ABSTRACT
Inhibition of the PD-1/PD-L1 pathway may induce anticancer immune responses in non-small cell lung cancer (NSCLC). Two PD-L1 immunohistochemistry (IHC) assays have been approved as companion diagnostic tests for therapeutic anti-PD-1 antibodies. However, many aspects of PD-L1 prevalence and association with genetically defined subtypes have not been addressed systematically. Here, we analyzed PD-L1 expression in 436 genetically annotated NSCLC specimens enriched for early stages using PD-L1 antibody 5H1. Expression of PD-L1 was detected in the tumor cells (TC) (34% of cases) and in associated immune cells (IC) (49%) across all stages of NSCLC, either alone or in combination. PD-L1 IHC-positive TC, but not IC showed significantly higher PD-L1 RNA expression levels. Expression in TC was associated with TP53, KRAS and STK11 mutational status in adenocarcinomas (AD) and with NFE2L2 mutations in squamous cell carcinomas (SQ). No correlations with histological subtype, clinical characteristics and overall survival were found. The presence of PD-L1-positive IC was significantly associated with patients' smoking status in AD. The findings are in agreement with the emerging concept that tumors with high mutational burden are more likely to benefit from immunotherapy, since TP53 and KRAS mutations are linked to smoking, increased numbers of somatic mutations and expression of neoantigens. Current clinical studies focus on stage IIIB and IV NSCLC; however, PD-L1 expression occurs in earlier stages and might be a predictive biomarker in clinical trials testing (neo-) adjuvant strategies.
Introduction
Anticancer immunotherapy targeting so-called immune checkpoints has become an established modality for advanced malignant melanoma and advanced pulmonary SQ.Citation1-3 Initial clinical approvals were not accompanied bycompanion diagnostic tests, given that unselected patients treated with immune checkpoint inhibitors achieved higher response rates and better clinical outcome compared to treatment with the standard-of-care, cytotoxic chemotherapy.Citation1,2,4-6 Two recent approvals for anti-PD-1 therapy in advanced NSCLC included the assessement of PD-L1 expression using PD-L1 immunohistochemistry (PD-L1 IHC) as obligatory companion diagnostic test.Citation7,8 Each of the two therapeutic antibodies features its own PD-L1 IHC assay and scoring criteria (Primary antibodies: 22C3; 28-8). Another two PD-L1 assays are currently tested in clinical trials (Primary antibodies: SP142; SP263). Many research trials have been conducted with other commercially available antibodies, most notably clone E1L3N.Citation9,10 Accordingly, results on prevalence of PD-L1 expression, predictive value and prognostic value differ considerably.Citation11-13
Here we performed PD-L1 IHC on a collection of 436 NSCLC specimens using antibody clone 5H1 ().Citation14 This antibody was among the first-monoclonal PD-L1 antibodies, and its potential predictive value is well established.Citation15,16 All cases were included in comprehensive NSCLC genomic studies and are clinically and genetically annotated.Citation17 PD-L1 protein expression was correlated with alterations in 27 genes, RNA expression as well as to clinical parameters.
Results
PD-L1 immunohistochemistry
IHC for PD-L1 using the 5H1 monoclonal antibody yielded previously described staining patternsCitation18: NSCLC specimens featured varying proportions of PD-L1-positive TC and tumor-associated IC. PD-L1-positive IC were found both, in combination with positive TCs and isolated (). A subset of 81 cases was stained for additional marker proteins by IHC. Most PD-L1-positive IC were found positive for CD163 and CD68 (), while IHC for T-killer, T-helper and B-cell marker proteins stained cell populations that were negative for PD-L1 (Figs. S1, 2). TCs and ICs were scored separately according to the Allred proportion scoreCitation19 and related to clinical and the tumors' genetic parameters.
Figure 1. PD-L1 IHC-staining patterns: expression was noticed in the tumor cells (A), in tumor-associated immune cells (B) or in both cell types (C; white arrow head: PD-L1-positive tumor cells, black arrow heads: PD-L1-positive immune cells). The proportion of positive cells varied with some cases showing few positive cells (A1–C1) while others showed mostly positive cells (A2–C2) (IHC PD-L1, Ab 5H1, 560x).
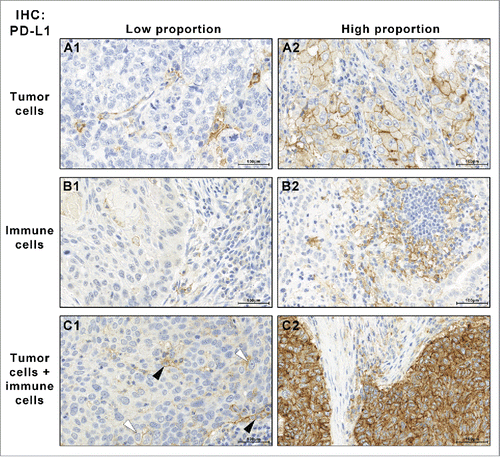
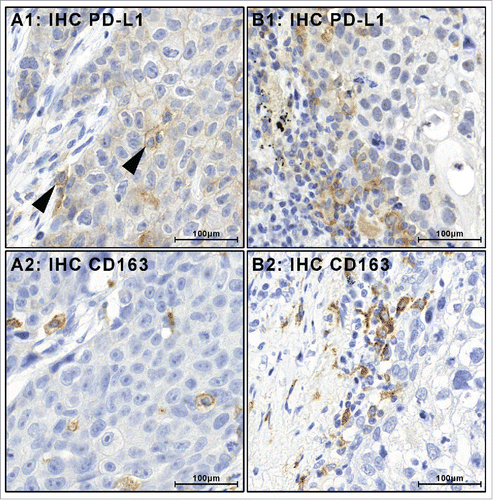
Figure 2. Tumor-associated PD-L1-positive immune cells stain positive for macrophage marker proteins. PD-L1-positive immune cells (black arrow heads) were encountered in combination with PD-L1-positive tumor cells (A1) or as the only PD-L1-positive cell population (B1). In all cases, PD-L1 was positive in immune cells that were also positive for macrophage marker proteins CD163 (A2, B2) and CD68 (Figs. S1, 2).
PD-L1 expression in tumor cells and clinical parameters
Among AD 34.4% (88/256) showed PD-L1-positive TCs (i.e., ≥ 1% positive cells, Allred proportion categories 2–5). Of the positive cases, 3.4% had 1–10% positive TCs (Allred category 2), 9.1% 10–33% (category 3), 20.5% 33–66% (category 4) and 67% > 66% (category 5). TC staining intensity was strong in 20.5% (18/88), intermediate in 47.7% (42/88) and weak in 31.8% (28/88).
Among SQ, 33.9% (61/180) showed PD-L1-positive TCs. The proportions scores 2–5 were 3.3%, 6.6%, 14.8% and 75.4%, respectively. Staining intensity was strong, intermediate and weak in 14.8% (9/61), 50.8% (31/61) and 34.4% (21/61), respectively (, ).
Figure 3. PD-L1 IHC positive (+) and negative (–) tumor cells (TC) and immune cells (IC) in pulmonary adenocarcinoma and squamous cells carcinoma. For each subgroup, absolute numbers and relative proportions are indicated. PD-L1-positive ICs are frequent and may occur isolated or in combination with positive TCs.
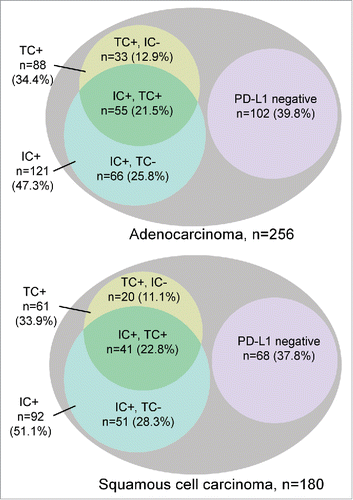
Table 1. Clinical data of the 436 NSCLC patients.
Table 2. Summary of PD-L1 protein expression, (A) in the tumor cells (TC) and (B) in tumor-associated immune cells (IC). Cases were classified PD-L1 IHC “positive” if ≥ 1% of the TCs or ICs were stained.
In both AD and SQ PD-L1-positive, TCs were present in early stage and in advanced state tumors. Conversely, no significant association between TC PD-L1 expression and UICC stage was detected (). Also, no associations were noticed for age, sex or smoking status (Tables S1, 2).
Table 3. In adenocarcinoma PD-L1 expression in TC was found in both early NSCLC (UICC stage I/II) and advanced NSCLC (UICC stage III/IV). Significant associations were found for KRAS, TP53 and STK11 mutations in univariate regression analysis (depicted) and in multivariate analysis (). All other investigated parameters did not show significant associations (Tables S1, 2).
The staining distribution among the three TMA cores of each case was evaluated. For AD, 57.9% of TC positive cases showed PD-L1 staining in all three TMA cores (51/88) and 26% showed two positive cores (23/88). In SQ, 39.3% of TC positive cases showed staining in all three cores, another 39.3% showed two positive cores (Table S3).
The median follow-up time for the AD cohort was 16.8 mo (range: 0–129 mo). The median follow-up time for the SQ cohort was 16.0 mo (range: 0–202 mo). Since UICC stage is one of the strongest predictors for outcome in NSCLC, independent survival analyses for each UICC stage were performed separately for AD and SQ. Kaplan–Meier analyses revealed no differences in survival between patients with PD-L1-positive and negative TCs with respect to tumor stage and histological subtype (data not shown).
PD-L1 expression in infiltrating immune cells
PD-L1-positive ICs were noticed in 47.3% of AD (121/256) and 51.1% of SQ (92/180). In AD, 45.5% of IC positive cases also featured positive TCs (55/121) while 54.5% were TC negative (66/121). A similar distribution was found in SQ; 44.6% were IC positive, TC positive (41/92) while 55.4% were IC positive, TC negative (51/92) (). Among TC positive cases, a majority also features positive ICs, 62.5% in AD (55/88) and 67% in SQ (41/61) ().
The staining distribution of the ICs among the three TMA cores per case was much more diverse compared to the TCs (Table S3). The most frequent scenario was one IC positive core in both AD (37/121) and SQ (44/92).
In AD, PD-L1 positivity of infiltrating ICs was significantly more frequent in patients with a history of ever-smoking (current smoker, former smoker) in univariate analysis ( p = 0.045; OR = 2.3). This association was not detected in SQ. For both AD and SQ, no significant associations between PD-L1-positive ICs and age, sex and UICC stage were found (data not shown).
PD-L1 expression in tumor cells and genetic alterations
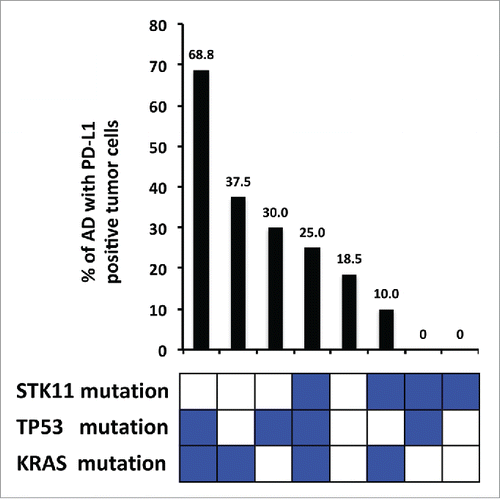
Expression of PD-L1 was related with the status of 27 genes by univariate and multivariate analysis. (, Table S1). In AD, PD-L1-positive TCs were significantly more frequent in cases with KRAS mutation (OR = 2.5, p = 0.018), TP53 mutation (OR = 2.4, p = 0.029), KRAS amplification (OR = 8.7, p = 0.042) and RB1 deletion (OR = 4.0, p = 0.009). On the other hand, PD-L1-positive TCs were significantly less frequent in the presence of STK11 mutations (OR = 0.2, p = 0.013). Multivariate logistic regression analysis yielded a combination of the mutational status of KRAS, TP53 and STK11 as best predictor of PD-L1 protein expression in TCs (). Accordingly, specimens with mutated KRAS, mutated TP53 and wild-type STK11 have the highest frequency of PD-L1-positive TCs (68.8%). Conversely, specimens with wild-type KRAS, wild-type TP53 and mutated STK11 have the lowest frequency (0%).
Figure 4. Percentage of PD-L1 expression in adenocarcinomas and mutational status of significantly associated genes. The combination of TP53 mutation, KRAS mutation and STK11 wildtype is associated with the highest percentage of PD-L1 expression in adenocarcinoma tumor cells. Conversely, STK11 mutations in the absence of TP53 and KRAS mutations are associated with the lowest percentage.
In SQ, PD-L1-positive TCs were significantly more frequent in specimens with a mutation in exon two of NFE2L2 (OR = 6.9, p = 0.007). No further significant associations of PD-L1-positive TCs and genetic alterations were detected; multivariate analysis did not yield a predictive combination of alterations (Table S2).
The genomic data were analyzed for copy-number (CN) variations of the PD-L1 locus (Chr.9 p24.1). Among the 1255 cases that contain all 436 cases of this study, only 4 cases showed CN ≥ 4 (AD n = 2, SQ n = 2). Given the low frequency of such events (0.3%) these 4 cases were not further investigated.
PD-L1 RNA expression
RNA expression array data were available for a subset of 65 cases. Dividing the cases by PD-L1 IHC score yielded significant differences in PD-L1 RNA-expression among the TC positive cases ( p = 0.0008) but not among the IC positive cases ( p = 0.33) (Fig. S4). If the cases were subdivided by the number of TMA cores with PD-L1 positive TCs (0–3, respectively), a trend toward increased RNA-expression was noticed for cases with 1, 2 and 3 positive cores. However, only the comparison of 0 and 3 IHC positive cores yielded a significant difference in RNA expression ( p = 0.0002) (Fig. S5).
PD-1 expression
IHC of the receptor PD-1 was performed using the antibody NAT105. Expression was found on ICs while TCs were negative, as expected. Cytomorphological appearance as well as additional marker proteins indicated that PD-1 positive ICs were predominantly tumor infiltrating lymphocytes (Fig. S3).
In AD, 57.0% (146/256) of the patients showed PD-1 positive ICs. This frequency was significantly higher in SQ with 69.3% (124/179; p = 0.010; OR = 1.7). For both histological subtypes no significant associations between PD-1 expression and clinical characteristics (age, sex, smoking status, UICC stage) were found (data not shown).
PD-1 positive ICs (i.e., lymphocytes) were significantly associated with PD-L1-positive ICs (i.e., macrophages) both in AD and in SQ. In AD expression of either PD-1, or PD-L1 was associated with a 2.7-fold increased probability of expression of the other protein ( p < 0.001). In SQ, the probability increased 3.5-fold if either protein was present ( p < 0.001).
Discussion
Four-hundred thirty-six cases of NSCLC were examined for protein expression of PD-L1 using the monoclonal antibody 5H1. One third of the cases showed PD-L1-positive TCs. Expression was noticed both in advanced- and early-stage cancer and significant association to specific gene mutations were found. Gene-amplification of PD-L1 was not present in significant numbers in the investigated cohort. Additionally, PD-L1 was detected in tumor-associated ICs in combination with PD-L1-positive TCs or alone.
PD-L1 IHC has now become a clinical companion diagnostic test for anti-PD-1 therapy in NSCLC.Citation7,8 Currently, two monoclonal antibodies are approved for advanced non-squamous NSCLC, each with its own PD-L1 IHC assay and cut-off criteria: i) Pembrolizumab with the 22C3 assay and ≥ 50% tumor cell cut-off; ii) Nivolumab with the 28-8 assay and ≥ 1% tumor cell cut-off. Furthermore, Nivolumab is approved for advanced squamous NSCLC without PD-L1 testing, and two more PD-L1 IHC assays (SP142; SP263) are tested in clinical trials.
Efforts for harmonization have been started since clinical treatment would be facilitated by reporting one PD-L1 status per patient, not one status for each approved companion diagnostic test.Citation13 The most critical parameters that have to be evaluated for harmonization include: antigen retrieval, primary antibody, IHC detection reagents and scoring criteria.
Here, we employed the well-established antibody 5H1 for PD-L1 detection.Citation14-16 In our hands, only one other commercially available antibody shows comparable staining patterns, clone E1L3N.Citation9,10 As scoring criteria, we used the proportion score developed by Allred DC in 1998 for the scoring of hormone receptors in breast cancer.Citation19 All clinical trial assays score the proportion of PD-L1-positive cells using cut-offs that define 2 or 3 categories. The cut-off values differ; however, ≥1% is commonly used as lowest cut-off that differentiates PD-L1 “positive” from “negative” cases. In the Allred score, ≥1% corresponds to proportion score ≥ 2 (score 2: 1/100–1/10). Immunohistochemical staining intensity, as is known from clinical Her2/Neu IHC scoring,Citation20 is currently not used for the scoring of PD-L1 IHC. For the 22C3 assay, ROC curves with different scoring modes have been published that indicate no additional predictive value of the intensity.Citation3 This does not preclude that upcoming indication might contain PD-L1 staining intensity.
Currently, PD-L1 IHC has the highest evidence as predictive biomarker for PD-1 directed therapy.Citation21 Another potential biomarker of interest is the second PD-1 ligand PD-L2,Citation22 while the PD-1 receptor itself has less predictive value.Citation23 Besides the analysis of checkpoint proteins, genomic techniques to evaluate the mutational burden and neoantigens are rapidly evolving and might have superior predictive value for anti-PD-1 as well as for anti-CTLA4 therapy.Citation24,25 Comprehensive genomic approaches are still challenging, as they require sufficient tumor material for DNA-extraction, whole exome sequencing and extensive computational processing.
Clinical NSCLC trials with checkpoint inhibitors focus on advanced, inoperable tumors.Citation1,2 Our patient cohort included almost 50% stage I tumors that had been resected with curative intention. PD-L1 expression was found as frequently in stages I and II, as in stages III and IV indicating that aberrant expression of this ligand might be an early event. Accordingly, one might conclude that early stage NSCLC might be sensitive to PD-1 or PD-L1 inhibition, if timely resection is not possible.
The frequencies of PD-L1-positive TCs in our study are comparable to another large NSCLC study by Velcheti et al., which also used antibody 5H1 (Ad: n = 226, PD-L1 TC positive 24.8%; Sq: n = 182, PD-L1 TC positive 29.7%).Citation26 Another study of squamous NSCLC with the 5H1 antibody observed fewer positive cases (n = 214, PD-L1 TC positive 19.6%).Citation27 Both studies also detected PD-L1 expression in early and advanced stage NSCLC.
Clinical trial assays are apparently optimized for sensitivity, as the reported PD-L1 frequencies are categorically higher: for the 22C3 assay 60.8% of the screening population of the Keynote-001 trial were PD-L1 TC positive, i.e., ≥ 1%Citation2 (n = 824, mixed AD and SQ). For the 28-8 assay, 54% of AD in the CheckMate-057 trial (n = 455)Citation5 and 52.8% of SQ in the CheckMate-017 trial (n = 225)Citation1 showed ≥ 1% PD-L1-positive TCs. In all three trials, roughly one third of cases was considered “PD-L1 highly positive” based on two different cut-offs (22C3: ≥ 50%; 28-8: ≥ 10%). Interestingly, the percentage of highly positive cases among the clinical trial assays seems to be in range of the percentage of all positive cases determined by antibody 5H1.
Another aspect when comparing studies may be the type of sampling: the cases in our study were represented as tissue microarrays (TMAs), which may underestimate heterogeneity. In fact, the majority of our cases are either completely negative or positive with a high proportion and only 25 cases (5.7%) featured one positive and two negative TMA cores. Thus, cases with focal PD-L1 expression may be underrepresented in our data.
Our data indicate no prognostic value for PD-L1 protein expression. Currently, the published data of PD-L1 and prognoses are controversial as both positiveCitation26 and negativeCitation10 prognostic value have been published. Recent data from a clinical trial that tested PD-L1 IHC assay 22C3 with n = 401 NSCLC patients as well as well as a large retrospective study with n = 678 NSCLC patients using the same assayCitation28 indicate a positive prognostic value.
In addition to PD-L1 expression in TCs, we also investigated expression of PD-L1 and PD-1 in ICs. PD-L1-positive ICs are found alone or in combination with positive TCs and may have independent predictive value for anti-PD-L1 therapyCitation29. We found PD-L1-positive ICs in over half of the cases, partly isolated, partly in combination co-ocurring with positive TCs. While detailed characterization of the tumor microenvironment was not the aim of this study, we performed additional IHC stainings for common immune marker proteins in 81 cases. The results correspond well with the cytomorphological appearance of the ICs and indicate that PD-L1-positive ICs are predominantly macrophages, while PD-1 positive ICs are mostly lymphocytes. These findings are in concordance with an immunosuppressive microenvironment mediated by aberrant activation of the pathway among antigen presenting cells known for their potential to express PD-L1 and susceptible immune effector-cells, which express the appropriate receptor.Citation30
Correlation studies of PD-L1 expression in TC and the status of 27 genes indicated distinct associations in AD and SQ. In AD, mutations in KRAS and TP53 and wildtype STK11 were strongly associated with PD-L1 expression, both as individual parameters and as combination in multivariate analysis. Previous studies did not report a correlation of PD-L1 IHC and KRAS status;Citation26,27 however, these studies only evaluated KRAS exon 2.
KRAS and TP53 are the most frequently mutated genes in AD.Citation17 Mutations have been linked to tobacco-smoking induced carcinomas that typically have a high mutational burden, i.e., express many neoantigens that might trigger an antitumoral immune response.Citation24 Thus, the association to PD-L1 expression might reflect the necessity of the neoplastic cells to compensate the high immunologic visibility by aberrant activation of an immune checkpoint that counteracts the cytotoxic effects of the incipient immune response.
The vast majority of the investigated patients in our cohort were ever smokers (405 of 432; 93.75%). In AD, history of smoking was associated with PD-L1 expression in IC. Smoking exposure may induce inflammatory as well as suppressive effects on the immune system.Citation31,32 Thus, PD-L1 expression by macrophages might reflect another mechanism of immunosuppression induced by chronic inflammatory stimulus.
PD-L1 expression is caused by transient induction of the gene in most cases. Activating mutations, however, have not been described. For certain subtypes of Hodgkin's lymphoma as well as triple negative breast cancer, gene amplification of the PD-L1 locus (Chr.9 p24.1) has been described.Citation32,34 Clinical studies with PD-1 inhibitors for these entities are ongoing.Citation35 In lymphoma, the amplified region may encompass the adjacent genes PD-L2 and JAK2. We searched the preexisting data of our cases as well as the as previously published cohort of NSCLC specimens (n = 1255) for CN variations of PD-L1. However, only four cases of AD and SQ showed CNs ≥ 4, i.e., 0.3%. Given the low frequency of such events, amplification-driven PD-L1 expression might be a rare constellation of PD-L1 expression or may be representing outliers due to technical limitations of the employed hybridization arrays.
In summary, our data indicate that PD-L1 is present in early stage NSCLC both in TCs and ICs. PD-L1 in TC was associated with the most frequently mutated genes in AD, KRAS and TP53. This strengthens the links between carcinogen exposure from tobacco smoke, deleterious KRAS activation and loss of genome surveillance by TP53 inactivation, high mutational burden and PD-L1 expression. PD-L1 expression was noticed in early and advanced stage NSCLC.
Materials and methods
Patient cohort
Formalin-fixed paraffin-embedded (FFPE) tumor specimens were collected for the Clinical Lung Cancer Genome Project (CLCGP) at two institutions (Department of Thoracic Surgery, Lung Clinic Merheim, Cologne, Germany; University Medical Center Groningen, Groningen, The Netherlands). Comprehensive molecular characterization of the complete CLCGP collection of 1255 NSCLC specimens has previously been reported.Citation17 In brief, specimens were obtained at first diagnosis of NSCLC prior to radiotherapy or systemic therapy. Clinical data on age at first diagnosis, sex, smoking status, UICC stage and overall survival were collected from patient records. Smoking history was defined as follows: never smoker (< 100 cigarettes per lifetime); former smoker (≥ 100 cigarettes and quit > 1 y prior to diagnosis of lung cancer); current smoker (≥ 100 cigarettes and quit ≤ 1 y prior to diagnosis of lung cancer or smoked at the time of lung cancer diagnosis).
In this study, a subset of 436 NSCLC specimens of Caucasian patients was used to construct TMAs. The subset encompassed 246 AD (median age 65 yr, 53.5% male, 10.2% never smokers) and 180 SQ (median age 68 yr, 78.3% male, 0.6% never smokers). The UICC stages in the AD cohort were stage I (47.3%), stage II (13.3%), stage III (32.8%) and stage IV (6.6%) and in the SQ cohort stage I (41.1%), stage II (32.8%), stage III (23.3%) and stage IV (2.8%) (). The study was approved by the local ethics committee and written informed consent was obtained from all patients.
Sample selection and TMA construction
The histopathological diagnoses were confirmed in accordance with the current classification systems by two board-certified pathologists (M.B., S.P.).Citation36,37
Each case was represented in the TMA by three cores of the primary carcinoma (core diameter 650 µm, approximately 1mm2 per case). The cores were sampled from different areas of well-preserved and vital TC. Adjacent normal lung tissue from 33.5% of the same surgical specimens was likewise included in each TMA block as internal on slide control. TMA construction was performed as described previously.Citation38
Genetic analysis and RNA expression
Data on mutations, translocations, amplifications and deletions were already generated as part of the CLCGP study. CN variations of the PD-L1 locus Chr.9 p24.1 were tested on pre-existing array-hybridization data that were part of the CLCGP study. RNA expression was assessed by llumina® Human HT-12 V3 Expression BeadChips using standard protocols. Detailed information on methods for nucleic acids extraction and quantification and the detection of genetic alterations were described previously.Citation17
Immunohistochemical analysis of PD-L1 and PD-1 expression
IHC was performed on 5 µm-thick sections of the TMA paraffin blocks. PD-L1 was stained using the primary antibody 5H1 (mouse monoclonal, kindly provided by Lieping Chen, Yale University, USA)Citation14 PD-1 was stained with the primary antibody NAT105 (mouse monoclonal, Abcam, Cambridge, UK). Detection was performed using the Bond Polymer Refine Kit (Leica Biosystems Newcastle Ltd, Newcastle, UK). Histopathological scoring was performed by two independent pathologists (A.M.S., A.H.S.) who were blinded to the clinical parameters and the genetic alterations of the tumor samples.
Scoring of the IHC stainings was performed separately for the TC and tumor-associated IC. The proportions of positive cells were scored by an adaptation of the six-step Allred-ScoreCitation19 (0; < 1%, 1–10%, 10–33%, 33–66%, 66–100%). For the TCs, intensities of the PD-L1 stainings were scored according to the “magnification rule” for Her2 scoring.Citation20 The maximum scores among the triplicates were used to classify each case. A case was considered as “positive” if ≥ 1% of the respective cells showed a specific staining of any intensity (Allred categories 2–5).
Immunohistochemistry of tumor-associated immune cells
PD-L1 and PD-1 positive IC were tested for additional common immune marker proteins in 81 of the cases (IHC: CD8+, CD4+, FoxP3, CD68, CD163, CD79a). PD-L1-positive ICs were predominantly found positive for CD68 and CD163 (Figs. S1, 2). Cytomorphology was compatible with PD-L1-positive monocytes / macrophages. PD-1 positive ICs co-localized with cells positive for lymphocytic marker proteins (CD4+, FoxP3, CD8+, CD79a) but not with monocytic markers (CD68, CD163) (Fig. S3). Cytomorphology was compatible with PD-1 positive lymphocytes, however, stainings were performed on individual, subsequent paraffin sections, precluding detailed co-localization studies.
Statistical analysis
Associations of IHC patterns and genetic alterations were evaluated by univariate cross-tabulation and by multivariate logistic regression. For AD, regression analysis yielded the model model: (logit(p) = −1.723 + 1.371 × [1 if KRAS mutation is present; else 0] – 1.982 × [1 if STK11 mutation is present; else 0] + 0.970 × [1 if TP53 mutation is present; else 0]) as best predictor for PD-L1 expression.
Odds ratios and corresponding 95% confidence intervals were calculated. Hypothesis testing was performed by tests appropriate for the respective data (Chi-squared test, Fisher's exact test) with α set to 5%. Patients' survival was investigated with the Kaplan–Meier estimator and log rank test. Median follow-up was calculated with the reverse Kaplan–Meier method.
Venn diagrams were computed with “R” package “venneuler” by Lee Wilkinson and Simon Urbanek (http://www.rforge.net/venneuler/).
In spite of the considerable sample size investigated (n = 436), the data may not exclude false-positive results among the 27 (AD) and 28 (SQ) planned pairwise comparisons due to the multiple comparisons problem. Using the Bonferroni correction, a p-value below 0.002 would be required for strong error control per histological subtype. With such strict interpretation all results are below significance. On the other hand, the two-step modeling approach (i.e., univariate significant associations were simultaneously considered in a multivariate regression equation) did account for multiple dependencies among the remaining genetic markers. Calculations were done with SPSS Statistics (IBM Corp., Armonk, NY, USA) and “R” statistical programming language version 3.1.0 (www.R-project.org).
Disclosure of potential conflicts of interest
AHS participates in advisory boards for BMS, MSD and Roche. WT participates in advisory boards for Roche and MSD (no personal fees, only financial compensation to institution (UMCG)). MVB received grants from Astellas, Amgen, Roche, MSD, Mologen and Miltenyi and received honoraria from Astellas, Amgen, Roche, MSD, Mologen and Alexxion. MVB is member of a Astellas speakers bureau. RB is a co-Founder of Targos Mol. Pathol. Inc., and serves on advisory boards for BMS, MSD, Roche, AstraZeneca, Novartis, BI, Lilly and QIAGEN. JW participates in compensated advisory boards and/or received lecture fees from AstraZeneca, BMS, Boehringer-Ingelheim, MSD, Clovis, Novartis, Pfizer and Roche. JR received research support from Bayer, Boehringer-Ingelheim, Novartis, Pfizer and Roche. The other authors declare no conflicts of interests.
KONI_A_1131379_supplementary_Material.docx
Download MS Word (12.4 MB)Acknowledgments
The authors would like to thank Susann Zupp, Corinna Seidel and Stefanie Marx for excellent technical assistance.
Funding
SA and AMS were supported by the Koeln Fortune Program / Faculty of Medicine, University of Cologne.
References
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non Small-Cell Lung Cancer. N Engl J Med 2015; 373(2):123-3; PMID:26028407; http://dx.doi.org/10.1056/NEJMoa1504627
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16(8):908-18; PMID:26115796; http://dx.doi.org/10.1016/S1470-2045(15)00083-2
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015. 372(21):2018-28; PMID:25891174; http://dx.doi.org/10.1056/NEJMoa1501824
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015; 373(17):1627-39; PMID:26412456; http://dx.doi.org/10.1056/NEJMoa1507643
- Paz-Ares L, Horn L, Borghaei H, Spigel DR, Steins M, Ready N, Chow LQM, Vokes EE, Felip E, Holgado E et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advancednon-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol 2015; 33 (suppl):LBA109.
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16(4):375-84; PMID:25795410; http://dx.doi.org/10.1016/S1470-2045(15)70076-8
- FDA News Release 2015-09-10, http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm466413.htm
- FDA News Release 2015-04-10, http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm412802.htm
- Schultheis AM, Scheel AH, Ozretić L, George J, Thomas RK, Hagemann T, Zander T, Wolf J, Buettner R. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015; 51(3):421-6; PMID:25582496; http://dx.doi.org/10.1016/j.ejca.2014.12.006
- Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim TM, Lee SH, Min HS, Kim YT, Kim DW et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol 2015; 28(9):1154-66; PMID:26183759; http://dx.doi.org/10.1038/modpathol.2015.63
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373(1):23-3; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015; 14(4):847-56; PMID:25695955; http://dx.doi.org/10.1158/1535-7163.MCT-14-0983
- Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, IASLC Pathology Committee. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol 2015; 10(7):985-9; PMID:26134220; http://dx.doi.org/10.1097/JTO.0000000000000526
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8(8):793-800; PMID:12091876; http://dx.doi.org/10.1038/nm730
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366(26):2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20(19):5064-74; PMID:24714771; http://dx.doi.org/10.1158/1078-0432.CCR-13-3271
- Seidel D, Zander T, Heukamp LC, Peifer M, Bos M, Fernandez-Cuesta L, Leenders F, Lu X, Ansen S, Gardizi M et al. A genomics-based classification of human lung tumors. Sci Transl Med 2013; 5(209):209ra153; PMID:24174329; http://dx.doi.org/10.1126/scitranslmed.3006802
- Gettinger SN, Kowanetz M, Koeppen H, Wistuba II, Kockx M, Kadel EE, Rizvi NA, Spira AI, Hirsch FR, Boyd Z et al. Molecular, immune and histopathological characterization of NSCLC based on PDL1 expression on tumor and immune cells and association with response to the anti-PDL1 antibody MPDL3280A. J Clin Oncol 2015; 33 (suppl; abstr 3015); PMID:25897158; http://dx.doi.org/10.1200/JCO.2014.58.3708
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11(2):155-68; PMID:9504686
- Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 2010; 457(3):299-307; PMID:20665045; http://dx.doi.org/10.1007/s00428-010-0952-2
- Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Cali∫ A, Cuppone F, Sperduti I, Giannarelli D, Chilosi M et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One 2015; 10(6); PMID:26086854; http://dx.doi.org/10.1371/journal.pone.0130142
- Yearly J, Gibson C, Yu N, Moon C, Murphy E, McClanahan T. PD-l2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Eur J Cancer 2015; 51(S3):18LBA.
- Taube JM. Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 2014 Dec 21; 3(11):e963413; PMID:25914862; http://dx.doi.org/10.4161/21624011.2014.963413
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124-8; PMID:25765070; http://dx.doi.org/10.1126/science.aaa1348
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MH, Goldinger SM et al. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science 2015; 350(6257):207-11; PMID:26359337; http://dx.doi.org/10.1126/science.aad0095
- Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014; 94:107-16; PMID:24217091; http://dx.doi.org/10.1038/labinvest.2013.130
- Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, Aubry MC. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013; 14:157-63; PMID:22868219; http://dx.doi.org/10.1016/j.cllc.2012.05.006
- Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, Yip P, Yu B, O'Toole SA, McCaughan BC et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015; 89(2):181-8; PMID:26024796; http://dx.doi.org/10.1016/j.lungcan.2015.05.007
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515(7528):563-7; PMID:25428504; http://dx.doi.org/10.1038/nature14011
- Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004; 10:5094-100; PMID:15297412; http://dx.doi.org/10.1158/1078-0432.CCR-04-0428
- Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol 2008; 122(6):1208-14; PMID:18926564; http://dx.doi.org/10.1016/j.jaci.2008.09.022
- Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 2005; 175(4):2684-91; PMID:16081845; http://dx.doi.org/10.4049/jimmunol.175.4.2684
- Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116(17):3268-77; PMID:20628145; http://dx.doi.org/10.1182/blood-2010-05-282780
- Barrett MT, Anderson KS, Lenkiewicz E, Andreozzi M, Cunliffe HE, Klassen CL, Dueck AC, McCullough AE, Reddy SK, Ramanathan RK et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative 635 breast cancer. Oncotarget 2015; 6(28):26483-93; PMID:26317899; http://dx.doi.org/10.18632/oncotarget.4494
- Kroemer G, Galluzzi L. Immunotherapy of hematological cancers: PD-1 blockade for the treatment of Hodgkin's lymphoma. Oncoimmunology 2015. 4(6):e1008853; PMID:26155425; http://dx.doi.org/10.1080/2162402X.2015.1008853
- Travis WD, Brambilla E, Müller-Hermelink H-K, Harris CC. World Health Organization Classification of Tumours. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Geneva: World Health Organization, 2004.
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G, Garg K et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011; 8(5):381-5; PMID:21926387; http://dx.doi.org/10.1513/pats.201107-042ST
- Remotti H. Tissue microarrays: construction and use. Methods Mol Biol 2013; 980:13-28; PMID:23359147; http://dx.doi.org/10.1007/978-1-62703-287-22
