ABSTRACT
Chronic lymphocytic leukemia (CLL) is characterized by an abnormal expansion of mature B cells in the bone marrow and their accumulation in blood and secondary lymphoid organs. Tumor CLL cells share expression of various surface molecules with many subsets of B cells and have several common characteristics with regulatory B cells (B regs). However, the identification of B regs and their role in CLL remain elusive. The aim of this review is to summarize recent works regarding the regulatory and phenotypic characteristic of B regs and their associated effects on the immune system. It is also meant to highlight their potential importance with regards to the immunotherapeutic response.
Introduction
CLL is a clinically heterogeneous disease, characterized by CD5 positive monoclonal B cell proliferation in the bone marrow and accumulation in blood and secondary lymphoid organs.Citation1 The origin of tumor cells in CLL is unknown and, based on their phenotypic characteristics several hypotheses have been formulated in the last few years. These cells can be linked to the B1 lineage found in a mouse model expressing the Ly1/CD5 antigen,Citation2 but they are also phenotypically close to naive B cells, mature B cells, and memory B cells.Citation3-6 Another way to classify CLL cells is related to their functional properties since several functional and phenotypic characteristics resemble the recently described B10 cell subset.Citation7 B10 cells produce interleukin (IL)-10 inducing, on the one hand, inhibition of proinflammatory cytokines by T cells, and, on the other hand, the development of regulatory T cells (T regs). Moreover, CLL cells are effective in modulating both the innate and adaptive immune system responses which, in part, could explain the immunodeficiency reported in CLL-prone mouse models and CLL patients, with an impact on the clinical course of the disease.Citation8
Last but not least, CLL remains an incurable malignant disease despite the recent development and use of therapeutic antibodies and chemotherapy mainly targeting B cells.Citation9 Since immunosuppression is observed in CLL, new strategies of treatment must be developed by taking into account the regulatory functions of CLL cells on cellular immunity. This review is aimed to summarize the functional and phenotypic characteristics of CLL cells to better understand their implication in the immunosuppressive responses, the disease progression, and thereby to point out the development of pertinent immunotherapeutic treatments.
Common activities of B regs and B-cell CLL: Regulation of immune cells
Regulatory functions of B regs
Generally speaking, B regs can be defined based on their immunosuppressive capacities on different effector cell types including T helper cells such as Th1, Th2 and Th17 cells,Citation10 and natural killer (NK) cells. Dendritic cells (DC) and macrophages can be negatively regulated, since B regs affect their phagocytic capacity both in vivo and in vitro.Citation11,12 In addition to the direct immunosuppressive function, the regulatory effect could be an indirect effect promoting T regs, along which conventional T regs and type 1 regulatory T (Tr1) cells.Citation13,14 In mice, NKT cells can also be activated by B regs. These B regs are characterized as marginal zone B cells and type 2 transitional B cells expressing the non-classical major histocompatibility complex (MHC) antigen CD1d and CD21 at high levels.Citation15 This B reg-dependent NKT activation can eliminate autoimmune or inflammatory responses.Citation16,17
New B regs have been identified in the case of solid tumors: they are named tumoral B regs (tB regs). These cells that strongly express CD19, CD25 and CD69 molecules, like their conventional counterpart, can inhibit Th cell proliferation and are able to activate T reg expansion by transforming naive CD4+ T cells.Citation18,19 Infiltrating tumor CD19+ CD38+ CD1d+ IgM+ CD147+ B cells can be considered as B regs due to their capacity to regulate T cell response via the production of granzyme B (GrB) which promotes T-cell receptor (TCR) ζ chain degradation.Citation20 Furthermore, GrB+ tB regs also express IL-10, CD25 and indoleamine-2, 3-dioxygenase (IDO). The latter enzyme mediates inhibition of T cell proliferation and promotes development of T regs. GrB-derived B regs are found in other human diseases such as tolerant graft recipients or allergy.Citation21,22
Table 1. Immune cells downregulated or upregulated that participate in the defective immune responses observed in CLL.
It is widely believed that acquisition of B regulatory functions is closely associated with the production of cytokines such as IL-10. The very first demonstrations of this association came from murine models of autoimmune or inflammatory diseases and were then confirmed in humans. Mizoguchi A et al. identified a subset of B cells in a murine model of chronic bowel inflammation that could act as B regs through their ability to produce IL-10.Citation23 This production is induced by stimulation of Toll-like receptor (TLR) 4 and TLR9.Citation24 It involves the transmission of a signal mediated by the myeloid primary response protein differentiation 88 (MyD88) adaptor, the activation of the nuclear factor κB-α kinase (NFκB), and the signal transducer and activator of transcription 3 (STAT3).Citation25,26 There are other mechanisms which induce IL-10 production by B cells that were subsequently identified. Stimulation of the B-cell receptor (BCR) via the B cell linker protein, and activation of calcium signaling via the stromal interaction molecule 1 (STIM-1), or expression of CD5 on the B-cell surface have been associated with elevated production of IL-10.Citation27,28 Moreover, various immunosuppressive cytokines can be produced by B regs. This is the case for the transforming growth factor (TGF)-β which triggers apoptosis of effector T cells and IL-21.Citation29,30 More recently, the secretion of IL-21 has been shown to be responsible for an upregulation of IL-10 production in a murine model for multiple sclerosis suggesting a more heterogeneous cytokine profile of the B regs than initially suspected.Citation31
Finally, several reports indicate that the activity of B regs can also be modulated. Thus, after the BCR engagement and CD40 stimulation, peripheral blood B cells proliferate and secrete the tumor necrosis factor (TNF)-α, lymphotoxin and IL-6, stimulating immune responses.Citation32 Alternatively, if B cells receive only CD40 stimulation in the absence of BCR activation, they produce negligible amounts of pro-inflammatory cytokines, but also a significant level of IL-10 and then can be identified as B regs.Citation32 Similarly, when treated with IL-2, marginal zone B cells can shift into a B reg phenotype since they produce a low level of TNF-α and an extremely high level of IL-10.Citation24 However, when activated by the extracellular signal-regulated kinase (ERK) pathway via the Ras proteins called nucleoside guanyl releasing proteins (RAS, GTP), the production of IL-10 by the marginal zone B cells is stopped while the synthesis of TNF-α and TNF-β is increased, leading to the acquisition of effector capacities for T cell stimulation.Citation33,34 Recent studies indicate that the number of B regs is decreases with age and contributing to the susceptibility for autoimmune reactions as described in murine models.Citation35
Regulatory functions of B-cell CLL
Acquisition of B reg functions in CLL is an important yet barely explored aspect in the disease. It has been suggested that it might be controlled by the tissue location of CLL cells rather than the CLL's origin, e.g. the unmutated vs. mutated status of IgVH. Consistent with this hypothesis, it has been demonstrated that CLL cells in lymph nodes over-expressed a high number of genes associated with immune tolerance (CAV1, MCM3, BATF) compared with those found in B cells from the bone marrow or the peripheral blood.Citation36 The influence of the microenvironment on cellular behavior in CLL is strongly supported by these observations.
Moreover, CLL cells are characterized by their plasticity related to their ability to produce IL-10. The regulatory functions and the capacity to produce IL-10 are maintained in cultured CLL cells when they are stimulated by TLR agonists such as CpG-ODN and/or CD40. However, these effects can be reversed in response to BCR stimulation or when using diacylglycerol agonists. In the latter conditions, CLL cells acquire the characteristics of effector B cells with the ability to activate T cells.Citation37
CLL cells can mediate an immunosuppressive effect on different effector cells and can induce T regs. However, CLL T cells were found deficient in their capacity to constitute competent immunological synapses with antigen-presenting cells (APC).Citation38 Gorgun et al. determined that this defect concerns CD4+ T cells with consequences such as defective cellular differentiation as well as defects in cytoskeletal formation and vesicle trafficking.Citation39 Moreover, the level of effector memory CD4+ T lymphocytes from CLL patients is increased when compared to controls, and it is positively associated with a more advanced stage of the disease with treatment requirements and with unfavorable genomic aberrations.Citation40 T cells from CLL patients express more programmed death-1 receptor (PD1) than normal T cells, a receptor involved in tumor-mediated immunosuppression, explaining the correlation between its expression and the progression of the disease. Surprisingly, the cross-talk between CLL cells and T helper cells through cell surface CD40-CD40L co-ligation is effective and induces malignant B cell proliferation.Citation41 Moreover, a defective cytotoxic activity of CD8+ T cells was observed which is related to the presence of CLL cells. Thus, healthy control CD8+ T cells can be rendered deficient when co-cultured with CLL cells.Citation39 These changes are mainly mediated by direct intercellular contact. CD8+ T cells also express PD-1, at their terminally differentiated stage, contributing to the immunosuppression observed in CLL ().Citation40 In addition, an elevated number of circulating CD4+ or CD8+ T regs has been noted.Citation42 These T regs are effective and induce transcriptional downregulation of the TCR in naive T cells responsible for defective maturation into Th cells. Furthermore, T regs are characterized by an elevated expression of CD25. It has been suggested that excessive soluble CD25 found in CLL patients could result from T reg cell surface release. As CD25 constitutes the α chain of the heterotrimeric IL-2 receptor, the soluble CD25 isoform could prevent IL-2 binding to the effective T cells and consequently could act as an antagonist of cytokine signaling, counteracting the IL-2 antitumor activities.Citation43 The T reg expansion is present very early in the course of CLL and the T reg level is positively associated with the disease progression ().Citation8,44
Table 2. Phenotypic characteristics of B CLL as regulatory B cells.
CLL cells can also regulate NKT and NK cells. They express more CD1d molecules on their surface than normal B cells and the expression density is correlated with the clinical outcome. High CD1d expression is associated with a poor outcome and unfavorable prognosis ().Citation45,46 According to their reactivity with the α-galactosylceramide glycolipid (GalCer) presented by CD1d and by their TCR, NKT cells can be divided into three subgroups.Citation47 Type I includes invariable cells called iNKT that in humans express a restricted TCR consisting of a Vα24-Jα18 invariant chain and a Vß11 variable chain.Citation48 These cells are dependent on the GalCer recognition by their TCR presented by CD1d. Type II NKT cells are also dependent on CD1d but express a more variable TCR α chain.Citation49 Finally, type III NKT cells, called NKT-like cells, are independent from the CD1d molecules and express semi-invariant TCR.Citation50 In CLL, type I and type II NKT cells recognize the α Gal-Cer antigen presented by the CD1d molecules of CLL cells, leading to tumor cell death.Citation51 A reverse correlation is found between the percentage of CD1d+ CLL cells and the percentage of type I iNKT cells. Although not regulated by CD1d, type III NKT-like cells are also suspected to play an immunoprotective role in CLL and this assertion is also supported by the inverse correlation observed between the number of these cells, the T regs frequency, and the CLL disease progression ().Citation52 In addition to their immunoregulatory function on NKT cells, CLL cells provide resistance to the NK cellular cytotoxic activity. Because CLL cells express the HLA-G tolerogenic molecule, they inhibit the NK cell killer function. It should be noted that the NK-dependent ADCC mechanism is also altered during B CLL.Citation53
Finally, CLL cells regulate myeloid phagocytic cells. Neutrophils play a major role in fighting infections through their ability to migrate, to produce cytokines, and to recruit immune cells. All these activities are deficient in CLL. The neutrophil migration and the chemotaxis mediated by C5a and N-formyl-methionylleucylphenylalanine (fMLP) are impaired.Citation54 Dysfunction in the bactericidal activity against key non-opsonized bacteria has been reported, while the micro bactericidal response against fungi seems normal ().Citation55 Besides, DC are subdivided into various subtypes of conventional DC and plasmacytoid DC (pDC). Both DC subtypes are effective in promoting normal antigen presentation to CD4+ T cells due to increased expressions of class II MHC molecules, co-stimulation molecules, and inflammatory cytokines.Citation56 However, a lower number and a reduced efficacy of pDC have been observed recently in progressive forms of CLL ().Citation57 Contrarily, the number of monocytes appears to be increased,Citation58 but it is associated with a qualitative impairment because monocytes dimly express HLA-DR MHC class II and CD86 molecules. These observations suggest that monocytes in CLL are not very efficient APCs and possess only a low capacity to stimulate the immune system, which would accelerate the disease progression (). These cells display several characteristics of the recently described monocytes myeloid-derived suppressor tumor cells (moMDSC) known to contribute to cancer development by supporting cancer growth; cancer scattering leads to metastasis and it has a negative impact on the regulation of the immune system. In agreement with a supplementary immunomodulatory effect, it has been recently reported that monocytes express high amounts of IDO in CLL.Citation59
Common phenotypic characteristics of B regs and B-cell CLL
Phenotypic comparisons of B regs and CLL cells highlight interesting common characteristics suggesting CLL cells' unexpected functional activities.
Phenotypic characteristics of B regs
The phenotypic signature of B regs remains controversial and no consensus has emerged to characterize B regs in contrast to their T cell counterparts.Citation60 Blair et al. and Lindner et al. defined human B regs as B cells expressing CD38+ CD1d+ IgM+ CD147+ and GrB within the B-cell immature subset CD19+ CD24+ CD38high, respectively.Citation20,61 In contrast, for Iwata et al., human B regs were related to memory B cells (CD24hiCD27+).Citation62 For others, they could be transitional precursor B cells of the marginal zone identified as CD19+ CD21+ CD23+ CD24+ IgM+ cells, but also B10 cells (CD19+ CD5+ CD1d+) expressing IL-10 ().Citation63
A distinct subtype of IL-10-producing B regs with the unique phenotype CD19hiFcγRIIbhi was recently observed in a murine model.Citation64 This phenotype is dependent on CD40-CD40L activation, known to play a major role in the induction of functional B regs and to enhance IL-10 producing transitional type 2-immature-like B reg subsets in the Mrl/lpr murine model ().Citation65
Recently, it has been pointed out that the expression of the BAFF receptor TACI as a phenotypic characteristic of B regs that mediates the B reg IL-10 production ().Citation66
Phenotypic characteristics of B-cell CLL
Some phenotypic modifications of B CLL cells result from cytogenetic alterations and mutations. Their identification provides better understanding of the origin and physiopathology of the disease.Citation67 Phenotypic studies highlight the identification of various sub-populations associated with the progression of the disease or with cytogenetic anomalies. Thus, CD19+ CD27− B cells derived from CD5+ mature B cells with unmutated IgVH seem to be clearly dissociated from the CD19+CD27+ B cells derived from post germinal center B cells with mutated IgVH.Citation5 However, this dichotomy has not been seen by other authors. So, Klein et al. demonstrated that the gene profiles and the CLL origin are independent from the IgVH genotype, suggesting a homogeneous phenotype of memory CD27+ B cells ().Citation4
CLL cells produce IL-10, via a pathway that involves CD5 through the downstream activation of the two transcription factors STAT3 and NFAT2.Citation28 The contribution of CD5 has been confirmed in experimental approaches in CLL cells to produce IL-10 by using small interfering (si)CD5 RNAs that lower the production of IL-10. On the other hand, forcing CD5 expression in human B cells, with plasmid technology, promotes IL-10 expression. Moreover, the IL-10 level in serum is increased in CLL compared to controls.Citation68
Another common characteristic of CLL cells and B regs is the expression of the cell surface CD38 ectoenzyme that promotes cytokine production, B CLL migratory potential through the hydrolysis of nicotinomide adenine dinucleotides (NAD) to cyclic adenosine dinucleotide-phosphate (ADP)-ribose, and the control of intracellular Ca2+ levels.Citation69,70 This marker is correlated with the CLL disease activity facilitating CLL cell circulation within the lymph nodes and favoring tumor growth and survival.Citation71,72
Furthermore, CLL cells can also express CD1d and produce GrB to gain cytotoxic potential after activation by IL-21.Citation73 GrB production is further enhanced following stimulation of TLR9 with CpG-ODN and in the presence of IL-21 ().Citation74,75 Interestingly, the level of TLR9 expression in CLL appears to be positively correlated with the expression of the immunosuppressive molecule HLA-G and its receptor CD85j.
CLL cells also express the FcγRIIb molecule similar to B regs. This expression is detrimental to the efficacy of rituximab (RTX), a therapeutic anti-CD20 antibody. In fact, FcγRIIb allows RTX internalization in a cis manner and after phosphorylation becomes internalized along with CD20: anti-CD20 complexes before lysosomal degradation.Citation76
Finally, as described for the B regs, BAFF stimulation promotes IL-10 production by CLL cells in a murine model of CLL but also in CLL patients.Citation66 The intracellular signaling pathway looks identical to that found in B regs and the expression of TACI on the surface of CLL cells is promoted by the stimulation of CD40 and IL-4R engagement.Citation77
What are recent immunotherapies ensuing from the Implication of B-cell CLL cells as B regs?
The actual gold standard for treating CLL patients is based on chemotherapy associated with monoclonal antibodies (mAb). RTX was the first chimeric anti-CD20 mAb approved by the Food and Drug Administration for the treatment of non-Hodgkin's lymphomas (NHL) and CLL. However, resistance and relapses are frequently observed.Citation78,79 This raises the problem of cell targeting and also the deleterious effect of CLL on the immune system which must be improved by efficient immunotherapy. Overall, clarification of functional and phenotypic characteristics of B CLL cells should facilitate the development of efficient therapeutic strategies based on the implications of B regs in CLL.
Treatments that reverse B CLL regulatory functions
Co-stimulatory molecules and T cell activation
Re-educating CLL cells from a B reg phenotype to effector B cells is an interesting approach. To this end, prerequisites are required, including CLL capacity to express important co-stimulatory molecules to activate competent T lymphocytes. The protein kinase C (PKC) agonist bryostatin is a possible candidate. Thus, treating CLL cells with bryostatin increases their capacity to stimulate a T cell response, Th1 cytokine production, and expression of co-stimulatory molecules ().Citation80
Figure 1. Immunotherapies based on regulatory B characteristics of B CLL cells. Several immunotherapies are based on the re-education of T cell activation. They are mediated by bryostatin with expression of co-stimulatory molecules and Th1 cytokine production, the T CAR targeting CD19 on B CLL cells, transfection of CD40L into B CLL cells, use of blinatumomab (anti-CD3xanti-CD19 bispecific Ab), polarized DC and lenalidomide leading Th17 expansion and production of pro-inflammatory cytokines. Other immunotherapies use the modulation of T regs. This could be realized by blockage of CD200 on B CLL cells and thalidomide, fludarabine, green tea constituents and mAb targeting CD25 or CTLA-4. Finally, stimulation of NK cells and increase of ADCC are realized by recombinant IL-15 and lenalidomide. NK cells can produce BAFF after binding of the anti-CD20 Ab rituximab to NK Fc receptor. A neutralizing anti-BAFF Ab, belimumab, prevents metabolism of CLL cells and restores efficacy of NK cells. Kuromamin, a CD38 inhibitor causes a decrease of CLL cells in the bone marrow and in the spleen but also, an increase in the peripheral blood. Ab, antibody; ADCC, antibody dependent cellular cytotoxicity; CAR, chimeric zntigen receptor T-cell therapy; CD40L, CD40 ligand; CLL, chronic lymphocytic leukemia; CTLA-4, (cytotoxic T-lymphocyte-associated protein 4); DC, dendritic cell; mAb, monoclonal antibody; NK, natural killer; Rhuman IL-15, recombinant IL-15; Th, T helper; T reg, regulatory T cells.
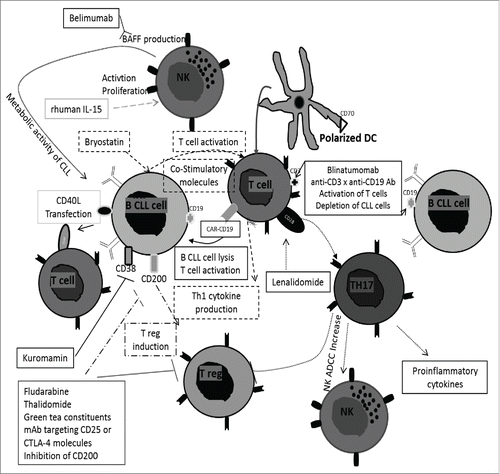
Moreover, re-educating T cells into effector T cells can be achieved by using T cell therapy. It is currently possible with chimeric antigen receptor (CAR) T-cell therapy. CARs are designed to recognize a specific antigen on target cells and are composed of intracellular T-cell signaling domains fused primarily to single-chain variable fragments (scFvs) from mAbs directed to this specific antigen. Retroviral construction leads to the expression of CAR on the T cell surface. A CD19-targeting T CAR has been successfully tested inducing CLL cell lysis by the activation of T cells.Citation81 A supplementary co-stimulatory signal by CD28 may be necessary to increase the antitumor efficacy of the T cells ().Citation82
CD40L
CD4+CD40L+ T cells in proliferative centers play an essential role in CLL cell activation, proliferation and survival. Triggering CD40 in B-cell lymphoma cell lines has been reported to suppress growth and to induce apoptosis in some cases.Citation83 However, treatment with Lucatumumab, an anti-CD40 antagonist Ab, appears to have minimal clinical efficacy.Citation84 In addition, CLL cells weakly express the costimulatory CD80/CD86 molecules, which makes them poorly effective APC. Genetic therapy based on transfection of CD40L in B-cell CLL was tested and found to promote CLL-specific autologous cytotoxic T lymphocytes in vitro () due to an increased expression of upregulating CD80/CD86 co-stimulatory and CD54 adhesion molecules.Citation85,86
CD200
Targeting CLL immune modulatory CD200 molecules is another interesting approach. Their blockage contributes to the inhibition of T reg activity and enhances the cytotoxic T cell response.Citation87 The expression of CD200 is upregulated on CLL cells and their stimulation is sufficient to induce T regs, and to downregulate the Th1 response by decreasing IL-2 and IFNγ pro-inflammatory cytokine synthesis ().Citation88,89
CD38
In CLL, expression of CD38 represents a marker of disease aggressiveness with a poor survival prognosis. For this reason, the effects of Kuromamin, a CD38 inhibitor, have been evaluated. Its use causes a decrease of CLL cells in the bone marrow and in the spleen but also, an increase in the peripheral blood (). Citation71
Treatments that restore immune regulation
Innate immune system
The ability of NK cells to lyse CLL cells by direct contact or through ADCC is compromised in CLL patients. Different strategies were developed to restore efficient killer NK activities. For example, the recombinant human IL-15 (rhIL-15) was used and showed the restoration of the trans-presentation between CLL cells and NK cells that further promotes the activation and proliferation of the latter cells leading to leukemic B cell depletion and ADCC reaction in the presence of anti-CD20 antibodies such as RTX (). Citation90-92 Moreover, binding of RTX to NK Fc receptor induces the production of BAFF by NK cells enhancing CLL metabolic activity which impairs the NK direct lysis and the RTX-induced cell lysis by RTX. These observations encourage the use of belimumab, the neutralizing anti-BAFF antibody, in CLL. The metabolism of CLL cells is prevented and the efficacy of NK cells is restored in allogeneic and autologous systems ().Citation93
Finally, the use of DCs to kill malignant cells is also supported by Junevik et al. DCs isolated from patients and expressing a strong functional rate of CD70 were identified and called α-type 1- polarized DC (α DC1s) ().Citation94 These cells produce IL-12p70 causing the activation of effector cells. To achieve the upregulation of CD70 expression on them, DCs are incubated in the presence of PGE2 (prostaglandin E2). This generates functionally activated cells leading to T-cell survival. Further research on pDCs demonstrated a possible increase of their activity using TGF-β and TNF inhibitors. Following this strategy, the tumor mass decreased.Citation57
An adaptive immune system
The deficient activity of effector T cells is partially due to their direct interactions with CLL cells.Citation38 Lenalidomide, a thalidomide analog, was studied based on its anti-neoplastic, anti-angiogenic, pro-erythropoietin and immunomodulatory activities. This drug stimulates T cells through CD28, leads to Th17 expansion and generates the production of pro-inflammatory cytokines, T reg repression and NK ADCC increased the effect ().Citation95 Adaptive therapy was recently elaborated.Citation96 A bispecific (CD3 × CD19) antibody, blinatumomab, has been designed, acting both as a T cell stimulator and a CLL depletion agent. Associated with the recombinant human IL-2, ex vivo peripheral blood mononuclear cells from CLL patients were incubated for 3 weeks with blinatumomab. T cells were mostly effector and central memory cells with the Th1 subset dominating and were efficiently cytotoxic against CLL in the presence of the bispecific Ab ().
T regs
Various therapeutic molecules demonstrated their ability to modulate T reg activity in CLL such as fludarabine, thalidomide and more recently green tea constituents, for patients in the early stage of the disease.Citation42,97,Citation98 Other treatments are currently in development including mAb targeting CD25 or CTLA-4 molecules ().Citation99,100 These antibodies are being tested in clinical trials for hematological or solid tumors. However, if T reg inhibition shows interesting results, this will become an area of intense research. It has been indeed reported in NHL that T reg cells were capable of killing the malignant cells with significant impact on survival.Citation101
Conclusions
A better comprehension of the immune responses and characterization of the impact of B-cell CLL as B regs in these responses are worthy of pursuit in order to establish new efficient treatments.
Disclosure of potential conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the “Ligue contre le Cancer,” “association Laurette Fugain,” Agence Nationale de la Recherche under the “Investissement d'Avenir” program with the reference ANR-11-LABX-0016-001, and the “Région Bretagne” for their support. Thanks are also due to Geneviève Michel and Simone Forest for their help typing of the paper. The editorial assistance of Dr. Wesley H. Brooks, University of South Florida, USA, is greatly appreciated.
References
- Dameshek W. Chronic lymphocytic leukemia–an accumulative disease of immunolgically incompetent lymphocytes. Blood 1967; 29: Suppl:566-84; PMID:6022294
- Phillips JA, Mehta K, Fernandez C, Raveche ES. The NZB mouse as a model for chronic lymphocytic leukemia. Cancer Res 1992; 52:437-43; PMID:1370214
- Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood 2011; 117:1781-91; PMID:21148333; http://dx.doi.org/10.1182/blood-2010-07-155663
- Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med 2001; 194:1625-38; PMID:11733577; http://dx.doi.org/10.1084/jem.194.11.1625
- Seifert M, Sellmann L, Bloehdorn J, Wein F, Stilgenbauer S, Durig J, Kuppers R. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J Exp Med 2012; 209:2183-98; PMID:23091163; http://dx.doi.org/10.1084/jem.20120833
- Forconi F, Potter KN, Wheatley I, Darzentas N, Sozzi E, Stamatopoulos K, Mockridge CI, Packham G, Stevenson FK. The normal IGHV1-69-derived B-cell repertoire contains stereotypic patterns characteristic of unmutated CLL. Blood 2010; 115:71-7; PMID:19887677; http://dx.doi.org/10.1182/blood-2009-06-225813
- DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, Matsushita T, Matta KM, Chen Y, Venturi GM et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 2013; 27:170-82; PMID:22713648; http://dx.doi.org/10.1038/leu.2012.165
- Jadidi-Niaragh F, Yousefi M, Memarian A, Hojjat-Farsangi M, Khoshnoodi J, Razavi SM, Jeddi-Tehrani M, Shokri F. Increased frequency of CD8+ and CD4+ regulatory T cells in chronic lymphocytic leukemia: association with disease progression. Cancer Invest 2013; 31:121-31; PMID:23286587; http://dx.doi.org/10.3109/07357907.2012.756110
- Hallek M. Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies. Blood 2013; 122:3723-34; PMID:24065239; http://dx.doi.org/10.1182/blood-2013-05-498287
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol 2011; 186:5569-79; PMID:21464089; http://dx.doi.org/10.4049/jimmunol.1100284
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol 2010; 185:2240-52; PMID:20624940; http://dx.doi.org/10.4049/jimmunol.1001307
- Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol 2010; 40:2296-307; PMID:20468007; http://dx.doi.org/10.1002/eji.200940288
- Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther 2012; 14:R32; PMID:22315945; http://dx.doi.org/10.1186/ar3736
- Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 2013; 5:173ra23; PMID:23427243; http://dx.doi.org/10.1126/scitranslmed.3005407
- Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med 1999; 190:75-89; PMID:10429672; http://dx.doi.org/10.1084/jem.190.1.75
- Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach JF, Monteiro RC. Overexpression of natural killer T cells protects Valpha14- Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med 1998; 188:1831-9; PMID:9815260; http://dx.doi.org/10.1084/jem.188.10.1831
- Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, Exley M, Snapper S, Balk SP, Hagen SJ et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology 2000; 119:119-28; PMID:10889161; http://dx.doi.org/10.1053/gast.2000.9114
- Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol 2011; 186:5656-62; PMID:21490155; http://dx.doi.org/10.4049/jimmunol.1100463
- He Y, Qian H, Liu Y, Duan L, Li Y, Shi G. The roles of regulatory B cells in cancer. J Immunol Res 2014; 2014:215471; PMID:24991577
- Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, Beyer T, Reister F, Fabricius D, Lotfi R et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res 2013; 73:2468-79; PMID:23384943; http://dx.doi.org/10.1158/0008-5472.CAN-12-3450
- Chesneau M, Michel L, Dugast E, Chenouard A, Baron D, Pallier A, Durand J, Braza F, Guerif P, Laplaud DA et al. Tolerant kidney transplant patients produce B cells with regulatory properties. J Am Soc Nephrol 2015; 26:2588-98; PMID:25644114; http://dx.doi.org/10.1681/ASN.2014040404
- Braza F, Chesne J, Castagnet S, Magnan A, Brouard S. Regulatory functions of B cells in allergic diseases. Allergy 2014; 69:1454-63; PMID:25060230; http://dx.doi.org/10.1111/all.12490
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002; 16:219-30; PMID:11869683; http://dx.doi.org/10.1016/S1074-7613(02)00274-1
- Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, Gray M. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci U S A 2012; 109:887-92; PMID:22207622; http://dx.doi.org/10.1073/pnas.1109173109
- Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 2013; 15 Suppl 1:S1; PMID:23566714; http://dx.doi.org/10.1186/ar3907
- Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, Xue Y, Zou H. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS One 2013; 8:e62855; PMID:23638156; http://dx.doi.org/10.1371/journal.pone.0062855
- Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 2011; 34:703-14; PMID:21530328; http://dx.doi.org/10.1016/j.immuni.2011.03.016
- Garaud S, Morva A, Lemoine S, Hillion S, Bordron A, Pers JO, Berthou C, Mageed RA, Renaudineau Y, Youinou P. CD5 promotes IL-10 production in chronic lymphocytic leukemia B cells through STAT3 and NFAT2 activation. J Immunol 2011; 186:4835-44; PMID:21398617; http://dx.doi.org/10.4049/jimmunol.1003050
- Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res 2011; 3:168-77; PMID:21738882; http://dx.doi.org/10.4168/aair.2011.3.3.168
- Snir A, Kessel A, Haj T, Rosner I, Slobodin G, Toubi E. Anti-IL-6 receptor antibody (tocilizumab): a B cell targeting therapy. Clin Exp Rheumatol 2011; 29:697-700; PMID:21813064
- Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012; 491:264-8; PMID:23064231; http://dx.doi.org/10.1038/nature11501
- Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol 2004; 172:3422-7; PMID:15004141; http://dx.doi.org/10.4049/jimmunol.172.6.3422
- Stang SL, Lopez-Campistrous A, Song X, Dower NA, Blumberg PM, Wender PA, Stone JC. A proapoptotic signaling pathway involving RasGRP, Erk, and Bim in B cells. Exp Hematol 2009; 37:122-34; PMID:19100522; http://dx.doi.org/10.1016/j.exphem.2008.09.008
- Tomic J, White D, Shi Y, Mena J, Hammond C, He L, Miller RL, Spaner DE. Sensitization of IL-2 signaling through TLR-7 enhances B lymphoma cell immunogenicity. J Immunol 2006; 176:3830-9; PMID:16517754; http://dx.doi.org/10.4049/jimmunol.176.6.3830
- Yokoyama T, Yoshizaki A, Simon KL, Kirby MR, Anderson SM, Candotti F. Age-dependent defects of regulatory B cells in Wiskott-Aldrich syndrome gene knockout mice. PLoS One 2015; 10:e0139729; PMID:26448644; http://dx.doi.org/10.1371/journal.pone.0139729
- Mittal AK, Chaturvedi NK, Rai KJ, Gilling-Cutucache CE, Nordgren TM, Moragues M, Lu R, Opavsky R, Bociek GR, Weisenburger DD et al. Chronic lymphocytic leukemia cells in a lymph node microenvironment depict molecular signature associated with an aggressive disease. Mol Med 2014; 20:290-301; PMID:24800836; http://dx.doi.org/10.2119/molmed.2012.00303
- Spaner DE, Shi Y, White D, Mena J, Hammond C, Tomic J, He L, Tomai MA, Miller RL, Booth J et al. Immunomodulatory effects of Toll-like receptor-7 activation on chronic lymphocytic leukemia cells. Leukemia 2006; 20:286-95; PMID:16341037; http://dx.doi.org/10.1038/sj.leu.2404061
- Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, Byrd JC, Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008; 118:2427-37; PMID:18551193; http://dx.doi.org/10.1172/JCI35017
- Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest 2005; 115:1797-805; PMID:15965501; http://dx.doi.org/10.1172/JCI24176
- Brusa D, Serra S, Coscia M, Rossi D, D'Arena G, Laurenti L, Jaksic O, Fedele G, Inghirami G, Gaidano G et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica 2013; 98:953-63; PMID:23300177; http://dx.doi.org/10.3324/haematol.2012.077537
- Os A, Bürgler S, Ribes Anna P, Funderud A, Wang D, Thompson Keith M, Tjønnfjord Geir E, Bogen B, Munthe Ludvig A. Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep 2013; 4:566-77; PMID:23933259; http://dx.doi.org/10.1016/j.celrep.2013.07.011
- Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005; 106:2018-25; PMID:15914560; http://dx.doi.org/10.1182/blood-2005-02-0642
- Lindqvist CA, Christiansson LH, Simonsson B, Enblad G, Olsson-Stromberg U, Loskog AS. T regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignancies. Immunology 2010; 131:371-6; PMID:20518821; http://dx.doi.org/10.1111/j.1365-2567.2010.03308.x
- Christopoulos P, Pfeifer D, Bartholome K, Follo M, Timmer J, Fisch P, Veelken H. Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Blood 2011; 117:3836-46; PMID:21270444; http://dx.doi.org/10.1182/blood-2010-07-299321
- Bojarska-Junak A, Hus I, Chocholska S, Tomczak W, Wos J, Czubak P, Putowski L, Rolinski J. CD1d expression is higher in chronic lymphocytic leukemia patients with unfavorable prognosis. Leuk Res 2014; 38:435-42; PMID:24418751; http://dx.doi.org/10.1016/j.leukres.2013.12.015
- Weinkove R, Brooks CR, Carter JM, Hermans IF, Ronchese F. Functional invariant natural killer T-cell and CD1d axis in chronic lymphocytic leukemia: implications for immunotherapy. Haematologica 2013; 98:376-84; PMID:23065503; http://dx.doi.org/10.3324/haematol.2012.072835
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol 2004; 4:231-7; PMID:15039760; http://dx.doi.org/10.1038/nri1309
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25:297-336; PMID:17150027; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141711
- Blomqvist M, Rhost S, Teneberg S, Lofbom L, Osterbye T, Brigl M, Mansson JE, Cardell SL. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur J Immunol 2009; 39:1726-35; PMID:19582739; http://dx.doi.org/10.1002/eji.200839001
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol 2010; 11:197-206; PMID:20139988; http://dx.doi.org/10.1038/ni.1841
- Fais F, Morabito F, Stelitano C, Callea V, Zanardi S, Scudeletti M, Varese P, Ciccone E, Grossi CE. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates alpha-galactosylceramide presentation to natural killer T lymphocytes. Int J Cancer 2004; 109:402-11; PMID:14961579; http://dx.doi.org/10.1002/ijc.11723
- Jadidi-Niaragh F, Jeddi-Tehrani M, Ansaripour B, Razavi SM, Sharifian RA, Shokri F. Reduced frequency of NKT-like cells in patients with progressive chronic lymphocytic leukemia. Med Oncol 2012; 29:3561-9; PMID:22669567; http://dx.doi.org/10.1007/s12032-012-0262-4
- Maki G, Hayes GM, Naji A, Tyler T, Carosella ED, Rouas-Freiss N, Gregory SA. NK resistance of tumor cells from multiple myeloma and chronic lymphocytic leukemia patients: implication of HLA-G. Leukemia 2008; 22:998-1006; PMID:18288133; http://dx.doi.org/10.1038/leu.2008.15
- Itala M, Vainio O, Remes K. Functional abnormalities in granulocytes predict susceptibility to bacterial infections in chronic lymphocytic leukaemia. Eur J Haematol 1996; 57:46-53; PMID:8698131; http://dx.doi.org/10.1111/j.1600-0609.1996.tb00489.x
- Kontoyiannis DP, Georgiadou SP, Wierda WG, Wright S, Albert ND, Ferrajoli A, Keating M, Lewis RE. Impaired bactericidal but not fungicidal activity of polymorphonuclear neutrophils in patients with chronic lymphocytic leukemia. Leuk Lymphoma 2013; 54:1730-3; PMID:23163595; http://dx.doi.org/10.3109/10428194.2012.750723
- Messmer D, Telusma G, Wasil T, Messmer BT, Allen S, Rai KR, Chiorazzi N. Dendritic cells from chronic lymphocytic leukemia patients are normal regardless of Ig V gene mutation status. Mol Med 2004; 10:96-103; PMID:16113842
- Saulep-Easton D, Vincent FB, Le Page M, Wei A, Ting SB, Croce CM, Tam C, Mackay F. Cytokine-driven loss of plasmacytoid dendritic cell function in chronic lymphocytic leukemia. Leukemia 2014; 28:2005-15; PMID:24721775; http://dx.doi.org/10.1038/leu.2014.105
- Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA, Zent CS, Dietz AB. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol 2012; 156:674-6; PMID:22050346; http://dx.doi.org/10.1111/j.1365-2141.2011.08902.x
- Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, Eckart MJ, Krause SW, Oefner PJ, Le Blanc K et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014; 124:750-60; PMID:24850760; http://dx.doi.org/10.1182/blood-2013-12-546416
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012; 30:221-41; PMID:22224776; http://dx.doi.org/10.1146/annurev-immunol-020711-074934
- Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010; 32:129-40; PMID:20079667; http://dx.doi.org/10.1016/j.immuni.2009.11.009
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117:530-41; PMID:20962324; http://dx.doi.org/10.1182/blood-2010-07-294249
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28:639-50; PMID:18482568; http://dx.doi.org/10.1016/j.immuni.2008.03.017
- Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L, Cao X. Regulatory dendritic cells program B cells to differentiate into CD19hiFcgammaIIbhi regulatory B cells through IFN-beta and CD40L. Blood 2012; 120:581-91; PMID:22692512; http://dx.doi.org/10.1182/blood-2011-08-377242
- Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol 2009; 182:3492-502; PMID:19265127; http://dx.doi.org/10.4049/jimmunol.0803052
- Saulep-Easton D, Vincent FB, Quah PS, Wei A, Ting SB, Croce CM, Tam C, Mackay F. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia 2015; PMID:26139429; http://dx.doi.org/10.138/leu.2015
- Puiggros A, Blanco G, Espinet B. Genetic abnormalities in chronic lymphocytic leukemia: where we are and where we go. Biomed Res Int 2014; 2014:435983; PMID:24967369; http://dx.doi.org/10.1155/2014/435983
- Karmali R, Paganessi LA, Frank RR, Jagan S, Larson ML, Venugopal P, Gregory SA, Christopherson KW, 2nd. Aggressive disease defined by cytogenetics is associated with cytokine dysregulation in CLL/SLL patients. J Leukoc Biol 2013; 93:161-70; PMID:23136257; http://dx.doi.org/10.1189/jlb.0612301
- Deaglio S, Capobianco A, Bergui L, Durig J, Morabito F, Duhrsen U, Malavasi F. CD38 is a signaling molecule in B-cell chronic lymphocytic leukemia cells. Blood 2003; 102:2146-55; PMID:12763926; http://dx.doi.org/10.1182/blood-2003-03-0989
- Vaisitti T, Aydin S, Rossi D, Cottino F, Bergui L, D'Arena G, Bonello L, Horenstein AL, Brennan P, Pepper C et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia 2010; 24:958-69; PMID:20220774; http://dx.doi.org/10.1038/leu.2010.36
- Vaisitti T, Audrito V, Serra S, Buonincontri R, Sociali G, Mannino E, Pagnani A, Zucchetto A, Tissino E, Vitale C et al. The enzymatic activities of CD38 enhance CLL growth and trafficking: implications for therapeutic targeting. Leukemia 2015; 29:356-68; PMID:24990614; http://dx.doi.org/10.1038/leu.2014.207
- Zupo S, Isnardi L, Megna M, Massara R, Malavasi F, Dono M, Cosulich E, Ferrarini M. CD38 expression distinguishes two groups of B-cell chronic lymphocytic leukemias with different responses to anti-IgM antibodies and propensity to apoptosis. Blood 1996; 88:1365-74; PMID:8695855
- Jahrsdorfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, Taylor CM, Weiner GJ. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood 2006; 108:2712-9; PMID:16809616; http://dx.doi.org/10.1182/blood-2006-03-014001
- Hagn M, Blackwell SE, Beyer T, Ebel V, Fabricius D, Lindner S, Stilgenbauer S, Simmet T, Tam C, Neeson P et al. B-CLL cells acquire APC- and CTL-like phenotypic characteristics after stimulation with CpG ODN and IL-21. Int Immunol 2014; 26:383-95; PMID:24497611; http://dx.doi.org/10.1093/intimm/dxu001
- Wlasiuk P, Tomczak W, Zajac M, Dmoszynska A, Giannopoulos K. Total expression of HLA-G and TLR-9 in chronic lymphocytic leukemia patients. Hum Immunol 2013; 74:1592-7; PMID:23994589; http://dx.doi.org/10.1016/j.humimm.2013.08.277
- Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, Beers SA, French RR, Cox KL, Davies AJ et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011; 118:2530-40; PMID:21768293; http://dx.doi.org/10.1182/blood-2011-01-330357
- Ferrer G, Bosch R, Hodgson K, Tejero R, Roue G, Colomer D, Montserrat E, Moreno C. B cell activation through CD40 and IL4R ligation modulates the response of chronic lymphocytic leukaemia cells to BAFF and APRIL. Br J Haematol 2014; 164:570-8; PMID:24245956; http://dx.doi.org/10.1111/bjh.12645
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998; 16:2825-33; PMID:9704735
- Huh YO, Keating MJ, Saffer HL, Jilani I, Lerner S, Albitar M. Higher levels of surface CD20 expression on circulating lymphocytes compared with bone marrow and lymph nodes in B-cell chronic lymphocytic leukemia. Am J Clin Pathol 2001; 116:437-43; PMID:11554173; http://dx.doi.org/10.1309/438N-E0FH-A5PR-XCAC
- Shaha SP, Tomic J, Shi Y, Pham T, Mero P, White D, He L, Baryza JL, Wender PA, Booth JW et al. Prolonging microtubule dysruption enhances the immunogenicity of chronic lymphocytic leukaemia cells. Clin Exp Immunol 2009; 158:186-98; PMID:19737143; http://dx.doi.org/10.1111/j.1365-2249.2009.04003.x
- Hosing C, Kebriaei P, Wierda W, Jena B, Cooper LJ, Shpall E. CARs in chronic lymphocytic leukemia – ready to drive. Curr Hematol Malig Rep 2013; 8:60-70; PMID:23225251; http://dx.doi.org/10.1007/s11899-012-0145-y
- Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJ. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res 2006; 66:10995-1004; PMID:17108138; http://dx.doi.org/10.1158/0008-5472.CAN-06-0160
- Benoit NE, Wade WF. Increased inhibition of proliferation of human B cell lymphomas following ligation of CD40, and either CD19, CD20, CD95 or surface immunoglobulin. Immunopharmacology 1996; 35:129-39; PMID:8956976; http://dx.doi.org/10.1016/S0162-3109(96)00138-5
- Byrd JC, Kipps TJ, Flinn IW, Cooper M, Odenike O, Bendiske J, Rediske J, Bilic S, Dey J, Baeck J et al. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk Lymphoma 2012; 53:2136-42; PMID:22475052; http://dx.doi.org/10.3109/10428194.2012.681655
- Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 2000; 96:2917-24; PMID:11049967
- Kato K, Cantwell MJ, Sharma S, Kipps TJ. Gene transfer of CD40-ligand induces autologous immune recognition of chronic lymphocytic leukemia B cells. J Clin Invest 1998; 101:1133-41; PMID:9486984; http://dx.doi.org/10.1172/JCI1472
- Pallasch CP, Ulbrich S, Brinker R, Hallek M, Uger RA, Wendtner CM. Disruption of T cell suppression in chronic lymphocytic leukemia by CD200 blockade. Leuk Res 2009; 33:460-4; PMID:18838168; http://dx.doi.org/10.1016/j.leukres.2008.08.021
- Gorczynski RM, Lee L, Boudakov I. Augmented induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation 2005; 79:488-91; PMID:15729177; http://dx.doi.org/10.1097/01.TP.0000152118.51622.F9
- McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, Ravey EP, Qin F, Bowdish KS. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci U S A 2006; 103:1041-6; PMID:16418292; http://dx.doi.org/10.1073/pnas.0510081103
- Laprevotte E, Voisin G, Ysebaert L, Klein C, Daugrois C, Laurent G, Fournie JJ, Quillet-Mary A. Recombinant human IL-15 trans-presentation by B leukemic cells from chronic lymphocytic leukemia induces autologous NK cell proliferation leading to improved anti-CD20 immunotherapy. J Immunol 2013; 191:3634-40; PMID:23997218; http://dx.doi.org/10.4049/jimmunol.1300187
- Moga E, Alvarez E, Canto E, Vidal S, Rodriguez-Sanchez JL, Sierra J, Briones J. NK cells stimulated with IL-15 or CpG ODN enhance rituximab-dependent cellular cytotoxicity against B-cell lymphoma. Exp Hematol 2008; 36:69-77; PMID:17959301; http://dx.doi.org/10.1016/j.exphem.2007.08.012
- Moga E, Canto E, Vidal S, Juarez C, Sierra J, Briones J. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp Hematol 2011; 39:1064-71; PMID:21864486; http://dx.doi.org/10.1016/j.exphem.2011.08.006
- Wild J, Schmiedel BJ, Maurer A, Raab S, Prokop L, Stevanovic S, Dorfel D, Schneider P, Salih HR. Neutralization of (NK-cell-derived) B-cell activating factor by Belimumab restores sensitivity of chronic lymphoid leukemia cells to direct and Rituximab-induced NK lysis. Leukemia 2015; 29:1676-83; PMID:25710310; http://dx.doi.org/10.1038/leu.2015.50
- Junevik K, Werlenius O, Fogelstrand L, Karlsson-Parra A, Andersson PO. High functional CD70 expression on alpha-type 1-polarized dendritic cells from patients with chronic lymphocytic leukaemia. Scand J Immunol 2014; 79:415-22; PMID:24684541; http://dx.doi.org/10.1111/sji.12172
- Riches JC, Ramsay AG, Gribben JG. Immune reconstitution in chronic lymphocytic leukemia. Curr Hematol Malig Rep 2012; 7:13-20; PMID:22231031; http://dx.doi.org/10.1007/s11899-011-0106-x
- Golay J, D'Amico A, Borleri G, Bonzi M, Valgardsdottir R, Alzani R, Cribioli S, Albanese C, Pesenti E, Finazzi MC et al. A novel method using blinatumomab for efficient, clinical-grade expansion of polyclonal T cells for adoptive immunotherapy. J Immunol 2014; 193:4739-47; PMID:25267972; http://dx.doi.org/10.4049/jimmunol.1401550
- D'Arena G, Simeon V, De Martino L, Statuto T, D'Auria F, Volpe S, Deaglio S, Maidecchi A, Mattoli L, Mercati V et al. Regulatory T-cell modulation by green tea in chronic lymphocytic leukemia. Int J Immunopathol Pharmacol 2013; 26:117-25; PMID:23527714
- Skorka K, Bhattacharya N, Wlasiuk P, Kowal M, Mertens D, Dmoszynska A, Giannopoulos K. Thalidomide regulation of NF-kappaB proteins limits Tregs activity in chronic lymphocytic leukemia. Adv Clin Exp Med 2014; 23:25-32; PMID:24596000; http://dx.doi.org/10.17219/acem/37018
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999; 163:5211-8; PMID:10553041
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007; 30:825-30; PMID:18049334; http://dx.doi.org/10.1097/CJI.0b013e318156e47e
- Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica 2008; 93:193-200; PMID:18223287; http://dx.doi.org/10.3324/haematol.11702
- Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin Cancer Res 2012; 18:678-87; PMID:22190592; http://dx.doi.org/10.1158/1078-0432.CCR-11-2630
- Anand M, Chodda SK, Parikh PM, Nadkarni JS. Dysregulated cytokine production by monocytes from chronic lymphocytic leukemia patients. Cancer Biother Radiopharm 1998; 13:43-8; PMID:10850341; http://dx.doi.org/10.1089/cbr.1998.13.43
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 2007; 178:7868-78; PMID:17548625; http://dx.doi.org/10.4049/jimmunol.178.12.7868
