ABSTRACT
The inducible T-cell co-stimulator (ICOS) belongs to the B7-CD28 immunoglobulin superfamily, which is currently the subject of intense study due to great successes gained in treatment of different malignancies by disrupting their family members. However, the role of ICOS played in colorectal cancer (CRC) remains poorly understood. A tissue microarray (n = 310) was stained with the ICOS specific antibody and ICOS expression is decreased in patients with either lymphatic or distant metastasis and inversely associated with CEA level and TNM stage of CRC patients. Importantly, high ICOS expression is significantly correlated with overall survival (OS) of CRC patients (n = 230, p < 0.001), and ICOS expression is also proved to be an independent prognostic factor by multivariate analysis. Surgical excised CRC specimens (n = 26) were enzymatically digested to get the tumor-infiltrating leukocytes and ICOS is mainly expressed on CD4+ T cells and its ligand ICOSL is detected on macrophages and tumor cells. ICOS expression level is associated with increased cytotoxic T lymphocyte antigen (CTLA)-4 (p < 0.001) and programmed death (PD-1) (p = 0.005) expression on T cells and more infiltrated CD8+ T cells (p < 0.001). Interestingly, ICOS+CD4+ cells isolated from tumor tissues have high T-bet and interferon (IFN)γ expression, the characteristics of Th1 cells, compared to ICOS−CD4+ cells. In addition, the correlation between the percentage of ICOS+CD4+ T cells in tumor tissue and peripheral blood was detected. Conclusively, expression of ICOS is associated with improved survival in CRC and percentage of ICOS+CD4+ cells acting as Th1 cells in either primary tumor tissue or peripheral blood may be a clinical biomarker for good prognosis of CRC patients.
Introduction
B7-CD28 family mediates the critically bidirectional signals on regulation of T-cell function: positive second signals that promote and sustain T cell responses and negative second signals that regulate T cell tolerance.Citation1 The significance of this family was highlighted by the FDA approval of ipilimumab, an antibody blocking CTLA-4, for cancer in 2011, which was reinforced by successes achieved by targeting CD28 family member PD-1 in different malignancies.Citation2
The CD28 family member ICOS, which interacts with a ligand ICOSL, was first reported on activated human T cells.Citation3 This pathway can promote T cell production of several cytokines including IL-10, IL-4, IL-5, IFNγ and IL-17, depending on which cell type the effect dominates.Citation1 A recent study showed that the ICOS mediates interaction between tumor-infiltrating CD4+ T cells and plasmacytoid dendritic cells (pDCs), which leads to the amplification of regulatory T cells (Tregs) and interleukin-10 secretion, then Tregs and pDCs that infiltrate primary breast tumors impair patient survival.Citation4,5 However, what is the role of ICOS signal in CRC has not been determined.
In this study, we utilized a well-documented CRC tissue microarray to investigate the expression of ICOS in CRC and its clinical significance. The expression pattern and function of ICOS signal were further examined in surgical excised tumor tissue and peripheral blood of CRC patients. We demonstrated that ICOS mainly expresses on Th1 cells and ICOS+ cell correlates with beneficial prognosis of CRC patients.
Results
Immunohistochemical detection of ICOS
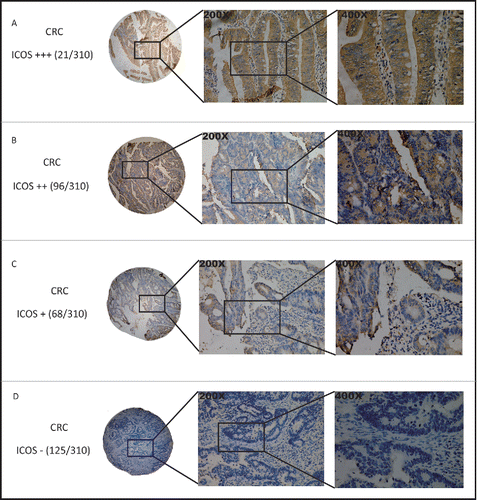
First, we evaluate the expression of ICOS in clinical CRC samples by immunohistochemistry (n = 310, paraffin-embedded tissues). Representative stainings of the tissues, incubated with monoclonal ab105227 Ab (Abcam) for specific for ICOS (). And ICOS expression was mainly found in stroma. The intensity of ICOS staining was determined by comparison with the staining of ICOS in human normal tonsil tissue, for ICOS is reported to be highly expressed on tonsillar T cellsCitation3 (Fig. S1).
ICOS expression is negatively associated with tumor metastasis and other pathological features of CRC patients
ICOS expression was low in the 157 (score < 6, 50.65%) of total 310 CRC samples while the rest 153 (score ≥ 6, 49.35%) samples remained a high-expression level (). What's important, ICOS expression is significantly negatively correlated with tumor metastasis, either lymph node or distance. Among the 154 CRC with lymph node metastasis, a low ICOS expression (score 0˜6) was observed in 109 cases (70.78%), whereas in 45 cases (29.22%), ICOS expression was high (score 7∼12) (). For distant metastasis, the more obvious tendency was observed, that is, in 29 distant metastatic cases (80.56%), ICOS expression was low or absent (). In univariate analysis, strong ICOS expression was associated with tumor size (p = 0.045), CEA level (p < 0.001), tumor classification (p = 0.03), lymph node metastasis (p < 0.001), distant metastasis (p < 0.001) and TNM stage (p < 0.001), whereas no significant relevance were found with age, gender and tumor location (, ). These results demonstrated that ICOS expression is negatively associated with the progress of CRC, especially tumor metastasis.
Figure 2. Expression of ICOS is associated with metastasis and other pathological features of CRC patients. The scores of ICOS staining in individual CRC punches (n = 310) were correlated with different status of lymphatic metastasis (A), distant metastasis (B), TNM stage (C) and CEA level (D).
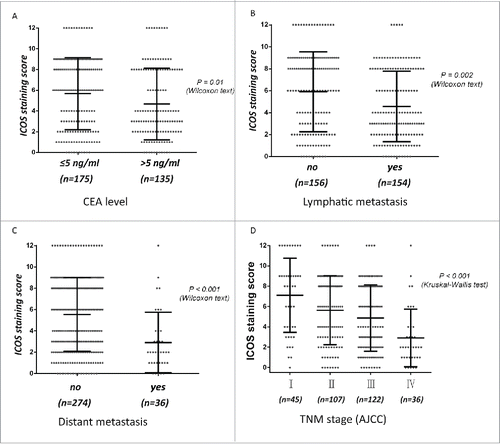
Table 1. Correlations between ICOS expression and clinicopathologic features in 310 colorectal cancer patients.
Prognostic significance of ICOS expression
The effect of ICOS expression on CRC prognosis was examined by constructing Kaplan–Meier curves and differences on OS and disease free survival (DFS) between groups were compared by Log Rank test. The results showed that ICOS expression was dramatically associated with OS (p < 0.001, ) and DFS (p < 0.001, ). These differences were also significant in univariate analysis (p < 0.001, HR = 0.471, ). Variables, including tumor size, CEA level, T classification, lymphatic metastasis and distant metastasis, that were significantly associated with OS in the univariate analysis were put into a Cox proportional hazards models. A dramatic significance (p = 0.002) indicating a correlation between high ICOS expression and improved survival in CRC could still be observed (). These results demonstrated a significant correlation between high ICOS expression and good prognosis, suggesting that low ICOS expression may be a predictor for progression of CRC patients.
Figure 3. Kaplan–Meier analysis of overall survival in colorectal cancer patients and differences were analyzed by Log Rank test. (A, B), High expression of ICOS is associated with a good overall survival (OS) (p < 0.001) and a longer DFS (p < 0.001). ICOS was detected by monoclonal antibody (ab105227, abcam).
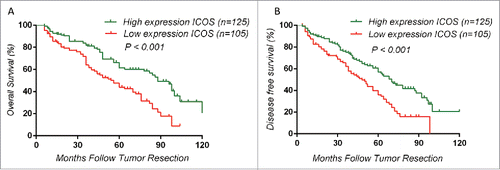
Table 2. Univariate and multivariate analyses of prognostic parameters for survival in 230 colorectal cancer patients.
ICOS is mainly expressed on CD4+ T cells
Previous studies demonstrated that ICOS is not expressed on resting T cells but is induced rapidly on T cells after TCR engagement.Citation6,7 To examine the expression pattern of ICOS on T cells in tumor tissues, surgical excised CRC specimens were minced finely, enzymatically digested and stained with Abs, following by flow cytometry analysis. As shown in , CD4+ T cell, but not CD8+ T cell, is the primary cell expressing ICOS, which is confirmed by the quantitation data. In addition to tumor tissue, a similar trend was detected in both pericarcinous tissue () and distal normal tissue (). T cells not only reside in tumor sites, but also migrate into the circulatory system. Then, we examined the ICOS expression pattern on circulating T cells, the results showed the majority of ICOS+ cells in peripheral blood are CD4+ T cells (). Collectively, in either tumor tissues or peripheral blood, ICOS is mainly expressed on CD4+ T cells.
Figure 4. ICOS expression pattern in tumor sites and peripheral blood. (A) CRC surgical tumor tissues were enzymatically digested and stained with fluorochrome labeled Abs, followed by flow cytometry analysis. (i) Representative figures of ICOS expression on CD4+ and CD8+ T cells, analyzed by flow cytometry, in tumor-infiltrated leukocytes. (ii) Quantitation of the percentage of ICOS+CD4+ or ICOS+CD8+ T cells among all of the CD4+ or CD8+ T cells gotten from tumor-infiltrated leukocytes, respectively (n = 26). (B) The similar protocol is as (A), except for the specimens from pericarcinous tissues (i) or distal normal tissues (ii). (C) The similar protocol is as (A), except for the specimens from peripheral blood. (D) CRC samples were stained with ICOS antibody by immunohistochemistry. At the same time, the samples were enzymatically digested to get the TILs and ICOS expression on CD4+ T cells were analyzed by flow cytometry. (E) Correlation between percentage of ICOS+ among CD4+ T cells and ICOS staining scores in the CRC tumor tissue (n = 13). Data are presented as averages ± SEM, statistical difference was evaluated by Student's t test. **p < 0.01; ***p < 0.001.
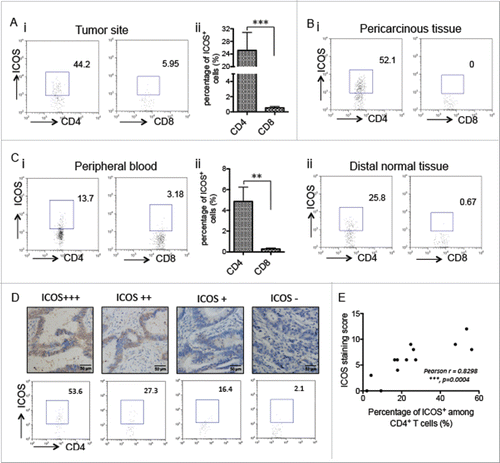
To further confirm that the ICOS staining scores gotten from paraffin-embedded tissues determined by IHC reflect the expression level of ICOS on CD4+ T cells in primary tumor sites, the CRC tumor tissues from same patients were both examined by IHC and enzymatically digested followed by flow cytometry analysis. The results demonstrated that ICOS staining scores are associated with the percentage of ICOS+ cells among CD4+ T cells in the tumor tissues ().
ICOSL is mainly expressed on macrophage and tumor cell
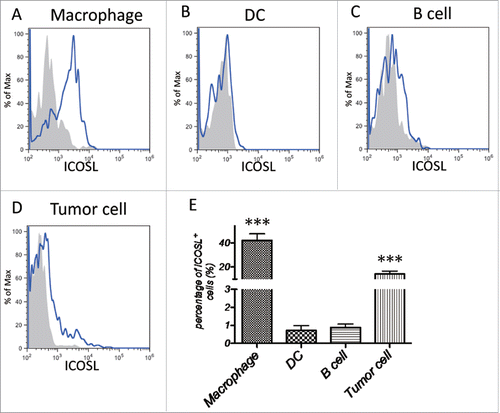
ICOSL is identified as a homolog of B7 and found to be the ligand of ICOS, although human ICOSL can also bind CD28 and CTLA-4.Citation8 It is constitutively expressed on B cells, DCs and macrophages, and can be induced on non-hematopoietic cells in response to inflammatory signals.Citation3 To figure out what kinds of cells regulate function of CD4+ T cells by ICOS–ICOSL signaling pathway, we analyzed the expression level of ICOSL on macrophages, DCs, B cells and tumor cells gotten from primary CRC tumor tissue. The data of flow cytometry demonstrated that ICOSL is mainly expressed on tumor-associated macrophages (TAMs) and tumor cells (), suggesting that ICOS–ICOSL signaling pathway plays a role in the interaction between TAMs (or tumor cells) and CD4+ T cells.
Figure 5. ICOSL expression pattern in tumor sites. CRC surgical tumor tissues were enzymatically digested and stained with fluorochrome labeled Abs, followed by flow cytometry analysis. (A) ICOSL expression in macrophages (CD11b+CD14+CD16−). (B) ICOSL expression in DCs (CD11c+MHC-II+). (C) ICOSL expression in B cells (CD19+CD3−). (D) ICOSL expression in tumor cells (EpCAM+CD45−). (E) Quantitation of the percentage of ICOSL+ cells among macrophages, DCs, B cells and tumor cells gotten from tumor-infiltrated leukocytes, respectively (n = 26). Data are presented as averages ± SEM, statistical difference was evaluated by Student's t test. **p < 0.01; ***p < 0.001.
ICOS expression is positively correlated with CTLA-4 and PD-1 expression on T cells
ICOS belongs to CD28 family checkpoint regulators, and expression of PD-1, another member of this family, has been demonstrated to be associated with improved survival of MMR-proficient CRC.Citation9 Then, we examined the correlation of ICOS expression with other molecules in this family, such as CTLA-4, CD28 and PD-1. The results showed that the number of ICOS+ T cells is associated with that of CTLA-4+ and PD-1+ T cells, but not CD28+ T cells (), suggesting that the expression of inducible checkpoint regulators reflects an immune-active microenvironment.
Figure 6. The expression of ICOS is associated with other immune checkpoints and immune cells. (A–C) Correlation between the percentage of ICOS+ T cells and that of CD28+ T cells (A), CTLA4+ T cells (B) and PD-1+ T cells (C) among total tumor-infiltrated leukocytes. (D–G) Correlation between the percentage of ICOS+ T cells and that of CD8+ T cells (D), CD4+ T cells (E), CD4+CD25+ T cells (F) and macrophages (CD11b+CD14+CD16−) (G) among tumor-infiltrated leukocytes.
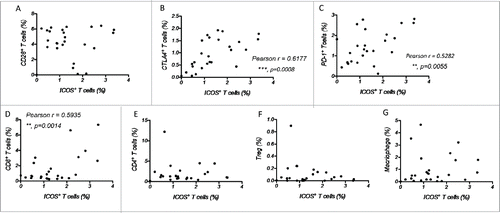
ICOS expression is associated with the number of CD8+ T cells in tumor tissues
We further checked the relationship between the number of ICOS+ T cells and that of CD8+ T cells, CD4+ T cells, CD4+CD25+ Treg cells or macrophages in primary tumor tissues, which have been proved to play important roles in CRC.Citation10 As shown in , ICOS expression is only associated with the amount of CD8+ T cells, not the number of other immune cells.
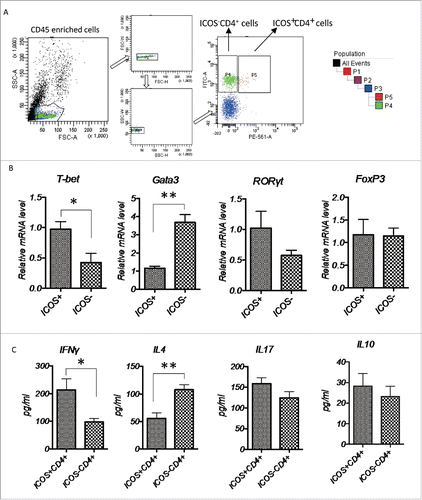
ICOS+CD4+ T cell is Th1 cell in CRC patients
Previous studies proved that ICOS signals exerts its effects mainly through regulating cytokine production by activated and effect T cells,Citation6,11 and it can induce T cells to produce cytokines, such as IL-10, IL-4, IL-5, IFNγ and IL-17.Citation7,12,13 To clarify the function of ICOS+ T cells in CRC, we isolated ICOS+CD4+ and ICOS−CD4+ T cells from primary tumor tissues of CRC patients by cell sorter (), and examined the mRNA expression of specific transcriptional factors by RT-PCR. At the same time, these isolated cells were ex vivo activated by leukocyte activation cocktail for 18 h, culture medium were collected and effector cytokines indicative of Th1, Th2, Th17 and Treg response were examined by ELISA. As shown in , T-bet (Th1) expression was significantly increased, meanwhile GATA3 (Th2) expression was dramatically decreased in ICOS+CD4+ T cells compared to ICOS−CD4+ T cells. While other transcriptional factors, RORγt (Th17) and FoxP3 (Treg), had no significant changes between these two groups. These results were echoed by the expression of effector molecules of a different subtype CD4+ T cells (), indicating that majority of ICOS+CD4+ T cells is Th1-polarized T cells.
Figure 7. Characterization of tumor-infiltrating ICOS+CD4+ T cells, (A) CD45+ cells were enriched from primary CRC tumor tissues and the ICOS+CD4+ and ICOS−CD4+ T cells were subsequently sorted by a cell sort. (B) Relative mRNA level of transcriptional factors T-bet (Th1), GATA3 (Th2), RORγt (Th17) and FoxP3 (Treg) of ICOS+CD4+ and ICOS−CD4+ T cells were determined by realtime-PCR. (C) Protein level of cytokines IFNγ (Th1), IL4 (Th2), IL17 (Th17) and IL10 (Treg) in the culture medium of ICOS+CD4+ and ICOS−CD4+ T cells activated by leukocyte activation cocktail for 18 h were examined by ELISA. Data are presented as averages ± SEM, statistical difference was evaluated by Student's t test. *p < 0.05; **p < 0.01.
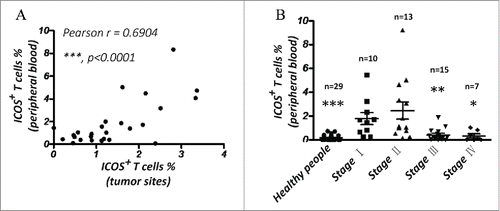
Higher percentage of ICOS+ T cells in peripheral blood is a marker for good prognosis
Our result showed that ICOS+ T cells are also present at peripheral blood of CRC patients (). Then, we determined if the percentage of ICOS+ T cells in peripheral blood could reflect the ICOS expression level in primary tumor tissue. The correlation analysis showed that the percentages of ICOS+ T cells in peripheral blood and that in primary tumor tissue are positively correlated in CRC patients (Pearson's r = 0.6904) (). What's more, the result demonstrated that amount of ICOS+ T cells in the peripheral blood is significantly higher in CRC patients at stage I and II as compared to stage III, IV and healthy control (). Maybe the inactive status of immune system kept in normal people could contribute the low level of ICOS expression. Collectively, ICOS expression level in peripheral blood is also a good indicator for OS of CRC patients.
Figure 8. The percentage of ICOS+ T cells in PBMC is a good marker for prognosis of CRC. (A) Correlation between percentage of ICOS+ T cells in peripheral blood and that in primary tumor tissues. (B) Quantity of ICOS+ T cells in peripheral blood of luminal breast cancer patients by flow cytometry. Data are presented as averages ± SEM, statistical difference was evaluated by Student's t test. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Since ICOS signal plays the opposite roles in different disease,Citation1 this pathway does not appear to be aggressively pursued for the translational study. Our results proved that ICOS+ cell, acting as Th1 cells, is associated improved survival of CRC patients. What's more, our data showed that ICOS expression is correlated with the inducible checkpoint inhibitors CTLA-1 and PD-1 expression on T cells, indicating that ICOS expression may be a useful marker for choice of anti-CTLA-4 or PD-1 therapy in CRC.
In contrast to another co-stimulatory receptor CD28 in this family, the effects of ICOS on activation of naïve T cells and T-cell proliferation are modest.Citation7,14 ICOS signal seem to play an important role on modulating cytokines production by activated and effector T cells, particularly Th1 and Th2 effector responses.Citation11 Since Th1 and Th2 cells play almost opposite roles in the immune system, disruption of ICOS may induce contrasting results depending on which effector function ICOS dominates. Our data showed that ICOS+ T cells produce more INFγ and less IL4, compared to ICOS− T cells, meanwhile expression of transcriptional factor of Th1 cells (T-bet) was upregulated in ICOS+ T cells, suggesting that ICOS promotes Th1 effector response in CRC patients (). Previous study reported that Th1 cells inhibit tumor cell invasion and metastasis by communicating with tumor associated myeloid cells, including TAMs and MDSCs,Citation15 which may contribute to the improved survival of CRC patients with high ICOS expression. The mechanism on how ICOS signal regulates Th1 effector response need to be clarified in future work.
ICOS is a co-stimulatory receptor homologous to the stimulatory receptor CD28 and the inhibitory receptor CTLA-4.Citation16 CD28 is constitutively expressed on T cells, whereas ICOS and CTLA-4 expression are induced rapidly after T-cell activation.Citation7,17,18 Previous studies demonstrated that expression of ICOS is regulated by both TCR and CD28 signals,Citation7,11,17 while these signals are inhibited by a negative signal delivered by CTLA-4 engaged.Citation19,20 In regards to PD-1, several studies showed that PD-1 is induced on activated T cells and PD-1/PD-L1 or PD-L2 signal inhibits TCR mediated T cell proliferation and cytokine production.Citation21,22 Our results demonstrated that the expression of ICOS is positively correlated to that of CTLA4 and PD-1 on T cells (). The phenomenon is echoed by a study showing that the active Th1/CTL immune-active microenvironment of CRC is counterbalanced by enhanced expression of multiple checkpoint inhibitors, especially in microsatellite instable CRC, suggesting that ICOS may be a useful biomarker for patient selection and response evaluation of anti-CTLA-4 or PD-1 therapy in CRC.
Materials and methods
Patients and tissue microarray (TMA) construction
Tissue microarrays consisting of 310 CRC specimens were obtained from Renji Hospital (Shanghai, China) from January 2003 to December 2012, and the pathological information was retrieved from the Pathology Department of Renji Hospital. The fresh CRC specimens and peripheral bloods were gotten from CRC patients from Renji Hospital (Shanghai, China) from January 2015 to September 2015. All the patients were provided with written informed consent before enrolment, and the study was approved by the Research Ethics Committee of Shanghai Jiao Tong University School of Medicine Renji Hospital. None of the patients had received tumor-specific therapy before diagnosis. The follow-up time was calculated from the date of surgery to CRC-related death, or December 31, 2012, the ultimate deadline. The TMA were constructed as previously described.Citation23 Briefly, tissue cores with a diameter of 0.6 mm were punched from paraffin-embedded tissue blocks using a Beecher microarrayer and were placed on a recipient paraffin block. Sections (4 mm) were cut and applied to APES-coated slides. The presence of tumor in the tissue core was confirmed by H&E staining.
Immunohistochemistry
The tissue microarray sections were rehydrated and treated with 3% hydrogen peroxide, followed by antigen retrieval. After being blocked with 10% normal goat serum for 30 min, the sections were incubated with primary antibodies at 4˚C overnight, followed by incubation with a peroxidase-labeled secondary antibody for 30 min at room temperature. Finally, Diaminobenzidine tetrahydrochloride (DAB; Maixin Biotech, China) was used for the color-reaction followed by nucleus counterstaining with hematoxylin. The following antibodies were used: rabbit anti-ICOS monoclonal antibody (ab105227, Abcam, UK), Elivision plus Polyer HRP (Mouse/Rabbit) IHC Kit (Maixin Biotech, China).
The slides were inspected independently by two investigators in a blinded manner. ICOS expression was evaluated according to the extent and intensity of staining (percentage of positive cells was measured on a scale of 0–4: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%; the intensity of staining was measured on a scale of 0–3: 0, no staining; 1, weak staining; 2, moderate staining, but weaker than that of tonsil; 3, strong staining, equivalent to or stronger expression than that of tonsil. And a final score was created to determine the cut-off value for low and high expression group by using grades of the extent × grades of intensity staining. Then, the protein expression was sorted into four categories: “−” for a score of 0–3, “+” for a score of 4–6, “++” for a score of 7–9 and “+++” for a score of >9; low expression was defined as a final score <6 and high expression with a final score ≥6.
Immune cell analysis and isolation
Surgical excised CRC specimens were mechanically dissociated and digested into single cells with collagenase type II (0.5 mg/mL), collagenase type IV (0.5 mg/mL), hyaluronidase (10 U/mL) and DNase I (0.01 mg/mL) for 2 h at 37°C. The dissociated cells were collected, lysed by RBC lysis buffer and then incubated with CD45 monoclonal antibody, followed by isolated with CD45+ cell isolation kit (Miltenyi Biotec). For ICOS+CD4+ or ICOS−CD4+ T cells, the cells were incubated with ICOS and CD4 monoclonal antibody. The positive cells were next sorted by flow cytometry on BD FACSAria using BD FACSDiva software. The purity of the isolated subpopulations regularly exceeded 90%. As previously described,Citation15 PBMCs from CRC patients were isolated by differential density gradient separation, and immune cells were analyzed by flow cytometry after labeling with fluorescence antibodies. The isotope antibodies for specific antibody on different fluorescence channels were used as negative control.
Statistical analysis
Student's t test was used to analyze the data. Results are given as mean ± SEM unless otherwise indicated. Probability values <0.05 were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1141857_s02.doc
Download MS Word (763.5 KB)Funding
The work were supported by the grants from Municipal Commission of Health and Family Planning of Shanghai, China (no. 201540031 to MZ), Science and Technology Commission of Shanghai, China (no.13DZ1942806 to MZ) and from the National Natural Science Foundation of China (81201524 to YZ).
References
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol 2002; 2:116-26; PMID:11910893; http://dx.doi.org/10.1038/nri727
- Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol 2013; 34:556-63; PMID:23954143; http://dx.doi.org/10.1016/j.it.2013.07.003
- Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 1999; 11:423-32; PMID:10549624; http://dx.doi.org/10.1016/S1074-7613(00)80117-X
- Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, Bachelot T, Treilleux I, Goddard-Leon S, Lavergne E et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res 2012; 72:6130-41; PMID:23026134; http://dx.doi.org/10.1158/0008-5472.CAN-12-2409
- Faget J, Sisirak V, Blay JY, Caux C, Bendriss-Vermare N, Menetrier-Caux C. ICOS is associated with poor prognosis in breast cancer as it promotes the amplification of immunosuppressive CD4 T cells by plasmacytoid dendritic cells. Oncoimmunology 2013; 2:e23185; http:dx.doi.org/10.4161/onci.23185
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraf R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999; 397:263-6; PMID:9930702; http://dx.doi.org/10.1038/16717
- Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 2000; 13:95-105; PMID:10933398; http://dx.doi.org/10.1016/S1074-7613(00)00011-X
- Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity 2011; 34:729-40; PMID:21530327; http://dx.doi.org/10.1016/j.immuni.2011.03.014
- Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013; 49:2233-42; PMID:23478000; http://dx.doi.org/10.1016/j.ejca.2013.02.015
- Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol 2003; 46:33-57; PMID:12672517; http://dx.doi.org/10.1016/S1040-8428(02)00159-2
- McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol 2000; 165:5035-40; PMID:11046032; http://dx.doi.org/10.4049/jimmunol.165.9.5035
- Detheux M, Standker L, Vakili J, Munch J, Forssmann U, Adermann K, Pohlmann S, Vassart G, Kirchhoff F, Parmentier M et al. Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J Exp Med 2000; 192:1501-8; PMID:11085751; http://dx.doi.org/10.1084/jem.192.10.1501
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365-9; PMID:10581077; http://dx.doi.org/10.1038/70932
- Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature 1999; 402:827-32; PMID:10617205; http://dx.doi.org/10.1038/45582
- Zhang Y, Lv D, Kim HJ, Kurt RA, Bu W, Li Y, Ma X. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res 2013; 23:394-408; PMID:23266888; http://dx.doi.org/10.1038/cr.2012.178
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol 2002; 20:29-53; PMID:11861596; http://dx.doi.org/10.1146/annurev.immunol.20.091101.091806
- Mages HW, Hutloff A, Heuck C, Buchner K, Himmelbauer H, Oliveri F, Kroczek RA. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur J Immunol 2000; 30:1040-7; PMID:10760791; http://dx.doi.org/10.1002/(SICI)1521-4141(200004)30:4<1040::AID-IMMU1040>3.0.CO;2-6
- Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996; 4:535-43; PMID:8673700; http://dx.doi.org/10.1016/S1074-7613(00)80480-X
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994; 1:405-13; PMID:7882171; http://dx.doi.org/10.1016/1074-7613(94)90071-X
- Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996; 183:2541-50; PMID:8676075; http://dx.doi.org/10.1084/jem.183.6.2541
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192(7):1027-34; PMID:11015443; http://dx.doi.org/10.1084/jem.192.7.1027
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2:261-8; PMID:11224527; http://dx.doi.org/10.1038/85330
- Sauter G, Simon R, Hillan K. Tissue microarrays in drug discovery. Nat Rev Drug Discov 2003; 2:962-72; PMID:14654795; http://dx.doi.org/10.1038/nrd1254