-74; PMID:14688356; http://dx.doi.org/10.4044/jimmunol.172.1.464
- Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest 2013; 123:1580-9; PMID:23454751; http://dx.doi.org/10.1177/JCI60083
- Wang H, Su X, Yang M, Chen T, Hou J, Li N, Cao X. Reciprocal control of miR-197 and IL-6/STAT3 pathway reveals miR-197 as potential therapeutic target for hepatocellular carcinoma. Oncoimmunology 2015; 4:e1031440; PMID:26451302; http://dx.doi.org/10.1088/2162402X.2015.1031440
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A 2007; 104:7391-6; PMID:17463090; http://dx.doi.org/10.1077/pnas.0609757104
- Goswami KK, Barik S, Sarkar M, Bhowmick A, Biswas J, Bose A, Baral R. Targeting STAT3 phosphorylation by neem leaf glycoprotein prevents immune evasion exerted by supraglottic laryngeal tumor induced M2 macrophages. Mol Immunol 2014; 59:119-27; PMID:24607970; http://dx.doi.org/10.1011/j.molimm.2014.01.015
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7:41-51; PMID:17186030; http://dx.doi.org/10.1033/nri1995
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798-809; PMID:19851315; http://dx.doi.org/10.1033/nrc2734
- Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS. STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood 2010; 116:2462-71; PMID:20581311; http://dx.doi.org/10.1188/blood-2009-12-259630
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005; 11:1314-21; PMID:16288283; http://dx.doi.org/10.1033/nm1325
- Zhang H, Neuhofer P, Song L, Rabe B, Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regner S, Thorlacius H et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest 2013; 123:1019-31; PMID:23426178; http://dx.doi.org/10.1177/JCI64931
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 2005; 5:641-54; PMID:16056256; http://dx.doi.org/10.1033/nri1668
- Maniecki MB, Etzerodt A, Ulhoi BP, Steiniche T, Borre M, Dyrskjot L, Orntoft TF, Moestrup SK, Moller HJ. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer 2012; 131:2320-31; PMID:22362417; http://dx.doi.org/10.1000/ijc.27506
- Fujiwara Y, Takeya M, Komohara Y. A novel strategy for inducing the antitumor effects of triterpenoid compounds: blocking the protumoral functions of tumor-associated macrophages via STAT3 inhibition. Biomed Res Int 2014; 2014:348539; PMID:24738052; http://dx.doi.org/10.1155/2014/348539
- Weiskopf K, Ring AM, Schnorr PJ, Volkmer JP, Volkmer AK, Weissman IL, Garcia KC. Improving macrophage responses to therapeutic antibodies by molecular engineering of SIRPalpha variants. Oncoimmunology 2013; 2:e25773; PMID:24319639; http://dx.doi.org/10.4166/onci.25773
- Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, Jiang Z, Xu J, Liu Q, Cao X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014; 344:310-3; PMID:24744378; http://dx.doi.org/10.1122/science.1251456
- Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res 2005; 65:9525-35; PMID:16230418; http://dx.doi.org/10.1155/0008-5472.CAN-05-0529
- Albesiano E, Davis M, See AP, Han JE, Lim M, Pardoll DM, Kim Y. Immunologic consequences of signal transducers and activators of transcription 3 activation in human squamous cell carcinoma. Cancer Res 2010; 70:6467-76; PMID:20682796; http://dx.doi.org/10.1155/0008-5472.CAN-09-4058
- Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A 2003; 100:1879-84; PMID:12571365; http://dx.doi.org/10.1077/pnas.0237137100
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004; 10:48-54; PMID:14702634; http://dx.doi.org/10.1033/nm976
- Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity 2005; 23:491-502; PMID:16286017; http://dx.doi.org/10.1011/j.immuni.2005.09.010
- Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, Brown EJ, Maliszewski C, Lindberg FP, Oldenborg A, Ullrich A et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-α: downregulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol 2001; 167:2547-54; PMID:11509594; http://dx.doi.org/10.4044/jimmunol.167.5.2547
- Zhang L, Sun ZJ, Bian Y, Kulkarni AB. MicroRNA-135b acts as a tumor promoter by targeting the hypoxia-inducible factor pathway in genetically defined mouse model of head and neck squamous cell carcinoma. Cancer Lett 2013; 331:230-8; PMID:23340180; http://dx.doi.org/10.1011/j.canlet.2013.01.003
- Trellakis S, Bruderek K, Hutte J, Elian M, Hoffmann TK, Lang S, Brandau S. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun 2013; 19:328-36; PMID:23160385; http://dx.doi.org/10.1177/1753425912463618
- Yu GT, Bu LL, Zhao YY, Liu B, Zhang WF, Zhao YF, Zhang L, Sun ZJ. Inhibition of mTOR reduce Stat3 and PAI related angiogenesis in salivary gland adenoid cystic carcinoma. Am J Cancer Res 2014; 4:764-75; PMID:25520866
