ABSTRACT
To improve prognosis of post-transplant lymphoproliferative disease (PTLD), a sequential therapeutic strategy that rituximab-based treatments followed by donor lymphocyte infusion (DLI) or autologous EBV-specific cytotoxic T lymphocytes (EBV-CTL) for biopsy-proven EBV-associated PTLD in recipients of allogeneic hematopoietic stem cell transplantation was designed. 84 patients with EBV-PTLD were enrolled in this prospective study. After two cycles of the rituximab-based treatments, 68 of 84 patients (81% [95% CI 71–88]) responded and 52 (62% [51–72]) had CRs. This increased to 73 of 77 patients (95% [87–98]) with completion of sequential cell infusions, and 70 of 77 (91% [82–96]) achieved CRs after DLI or autologous EBV-CTL infusion. 22 patients experienced acute GVHD (aGVHD) (grade I in 5 and grade II in 13, grade III in 4) and 13 chronic GVHD (limited cGVHD in 7 and extensive cGVHD in 6) in 62 patients undergoing a median of three doses of DLI. The incidences of GVHD were similar between DLI and EBV-CTL group (aGVHD 35% vs. 33%, p = 0.876; cGVHD 21% vs. 13%; p = 0.503). EBV-CTL activity after the rituximab-based treatments did not change, while increased after cell infusions and reached its maximum in the 3rd or 6th month after EBV-CTL or DLI treatment, respectively. The 5-y cumulative incidence of PTLD relapse was 4.5% ± 3.3%. The 5-y overall survival (OS) and progression-free survival (PFS) after PTLD were 70.7% ± 5.2% and 68.9% ± 5.3%, respectively. Rituximab-based treatments combined with adoptive cellular immunotherapy might elevate CR rates and reduce relapse of PTLD after allo-HSCT.
Abbreviations
| allo-HSCT | = | allogeneic hematopoietic stem cell transplantation |
| BMT | = | bone marrow stem cell transplantation |
| CNS | = | central nervous system |
| CR | = | complete remission/response |
| DCs | = | dendritic cells |
| DLI | = | Granulocyte colony-stimulating factor-mobilized donor lymphocyte infusion |
| EBV-CTL | = | Epstein–Barr virus-specific cytotoxic T lymphocytes |
| EBV-LCL | = | EBV-transformed lymphoblastoid cell line |
| GVHD | = | graft versus host disease |
| MODS | = | multiple-organ disfunction syndrome |
| NR | = | no remission/response |
| OS | = | overall survival |
| PBMC | = | peripheral blood mononuclear cells |
| PBSCT | = | peripheral blood stem cell transplantation |
| PD | = | progression of disease |
| PFS | = | progression-free survival |
| PGE2 | = | prostaglandin E2 |
| PR | = | partial remission/response |
| PTLD | = | post-transplant lymphoproliferative disease |
| R | = | rituximab |
| R-COP | = | rituximab + cyclophosphamide + vincristine + prednisolone |
| R-CHOP | = | R-COP + epirubicin |
| rhGM-CSF | = | recombinant human granulocyte-macrophage colony stimulating factor |
| rhIL-4 | = | recombinant human interleukin-4 |
| rhTNF-α | = | recombinant human tumor necrosis factor-α |
| RI | = | reduction of immunosuppression. |
Introduction
Epstein–Barr virus (EBV)-associated PTLD is a life-threatening complication in the recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT). With rituximab introduced as preemptive therapy in high-risk patients and treatment for PTLD, the morbidity and mortality of PTLD have been reduced. Rituximab administered preemptively can induce sustained reversal of EBV-emia in up to 90% of patients,Citation1 but only about 50% of established PTLD achieve complete remission (CR) with the treatment of rituximab.Citation2,3 Meanwhile, rituximab has side effects, such as an increase in immunocompromise, and not restoring EBV-specific immunity, making PTLD at risk of relapse.Citation4,5 In the recipients of allo-HSCT, we observed that two of nine PTLD patients treated with rituximab-based treatment experienced PTLD relapse within two yearsCitation6
Adoptive cellular immunotherapies, including EBV-CTL and DLI, may induce durable remissions of PTLD, with a response rate of up to 90%.Citation7-10 With adoptive cellular immunotherapies, no documented cases of PTLD relapse have been reported. Despite high CR rates, the disadvantages of adoptive cellular immunotherapies should be taken into account that ex vivo generation of EBV-CTL requires time and facilities, leading to treatment delay which is associated with a mortality of > 90%Citation8,11 and DLI may increase the risk of graft vs. host disease (GVHD)Citation12 and contraindicated in patients with pre-existing GVHD, compared with rituximab. Thus, rituximab is recommended as the highest priority for PTLD, and EBV-CTL or DLI should be taken into account when available or as a second-line therapy according to the Second European Conference on Infections in Leukemia.Citation11
Based on the aforementioned problems, in this prospective study, a sequential therapeutic strategy of rituximab-based treatments followed by adoptive cellular immunotherapy was devised for biopsy-proven PTLD. The aim of this study was to investigate whether the strategy can elevate CR rate and reduce relapse of PTLD, and overcome the drawbacks of the adoptive cellular immunotherapy.
Results
Patient, transplant and clinical characteristics
84 patients with EBV-associated PTLD were enrolled in this trial. Thirty patients were females and 54 males. The median age was 23.5 (range, 9–49) y old. Primary diseases included acute myeloblastic leukemia (n = 39), acute lymphoblastic leukemia (n = 30), myelodysplastic syndrome (n = 4), chronic myeloid leukemia (n = 1), non-Hodgkin lymphoma (n = 1), and severe aplastic anemia (n = 9). Sixty-five patients received related and nineteen unrelated donor transplants. Seventeen patients received HLA-matched and 67 HLA-mismatched transplants. Fifty-three patients received anti-thymocyte globulin as prophylaxis of GVHD.
The median time to PTLD onset was 58 (range, 29–867) d post-transplantation and PTLD was diagnosed within 3 (range, 1–19) d after onset. Fifty-three patients developed nodal disease, twenty-eight had lymph nodes accompanying extranodal tissues involved, and three developed primary extranodal PTLD. The involved extranodal sites included lung (n = 18), central nervous system (CNS, n = 10), Waldeyer ring (n = 8), liver (n = 5), spleen (n = 6), nasal cavity (n = 2) and stomach (n = 3). The histopathology included diffuse large B-cell lymphoma (n = 54), polymorphy PTLD (n = 21), early lesions (infectious mononucleosis-like lesion, n = 7; plasmacytic hyperplasia, n = 1), and Hodgkin lymphoma (n = 1).
Treatment and response
At the time of rituximab-based treatments initiated, 59 patients were receiving single-agent and 7 multiple immunosuppressants as prophylaxis or treatment for GVHD, including ciclosporin (n = 48), tacrolimus (n = 11) and meprednisone plus tacrolimus (n = 7). At the time of the adoptive cellular immunotherapies, 24 patients were receiving single-agent and 3 multiple immunosuppressants, including ciclosporin (n = 19), tacrolimus (n = 5) and meprednisone plus tacrolimus (n = 3). There were no differences in immunosuppressant treatment between patients receiving rituximab and rituximab plus chemotherapy or DLI and EBV-CTL (data not shown).
Forty (48%) patients accepted reduction of immunosuppression (RI), and 46 rituximab monotherapy and 38 rituximab combined with chemotherapy. Seventy-seven received DLI (n = 62) or autologous EBV-CTL (n = 15), including 27 for inducing CR after rituximab-based treatment failure and preventing relapse and 50 for only relapse prophylaxis after the planed rituximab-based treatments. In 4 of 7 patients with PD during or after rituximab-based treatment, DLI or autologous EBV-CTL was applied ahead of schedule by a median of 15 d Seven (8%) of 84 patients did not receive the planned DLI or EBV-CTL treatment: five (6%) because of death from disease progression (PD) during rituximab-based treatments, two CR patients died from infections and multiple-organ disfunction syndrome (MODS), respectively.
After two cycles of rituximab-based treatment, the donor lymphocytes or EBV-CTL was available and no patient experienced EBV-CTL production failure. A total of 256 doses of DLI or EBV-CTL were administered to prevent PTLD relapse, including 170 doses of DLI in 52 cases, with a median of 3 (range, 1–4) doses per patient and 86 doses of EBV-CTL in 14 cases, with a median of 6 (range, 3–8) doses per patient. The initial administration of DLI or EBV-CTL for inducing CR or preventing relapse was performed on day 121 (range, 47–1172) post-transplantation.
After the rituximab-based treatments, 68 of 84 patients (81% [95% CI 71–88]) had either a complete or partial response (PR) after rituximab-based treatment. This increased to 73 of 77 patients (95% [87–98]) with completion of sequential cell infusions. Fifty-two of 84 (62% [51–72]) reached CR after rituximab-based treatments and 70 of 77 (91% [82–96]) obtained CR following rituximab-based treatments and DLI or EBV-CTL. Fourteen (88%) of sixteen patients in PR after rituximab-based treatments achieved CR with cellular immunotherapy. Eleven of sixteen rituximab-based treatment non-responders received DLI or EBV-CTL and seven responded (six CR and one PR), and four cell therapy non-responders died of PD.
In addition, intrathecal rituximab (sequential dose-escalation schedule [10 mg, 20 mg, 30 mg, 40 mg and 50 mg/time] weekly) was used in six patients with CNS involvement, who had failed intravenous rituximab-based treatments, from day 7–15 after the intravenous rituximab-based treatments, and finally they all obtained CR. The efficacy of rituximab alone was similar to that of rituximab plus chemotherapy (CR rate: 27 [59%] of 46 vs. 25 [66%] of 38, p = 0.505). Patients with isolated lymph node involved were more responsive to the rituximab-based treatments than those with extranodal involvement (CR rate: 38 [72%] of 53 vs. 14 [45%] of 31, p = 0.016). The CR rates were not different between the patients with early lesion/polymorphic PTLD and those with monomorphic PTLD (21 [72%] of 29 vs. 31 [56%] of 55, p = 0.097). The DLI efficacy was similar to that of autologous EBV-CTL (CR rate 13[68%] of 19 vs. 7[88%] of 8, p = 0.302) (, ).
Table 1. Characteristics of patients receiving rituximab-monotherapy or rituximab+chemotherapy
Table 2. Characteristics of patients receiving DLI or EBV-CTL for inducing CR
GVHD
During the RI and rituximab-based treatments, 13 patients developed de novo aGVHD (grade I, n = 3; grade II, n = 5; grade III, n = 4; grad IV, n = 1), 8 were controlled with GVHD treatments. Of the 62 patients undergoing DLI, aGVHD occurred in 22 patients (35% [95% CI 25–48]; grade I in 5 and grade II in 13, grade III in 4) and cGVHD occurred in 13 (21% [13–33]; limited cGVHD in 7 and extensive cGVHD in 6). Of the 15 patients undergoing EBV-CTL, 5 patients (33% [15–59]) developed aGVHD (grade I in 1, grade II in 3 and grade III in 1) and 2 (13% [2–39]) limited cGVHD. There were no difference in the incidence of aGVHD (p = 0.876) and cGVHD (p = 0.503) between the patients received DLI and EBV-CTL, and the severity of aGVHD and cGVHD was similar in both groups (Grade III/IV, 4 [6%] of 62 vs. 1[7%] of 15; extensive cGVHD 6 [10%] of 62 vs. 0 of 15). No patient died of GVHD, while two patients who developed grade II aGVHD after DLI died of CMV pneumonia during GVHD treatments.
Lymphocyte subsets and EBV-CTL activity
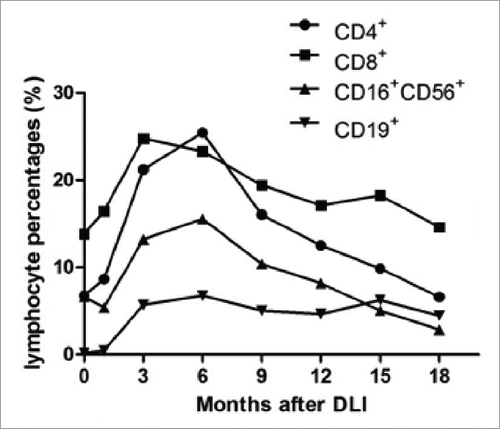
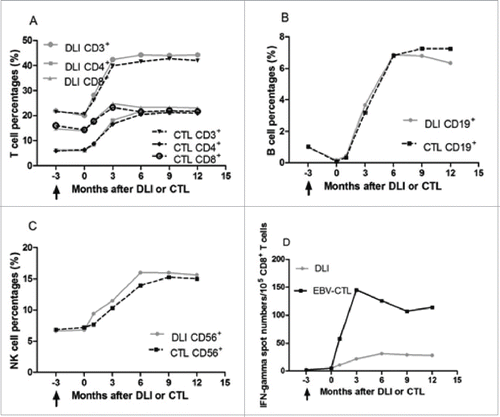
Lymphocyte subset analyses showed that the percentages of CD3+ T cells, and CD16+CD56+ NK cells after the rituximab-based treatments were not different from those before the treatment, but began to increase consistently following cell infusions and reached their maximal at the 3rd and 6th month after cellular immunotherapies, respectively, and no difference among those of the 6th, 9th, 12th was evident. The CD3+CD4+:CD3+CD8+ T cell ratio increase consistently after the 3rd month and reached its maximal at the 6th month after cell therapy. The percentage of CD19+ B cells decreased rapidly after the rituximab-based treatments, while increased after cell infusions till the 6th month and did not change significantly thereafter. The percentages of T lymphocyte subsets, NK cells and B cells were not different between patients undergoing DLI and EBV-CTL at every time point. EBV-CTL activity after the rituximab-based treatments was not different from those before the treatments, while increased after cell infusions. In the EBV-CTL group, the spot numbers in the Elispot assay reached their maximum in the 3rd month since the first infusion and did not change significantly thereafter. In the DLI group, the spot numbers reached their maximum in the 6th month and stabilized thereafter. The EBV-CTL activity was significantly higher in the EBV-CTL treatment group than that in the DLI group at every time point. The details are shown in . In one relapsed PTLD case, CD4+/CD8+ reverse and a steady decrease in percentage of NK cells were observed since the 9th month after DLI () and EBV-CTL activity was not detectable when relapse occurred.
Figure 1. Study profile. DLI, Granulocyte colony-stimulating factor-mobilized donor lymphocyte infusion; EBV-CTL, Epstein-Barr virus-specific cytotoxic T lymphocytes; PD, progression of disease; CR, complete remission; MODS, multiple-organ disfunction syndrome; PFS, progression-free survival; OS, overall survival. *: included two CR patients not receiving DLI or EBV-CTL.
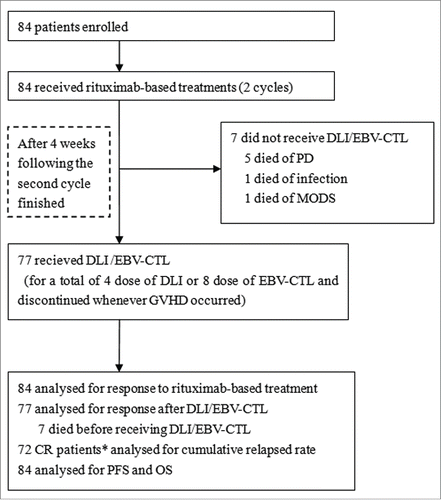
Figure 2. Lymphocyte percentages in circulation and EBV-specific cytotoxic lymphocyte activity of patients who received DLI and EBV-CTL infusion. No significant difference was observed between patients received DLI and EBV-CTL in lymphocytes (CD3+ T cells, p = 0.552; CD16+CD56+ NK cells, p = 0.549; CD19+ B cells, p = 0.704). (A), (B) and (C): The percentages of CD3+ T cells, and CD16+CD56+ NK cells before cellular immunotherapies were not different from those before rituximab-based treatment (p = 0.471 and p = 0.603, respectively), while the CD19+ B cells before the cell infusions were less than those before the rituximab-based treatments (p < 0 .001). The percentages of CD3+ T cells, CD16+CD56+ NK cells, CD19+ B cells were increased in the 1st month after cellular immunotherapies than those before cell infusions (p = 0.001, p = 0.01, p < 0 .001, respectively), higher in the 3rd month than those in the 1st month after DLI or EBV-CTL (p < 0 .001, p = 0.009, p < 0 .001, respectively), and reached their maximal in the 3rd, 6th, 6th month, respectively. The CD4+ T cell to CD8+ T cell ratio increased since the 3rd month (p < 0.001), no differences among the 6th, 9th and 12th month. (D) In the spot assay, the IFNγ spot numbers before the cell infusion were not different from those before rituximab-based treatments (p = 0.296), while more in the 1st month than those before cell infusion (p < 0 .001), more in the 3rd month than those in the 1st month after cellular immunotherapies (p < 0 .001) and more in the 6th month than those in the 3rd month (p < 0 .001), no differences among the 6th, 9th and 12th month after cellular immunotherapies. The EBV-CTL activity in EBV-CTL treatment group was higher than that in the DLI group (p = 0.001). Notes: Arrows denote the time of rituximab-based treatments.
Survival and relapse
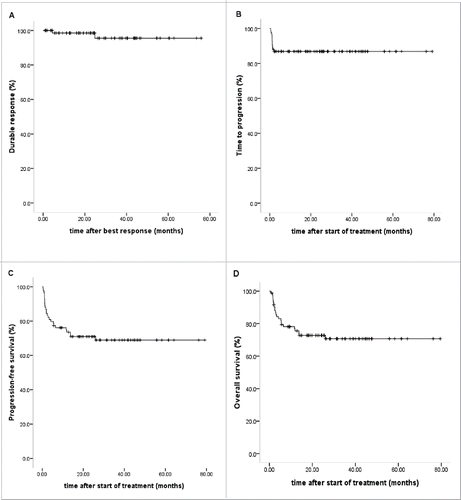
Median response duration had not been reached (>75 .7 mo) at the study cut-off date (). Within a median of 24.0 (0.9–75.8) mo, two of the 72 CR patients had a relapse at 4.5 mo and 2.1 y after treatment, respectively, and died despite second-line chemotherapy. The 5-y cumulative incidence of PTLD relapse was 4.5% ± 3.3%.
Median time to progression had not been reached (>79 .2 mo) (). Cox regression analysis of time to progression was performed taking into account the following covariates: age, sex, presence of extranodal disease, stage of disease, monomorphic disease, rituximab plus chemotherapy, lung involvement and CNS involvement. Rituximab plus chemotherapy were associated with extended time to progression intervals (HR 0.059 [95% CI 0.010–0.361], p = 0.002), a higher risk of disease progression was observed in patients with lung involvement (HR 13.157 [95% CI 1.040–166.484], p = 0.047).
Within a median follow-up of 23.1 (range, 0.7 to 79.5) mo after treatment, median PFS and median OS had not been reached (), 61 patients survived and 23 died. Cox regression analysis of survival were performed taking into account the following factors: age, sex, presence of extranodal disease, stage of disease, monomorphic disease, rituximab plus chemotherapy, lung involvement and CNS involvement, rituximab combined with chemotherapy was associated with superior PFS and OS compared with rituximab monotherapy (HR 0.208 [95% CI 0.070–0.615], p = 0.005; HR 0.238 [95% CI 0.076–0.743], p = 0.013), and shorter PFS and OS were evident in patients with lung involvement (HR 6.502 [95% CI 1.271–33.254], p = 0.025; HR 5.941 [95% CI 1.128–31.299], p = 0.036).
Five-year PFS was 68.9% ± 5.3%, and OS was 70.7% ± 5.2%. The causes of death included PTLD progression (n = 9), infection (n = 9; CMV pneumonia in 2; viral myocarditis in 1, bacterial infection in 4, fungal infection in 2), MODS (n = 1), PTLD relapse (n = 2) and primary malignancy relapse (n = 2). Up till now, two experienced primary malignancy relapse. The 5-y cumulative incidence of primary malignancy relapse post-transplantation was 5.9±4.0%.
Discussion
The introduction of rituximab has improved the prognosis of PTLD, and it is readily available and favorably tolerated,Citation2 but the efficacy is inferior to cellular immunotherapy and cannot restore EBV-specific immunity. The efficacy of rituximab-based treatment for PTLD depends on the early diagnosis and prompt administration, and the location and degree of organ involvement.Citation9,13-15 PTLD with extranodal involvement carries worse prognosis than those with isolated lymph node involvement, which might result from the difficulty in early diagnosis and low concentrations of medication distributed in affected tissues,Citation16,17 in this cohort, lung involvement associated with a higher risk of disease progression and poorer survival. Our results also revealed that the patients with extranodal involvement were less responsive to rituximab than those with isolated lymph node involvement, but had a higher response rate than those with extranodal involvement in reports,Citation14,18 which might be attributed to early diagnosis and prompt treatment as well as special medication approach. In our centers, EBV-DNA monitoring is routinely performed, which is helpful to early diagnose PTLD.Citation19 Six patients with CNS involvement who had failed intravenous rituximab-based treatments all obtained CR after intrathecal rituximab combination. In addition, some novel B cell-targeted agents has been proved to mediate potent antitumor activity in B cell malignancy such as ibrutinib in chronic lymphocytic leukemia, blinatumomab in relapsed/refractory precursor B cell acute lymphoid leukemia, whether these agents are efficacious in EBV-associated PTLD needs further study.Citation20-22
EBV-CTL or DLI alone induce high CR rates for PTLD.Citation7,9,10 Moreover, they are also effective for rituximab-resistant PTLD. However, as aforementioned, they also have disadvantages. For example, the long time required for EBV-CTL production limits its prompt application, and DLI result in high risk of GVHD. The efficacy of cellular immunotherapy depends on effector cell to targeted tumor cell ratio.Citation9 There is a lack of randomized controlled trials for comparing efficacy between rituximab and cellular immunotherapy. There is also no comparison between rituximab combined with cellular immunotherapy and rituximab alone or cellular immmunotherapy alone. Doubrovina et al Citation9 observed equivalent efficacy of DLI and EBV-CTL for PTLD. In this study, we designed a sequential therapeutic strategy of the rituximab-based treatments followed by adoptive cellular immunotherapy. In the patients who had not achieved CR after rituximab-based treatments, the cellular immunotherapies were administrated, which included DLI used in the patients without pre-existing GVHD, and autologous EBV-CTL administrated in the patients with pre-existing GVHD or unavailability of the original donor. The strategy decreased the risk of GVHD because of rituximab-based treatment-induced immunosuppressionCitation23-25 and reserved the time to product EBV-CTL. Furthermore, the protocol of rituximab-based treatments followed by cellular immunotherapy might increase the effector cell to targeted tumor cell ratio, the former decrease the tumor loads so that the effector cells can fully exert anti-residual tumor effect. Our results showed that 20 of the 27 non-CR patients after rituximab-based treatments upgraded to CR following cellular immunotherapy, resulted in an increase of 29% (from 62% to 91%) in CR rate as expected. The efficacy was not different between rituximab-based treatments combined with DLI and rituximab-based treatments combined with EBV-CTL, which was consistent with the literature.Citation9
The introduction of rituximab has improved PTLD remission, thus PTLD relapse might become a crucial factor on long-term survival. The incidence of PTLD relapse varies from 12% to 50% in recipients of solid organ transplant (SOT) treated with rituximab,Citation5,26,27 while there is absence of large sample data in recipients of HSCT. The development of PTLD is closely related to immune status.Citation28 Reconstituting EBV-specific immunity is essential to reduce PTLD relapse. Immunosuppressant discontinuation is an ideal method to restore EBV-specific immunity, but is not feasible in the recipients of allo-HSCT because of high morbidity and mortality of GVHD resulted from immunosuppressant withdrawal. The complete reconstitution of immune function needs generally 3–5 y in recipients of HSCT.Citation29 EBV-specific T-cell recoveries can be influenced by many factors, such as global T-cell recovery.Citation30 Some studies indicated that the majority of EBV-CTL generated ex vivo show an effector memory phenotype,Citation31,32 and adoptive cell infusion can result in sustained expansion of EBV-CTL at high frequency in vivo and durable remission of PTLD.Citation7,9,28 In this study, to eradicate minimal residual tumor, the adoptive cellular immunotherapies were administrated as prophylaxis in patients with CR. Our study demonstrated that the EBV-specific CD8+ T cell reactivity improved significantly after autologous EBV-CTL or DLI treatment, additionally, NK cells and CD4+ to CD8+ T cell ratio showed remarkable increases in these patients after cell infusions. Within a median follow-up of 24.0 mo, only two of the 72 CR patients experienced relapse. The incidence of PTLD relapse was lower than those of PTLD patients after SOT and that of historical PTLD patients in our center.Citation5,6,26,27 The late-relapsed case had steady decreases in lymphocytes from the 9th month after DLI and the EBV-CTL activity was undetectable when PTLD relapsed, which might be attributable to the long-term immunosuppressive therapies because of extensive cGVHD. These data suggested that adoptive cell infusion can restore EBV-specific immunity against minimal residual tumor. As far as we know, this is the first report to employ the adoptive cellular immunotherapy for preventing PTLD relapse.
The main adverse effect of DLI is associated with the risk at increasing the morbidity and mortality of GVHD. Our previous studies and other reports indicated that G-CSF-mobilized DLI had a lower risk of GVHD compared with steady DLI.Citation33-35 In this study, G-CSF-mobilized DLI instead of steady DLI was performed, the incidences of aGVHD and cGVHD were 35% and 21%, respectively, after DLI, which were similar to those of the general recipients in our institution.Citation36 No patient died of GVHD. There were no differences in incidence and severity of GVHD between the patients received DLI and EBV-CTL. These results suggested that rituximab-based treatment followed by G-CSF-mobilized DLI was safe without increasing morbidity and mortality of GVHD.
Interestingly, we observed that only two patients experienced primary malignancy relapse. The incidence of primary malignancy relapse was lower than other population in our transplant centers and literatures.Citation36,37 A reasonable interpretation of our findings is that adoptive cells mediate graft-versus-tumor (GVT) response. It is recognized that DLI induce GVT effect.Citation38,39 The antitumor activity of EBV-CTL may be ascribed to that the lymphocytes used to generate EBV-CTL might contain tumoricidal effector T cells targeting primary malignancy and the high levels of interferon-γ secreted by EBV-CTL also induce antitumor response.Citation40 In addition, the rapid reconstitution of immunity attributable to RI might also be one cause of lower primary malignancy relapse.
In summary, rituximab-based treatments combined with adoptive cellular immunotherapy might elevate CR rates for PTLD, and rituximab-based treatments followed by adoptive cellular immunotherapy might decrease PTLD relapse and improve survival. Considering the limitation of the small number of patients studied, our results need to be further confirmed in more patients and prospective, two-arm trials.
Patients and methods
Ethics statement
The prospective, open-labeled, phase 2 study was conducted in four centers. The study was performed in accordance with the modified Helsinki Declaration, and the protocol was approved by the Ethics Boards of our centers. All recipients, donors and/or guardians provided written informed consent to participate in the study.
Eligibility and PTLD diagnosis
Treatment-naive EBV-associated PTLD Patients after allo-HSCT were enrolled from March 2008 to June 2014 if confirmed by biopsy, and patients with the following criteria were excluded: irreversible organ failure not related to PTLD or active visceral hemorrhage or Eastern Cooperative Oncology Group (ECOG) performance status higher than 2. EBV-associated PTLD was diagnosed according to the WHO criteria.Citation41 EBV-DNA in blood and secretions was detected by Real-time quantitative polymerase chain reaction assay (RQ-PCR) and EBV in tissues was verified by in situ hybridization for EBV-encoded small nuclear RNA (EBV-EBER) in lymphocytes of biopsy specimens.Citation42
Study treatment
The study treatments included reducing immunosuppression (RI) and rituximab-based treatments followed by adoptive cellular immunotherapies. Immunosuppressants were withdrawn in a stepwise fashion (i.e., total dose reduced by 20%/week) if tolerated. Rituximab alone (375 mg/m2 intravenously once a week, 4 doses as one cycle with an cycle interval of 2 weeks, for a total of 2 cycles) or rituximab combined with chemotherapy (two 21-day cycles of COP consisting of rituximab 375 mg/m2 intravenously on day 0, cyclophosphamide 600 mg/m2 intravenously on day 1, vincristine 1.4 mg/m2 intravenously on day 1 and limited to 2 mg absolute per cycle, and prednisolone 60 mg orally on days 1–5 or two 21-day cycles of CHOP consisting of COP regimen and epirubicin 75 mg/m2 intravenously on day 1) were given based on PTLD histopathology and blood counts. Generally, rituximab monotherapy was administrated in the patients with early lesions or polymorphic PTLD or white blood cells less than 4 × 109 /L and/or platelets less than 50 × 109 /L and R-CHOP/R-COP in those with monomorphic PTLD and white blood cells no less than 4 × 109 /L and platelets no less than 50 × 109 /L. After two cycles of rituximab-based treatments (starting 4 weeks after the last dose of rituximab or R-COP/ R-CHOP), the adoptive cellular immunotherapies would be administrated for improving response and preventing relapse, including donor lymphocytes given at a dose of 2.0 × 107 /kg CD3+ T cells/kg for the patients with availability of the original donor and no pre-existing GVHD, and autologous EBV-CTL given at a dose of 1 × 106 cells/kg for the patients had pre-existing GVHD or lack of original donor access. In those presenting clinical signs of disease progression during rituximab-based treatments or interval, DLI or autologous EBV-CTL treatment was started immediately if the adoptive cells were prepared and disease progression was verified. DLI was performed once monthly for a total of four doses and EBV-CTL every two weeks for a total of eight doses. When GVHD occurred, DLI or EBV-CTL infusion would be discontinued. The Trial profile is shown in .
Donor lymphocytes and EBV-CTL preparation
Donor lymphocytes were obtained from the original donor. Collections were began on day 5 after Granulocyte colony-stimulating factor mobilized, and consecutive daily collections were performed until CD3+ T cell yields were more than 8.0 × 107 /kg (recipient body weight). A dose of 2.0 × 107 /kg CD3+ T cells was infused every time. Because of unavailability of the original donor, EBV-CTL were generated from autologous peripheral blood mononuclear cells (PBMC) which were isolated from recipients themselves by blood cell separator. To isolated adequate PBMC, we waited the white blood cells to increase to 1 × 109 /L and cycled enough times using blood cell separator. For generating dendritic cells (DCs), PBMC were washed 4 to 5 times in RPMI 1640 and incubated in serum-free medium AIM-V (Gibco, Life Technologies, Grand Island, NY) in 75-cm2 tissue culture flasks (37°C, 5% CO2). After 2 h of incubation, plastic adherent cells were cultured (37°C, 5% CO2) in 10 mL of DC medium (AIM-V medium supplemented with 1000 U/mL granulocyte-macrophage colony stimulating factor [GM-CSF] and 1000 U/mL recombinant human interleukin-4[rhIL-4], both from Schering-Plough) after removal of non-adherent cells. After 5 d, non-adherent cells were rinsed off the flasks and cultured in six-well plates (Costar, Corning, NY) at a final concentration of 5 × 105 cells per well in 3 mL of DC medium and a cytokine cocktail consisting of 10 ng/mL recombinant human tumor necrosis factor-α (rhTNF-α; Sigma), 10 ng/mL interferon-α (Genzyme, Cambridge, MA), 1000 U/mL rhIL-6 (Genzyme), and 1 mg/mL prostaglandin E2 (PGE2; Sigma) were added to promote DC maturation, and 24 h later, 3 ug/mL lysates (freeze–thaw lysates) of EBV-transformed lymphoblastoid cell line (EBV-LCL) were added and incubated for 4 h. The EBV-CTL were generated by stimulating the recipient's lymphocytes with autologous EBV-LCL-pulsed DCs at a responder-to-stimulator ratio of 30:1 in AIM-V medium containing 10 IU/mL rhIL-2, 10 ng/mL rhIL-7 and 10 ng/mL rhIL-15 (Genzyme), then harvested after 10 d, and administered to the recipient. A dose of 1.0 × 106 autologous EBV-CTL/kg was infused every time. EBV-CTL was defined as CD4+ or CD8+ T-cell responsive to EBV-LCL stimulation that secrete interferon-gamma observed in Elispot assays.Citation43
EBV-DNA monitoring in blood
EBV-DNA monitoring of blood was based on our previous description in the recipients post-transplantation.Citation44 During study treatments, EBV-DNA was monitored twice weekly till CRs achieved. EBV-DNA monitoring was performed once weekly for 3 mo after CRs; the frequency was once every 2 weeks in the 4th to the 9th month, once monthly from the 10th to the 24th month and once every 2 mo from the 25th to the 36th month after CRs.
Detection of lymphocyte subsets and EBV-CTL activity
T lymphocyte subsets (CD3+, CD3+CD4+, CD3+CD8+), CD19+ B cells and CD16+CD56+ NK cells in peripheral blood were analyzed by flow cytometry and EBV-CTL activity was monitored by Elispot assay before study treatments, and at different time points after treatment initiated.
Evaluation points and statistical analysis
Our data were analyzed on June 15, 2015. The primary endpoints included efficacy of sequential treatment measured as CR rates and relapse rates. Secondary endpoints included the incidence and severity of GVHD attributable to cellular immunotherapy and survival. To determine the CR rate and the duration at 2 y with a CI of 10% on a 95% confidence level, 62-97 patients were necessary. Our assumptions were that rituximab-based treatment would result in a CR rate of 50%, and that sequential application of CHOP would increase the CR rate by 30%.
Interim response assessments were scheduled 3–4 weeks after rituximab-based treatment, 4 weeks after the second dose of DLI and 2 weeks after the fourth dose of EBV-CTL, final response was assessed 1 mo after the last dose of DLI/EBV-CTL. The responses and relapse of PTLD were according to the revised response criteria for malignant lymphoma,Citation45 the responses were classified into CR, partial remission (PR) and no remission (NR). Stable disease and progressive disease (PD) were classified as NR. In addition, EBV-DNA negativity in blood and secretions of affected tissues were required in the designation of CR. Isolated EBV-DNA viremia was not regarded as an evidence of relapse. GVHD diagnosis was according to the literatures.Citation46,47 The data were analyzed on SPSS 19.0. We calculated CIs and best point estimate for observed response rates using the adjusted Wald method. The Categorical values were compared by the Chi-squared test. Lymphocyte percentages and EBV-CTL activity at different times were compared using Repeated-Measures Analysis of Variance. Kaplan–Meier analysis was used to estimate relapse incidence and survival. Cox proportional hazard model with stepwise backward selection was applied to survival to identify potential prognostic factors. A p value <0 .05 was considered statistically significant, tests are two-tailed.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics statement
The study was performed in accordance with the modified Helsinki Declaration, and the protocol was approved by the Ethics Boards of our centers.
Funding
This work was supported by the National High Technology Research and Development Program of China (863 Program) (2011AA020105), the National Natural Science Foundation of China (81270647, 81000231), the National Public Health Grand Research Foundation of China (201202017), the Natural Science Foundation of Guangdong, China (S2012010009299) and the Project of Guangzhou Science and Technology Plan (11A72121174). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The EBV-CTL were generated and provided by Daopei Lu and Chunrong Tong, Hematology and Oncology Center of Lu Daopei, YaDa Hospital.
References
- Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis 2009; 11:383-92; PMID:19558376; http://dx.doi.org/10.1111/j.1399-3062.2009.00411.x
- Choquet S, Leblond V, Herbrecht R, Socie G, Stoppa AM, Vandenberqhe P, Fischer A, Morschhauser F, Salles G, Feremans W et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood 2006; 107:3053-7; PMID:16254143; http://dx.doi.org/10.1182/blood-2005-01-0377
- Blaes AH, Peterson BA, Bartlett N, Dunn DL, Morrison VA. Rituximab therapy is effective for posttransplant lymphoproliferative disorders after solid organ transplantation: results of a phase II trial. Cancer 2005; 104:1661-7; PMID:16149091; http://dx.doi.org/10.1002/cncr.21391
- Choquet S, Oertel S, LeBlond V, Riess H, Varoqueaux N, Dörken B, Trappe R. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol 2007; 86:599-607; PMID:17522862; http://dx.doi.org/10.1007/s00277-007-0298-2
- Savoldo B, Rooney CM, Quiros-Tejeira RE, Caldwell Y, Wagner HJ, Lee T, Finegold MJ, Dotti G, Heslop HE, Goss JA. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am J Transplant 2005; 5:566-72; PMID:15707412; http://dx.doi.org/10.1111/j.1600-6143.2004.00693.x
- Fang ZP, Yu XF, Liu QF. Post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. NatI Med J China 2006; 86:2975-7
- Heslop HE, Slobod KS, Pule MA, hale GA, Smith CA, Bollard CM, Liu H, Wu MF, Rochester RJ et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115:925-35; PMID:19880495; http://dx.doi.org/10.1182/blood-2009-08-239186
- Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis 2009; 11:383-92; PMID:19558376; http://dx.doi.org/10.1111/j.1399-3062.2009.00411.x
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, kernan NA, Abramson S, Teruya-Feldstein J, Hedvat C, Chou JF, Heller G, Barker JN et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 2012; 119:2644-56; PMID:22138512; http://dx.doi.org/10.1182/blood-2011-08-371971
- Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994; 330:1185-91; PMID:8093146; http://dx.doi.org/10.1056/NEJM199404283301703
- Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, Ljungman P, Engelhard D. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43:757-70; PMID:19043458; http://dx.doi.org/10.1038/bmt.2008.386
- Klyuchnikov E, Holler E, Bornhäuser M, Kobbe G, Nagler A, Shimoni A, Könecke C, Wolschke C, Bacher U, Zander AR et al. Donor lymphocyte infusions and second transplantation as salvage treatment for relapsed myelofibrosis after reduced-intensity allografting. Br J Haematol 2012; 159:172-81; PMID:22909192; http://dx.doi.org/10.1111/bjh.12013
- Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, kircher SM, Gimelfarb A, Hattersley E, Mauro LA, Jjovanovic B et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol 2010; 28:1038-46; PMID:20085936; http://dx.doi.org/10.1200/JCO.2009.25.4961
- Hou HA, Yao M, Tang JL, Chen YK, Ko BS, Huang SY, Tien HF, Chang HH, Lu MY, Lin TT et al. Poor outcome in post transplant lymphoproliferative disorder with pulmonary involvement after allogeneic hematopoietic SCT: 13 years' experience in a single institute. Bone Marrow Transplant 2009; 43:315-21; PMID:18836488; http://dx.doi.org/10.1038/bmt.2008.325
- Faye A, Quartier P, Reguerre Y, Lutz P, Carret AS, Dehée A, Rohrlich P, Peuchmaur M, Matthieu- Boué A, Fischer A et al. Chimaeric anti-CD20 monoclonal antibody (rituximab) in post-transplant B-lymphoproliferative disorder following stem cell transplantation in children. Br J Haematol 2001; 115:112-8; PMID:11722420; http://dx.doi.org/10.1046/j.1365-2141.2001.03041.x
- Maximiano AC, Sanchez RA, Cantos SDIB, Mendez GM, Ronco IS, Provencio PM. Ocular relapse of primary brain lymphoma in immunocompetent patient, treated with intrathecal rituximab. Clin Transl Oncol 2010; 12:701-3; PMID:20947485; http://dx.doi.org/10.1007/s12094-010-0580-y
- Kikuchi A, Kawada H, Iwaki Y, Machida S, Tsuchiya T, Fukuda R, Hotta T. [Measurement of rituximab concentration in the cerebrospinal fluid in CNS lymphoma]. Rinsho Ketsueki 2004; 45:1255-7; PMID:15678918
- Bonney DK, Htwe EE, Turner A, Kelsey A, Shabani A, Hughes S, Hughes I, Wynn RF. Sustained response to intrathecal rituximab in EBV associated Post-transplant lymphoproliferative disease confined to the central nervous system following haematopoietic stem cell transplant. Pediatr Blood Cancer 2012; 58:459-61; PMID:21584931; http://dx.doi.org/10.1002/pbc.23134
- Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, Meijer CJ, van Den Brule AJ, Middeldorp JM. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood 2001; 97:1165-71; PMID:11222357; http://dx.doi.org/10.1182/blood.V97.5.1165
- Rai KR. Therapeutic potential of new B cell-targeted agents in the treatment of elderly and unfit patients with chronic lymphocytic leukemia. J Hematol Oncol 2015; 8:85; PMID:26170206; http://dx.doi.org/10.1186/s13045-015-0165-x
- Novero A, Ravella PM, Chen Y, Dous G, Liu D. Ibrutinib for B cell malignancies. Exp Hematol Oncol 2014; 3:4; PMID:24472371; http://dx.doi.org/10.1186/2162-3619-3-4
- Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol 2015; 8:104; PMID:26337639; http://dx.doi.org/10.1186/s13045-015-0195-4
- Clavert A, Chevallier P, Guillaume T, Delaunay J, Le Gouill S, Mahe B, Dubruille V, Gastinne T, Blin N, Moreau P et al. Safety and efficacy of rituximab in steroid-refractory chronic GVHD. Bone Marrow Transplant 2013; 48:734-6; PMID:23085828; http://dx.doi.org/10.1038/bmt.2012.203
- Crocchiolo R, Castagna L, El-Cheikh J, Helvig A, Furst S, Faucher C, Vazquez A, Granata A, Coso D, Bouabdallah R et al. Prior rituximab administration is associated with reduced rate of acute GVHD after in vivo T-cell depleted transplantation in lymphoma patients. Exp Hematol 2011; 39:892-6; PMID:21703987; http://dx.doi.org/10.1016/j.exphem.2011.06.006
- Kanakry JA, Kasamon YL, Bolanos-Meade J, Borrello IM, Brodsky RA, Fuchs EJ, Ghosh N, Gladstone DE, Gocke CD, Huff CA et al. Absence of Post-Transplantation Lymphoproliferative Disorder after Allogeneic Blood or Marrow Transplantation Using Post-Transplantation Cyclophosphamide as Graft-vs.-Host Disease Prophylaxis. Biol Blood Marrow Transplant 2013; 19:1514-7; PMID:23871780; http://dx.doi.org/10.1016/j.bbmt.2013.07.013
- Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol 2012; 13:196-206; PMID:22173060; http://dx.doi.org/10.1016/S1470-2045(11)70300-X
- Gupta S, Fricker FJ, Gonzalez-Peralta RP, Slayton WB, Schuler PM, Dharnidharka VR. Post-transplant lymphoproliferative disorder in children: recent outcomes and response to dual rituximab/low-dose chemotherapy combination. Pediatr Transplant 2010; 14:896-902; PMID:20642490; http://dx.doi.org/10.1111/j.1399-3046.2010.01370.x
- Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med 1996; 2:551-5; PMID:8616714; http://dx.doi.org/10.1038/nm0596-551
- Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol 2008; 21:579-96; PMID:18790456; http://dx.doi.org/10.1016/j.beha.2008.06.003
- D'Aveni M, Aissi-Rothe L, Venard V, Salmon A, Falenga A, Decot V, virion JM, Wang Y, Clement L, Latger-Cannard V et al. The clinical value of concomitant Epstein Barr virus (EBV)-DNA load and specific immune reconstitution monitoring after allogeneic hematopoietic stem cell transplantation. Transpl Immunol 2011; 24:224-32; PMID:21440066; http://dx.doi.org/10.1016/j.trim.2011.03.002
- Xu L, Zhang Y, Luo G, Li Y. The roles of stem cell memory T cells in hematological malignancies. J Hematol Oncol 2015; 8:113; PMID:26462561; http://dx.doi.org/10.1186/s13045-015-0214-5
- Ricciardelli I, Blundell MP, Brewin J, Thrasher A, Pule M, Amrolia PJ. Towards gene therapy for EBV-associated posttransplant lymphoma with genetically modified EBV-specific cytotoxic T cells. Blood 2014; 124:2514-22; PMID:25185261; http://dx.doi.org/10.1182/blood-2014-01-553362
- Zeng D, Dejbakhsh-Jones S, Strober S. Granulocyte colony-stimulating factor reduces the capacity of blood mononuclear cells to induce graft-versus-host disease: impact on blood progenitor cell transplantation. Blood 1997; 90:453-63; PMID:9207483
- Huang XJ, Liu DH, Xu LP, Chen H, Han W, Liu KY, Lu DP. Prophylactic infusion of donor granulocyte colony stimulating factor mobilized peripheral blood progenitor cells after allogeneic hematological stem cell transplantation in patients with high-risk leukemia. Leukemia 2006; 20:365-8; PMID:16331281; http://dx.doi.org/10.1038/sj.leu.2403995
- Vendramin A, Gimondi S, Bermema A, longoni P, Rizzitano S, Corradini P, Carniti C. Graft monocytic myeloid-derived suppressor cell content predicts the risk of acute graft-versus-host disease after allogeneic transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Biol Blood Marrow Transplant 2014; 20:2049-55; PMID:25246295; http://dx.doi.org/10.1016/j.bbmt.2014.09.011
- Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, Zhang Y, Huang F, Zhou H, Fan Z et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol 2013; 6:15; PMID:23394705; http://dx.doi.org/10.1186/1756-8722-6-15
- Zhang H, Chen J, Que W. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in acute leukemia patients. Biol Blood Marrow Transplant 2012; 18:1164-73; PMID:22289799; http://dx.doi.org/10.1016/j.bbmt.2012.01.015
- Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, Han W, Wang Y, Qin YZ, Huang XJ et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119:3256-62; PMID:22337715; http://dx.doi.org/10.1182/blood-2011-09-380386
- Krishnamurthy P, Potter VT, Barber LD, Kulasekararaj AG, Lim ZY, Pearce RM, de Lavallade H, Kenyon M, Ireland RM, Marsh JC et al. Outcome of donor lymphocyte infusion after T cell-depleted allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 2013; 19:562-8; PMID:23266740; http://dx.doi.org/10.1016/j.bbmt.2012.12.013
- Lu Y, Waller EK. Dichotomous role of interferon-gamma in allogeneic bone marrow transplant. Biol Blood Marrow Transplant 2009; 15:1347-53; PMID:19822293; http://dx.doi.org/10.1016/j.bbmt.2009.07.015
- Mucha K, Foroncewicz B, Ziarkiewicz-Wroblewska B, Krawczyk M, Lerut J, Paczek L. Post-transplant lymphoproliferative disorder in view of the new WHO classification: a more rational approach to a protean disease? Nephrol Dial Transplant 2010; 25:2089-98; PMID:20576725; http://dx.doi.org/10.1093/ndt/gfq231
- Shapiro NL, Tang CG, Bhattacharyya N. Association between Epstein-Barr virus seroconversion and immunohistochemical changes in tonsils of pediatric solid organ transplant recipients. Laryngoscope 2011; 121:1718-25; PMID:21792960; http://dx.doi.org/10.1002/lary.21871
- Herr W, Ranieri E, Olson W, Zarour H, Gesualdo L, Storkus WJ. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4(+) and CD8(+) T lymphocyte responses. Blood 2000; 96:1857-64; PMID:10961887
- Liu Q, Xuan L, Liu H, Huang F, Zhou H, Fan Z, Zhao K, Wu M, Xu L, Zhai X et al. Molecular monitoring and stepwise preemptive therapy for Epstein-Barr virus viremia after allogeneic stem cell transplantation. Am J Hematol 2013; 88:550-5; PMID:23564232; http://dx.doi.org/10.1002/ajh.23452
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25:579-86; PMID:17242396; http://dx.doi.org/10.1200/JCO.2006.09.2403
- Przepiorka D, Weisdorf D, Martin P, klingemann HG, Beatty P, Hows J, Thomas ED. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15:825-8; PMID:7581076
- Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, Schubert MM, Atkinson K, Thomas ED. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981; 57:267-76; PMID:7004534