ABSTRACT
Background: Untreated metastatic uveal melanoma (UM) carries a grave prognosis. Unlike cutaneous melanoma (CM), there are no established treatments known to significantly improve outcomes for a meaningful proportion of patients. Inhibition of the PD1–PDL1 axis has shown promise in the management of CM and we here report a two center experience of UM patients receiving pembrolizumab. Methods: To assess the efficacy and safety of pembrolizumab, we retrospectively analyzed outcome data of 25 consecutive UM patients participating in the MK3475 expanded access program (EAP) who received pembrolizumab at 2 mg/kg 3 weekly. Tumor assessment was evaluated using RECIST 1.1 and immune-related Response Criteria (irRC) by CT scanning. Toxicity was recorded utilizing Common Terminology Criteria for Adverse Events (“CTCAE”) v4.03. Results: Twenty-five patients were identified receiving a median of six cycles of treatment. Two patients achieved a partial response and six patients stable disease. After a median follow-up of 225 d median progression free survival (PFS) was 91 d and overall survival (OS) was not reached. There was a significant trend for improved outcomes in patients with extrahepatic disease progression as opposed to liver only progression at the outset. Five patients experienced grade 3 or 4 adverse events (AEs); there were no treatment related deaths. Conclusions: Pembrolizumab 2mg/kg q3w is a safe option in UM patients. Disease control rates, particularly in the subgroup of patients without progressive liver disease at the outset are promising; these results merit further investigation in clinical trials possibly incorporating liver targeted treatment modalities.
Abbreviations
| AE | = | Adverse Event |
| ALT | = | Alanine aminotransferase |
| AST | = | Aspartate aminotransferase |
| CM | = | Cutaneous Melanoma |
| CTCAE | = | Common Terminology Criteria for Adverse Events |
| EAP | = | Expanded Access Program |
| ECOG | = | Eastern Cooperative Oncology Group |
| irAE | = | immune-related AE |
| irRC | = | immune-related Response Criteria |
| LDH | = | Lactate Dehydrogenase |
| OS | = | Overall Survival |
| PD | = | Progressive disease |
| PFS | = | Progression Free Survival |
| PS | = | Performance Status |
| RFA | = | Radiofrequency ablation |
| SIRT | = | Selective internal radiation therapy |
| TACE | = | Transcatheter arterial chemoembolization |
| TIL | = | Tumor infiltrating lymphocytes |
| ULN | = | Upper Limit of Normal |
| UM | = | Uveal Melanoma |
Introduction
UM is the most common malignancy of the eye; it is significantly rarer and carries a genetic profile that is markedly discordant from CM. Citation1 UMs typically demonstrate a relatively lower degree of aneuploidy and genomic instability compared with other cancer types Citation2-4 and a mutational load significantly lower than CM. Citation5,6
Even in the pre-immunotherapy and targeted therapy era, outcomes of metastatic UM were worse than metastatic CM with median survival in unselected series as low as 4 mo. Citation7 This possibly relates to the predilection of UM for hepatic metastases—seen in >85% of cases with liver the sole site of metastatic disease in around 50%,Citation7 compared to 25% in CM. Citation8
Cytotoxic chemotherapy offers little benefit in UM and is no more effective than in CM when site and extent of disease is considered. Citation9 The absence of activating BRAF mutations in the majority of UM patients limits the use of BRAF inhibitors. Alternative targeted approaches are sought, e.g. the MEK inhibitor selumetinib,Citation10 however, at the time of writing none have demonstrated significant activity in large Phase III trials.
Immunotherapy has revolutionized the treatment of metastatic CM; immune checkpoint inhibition with ipilimumab Citation11—an anti-CTLA4 antibody—and anti-PD1 agents alone Citation12 or in combination Citation13,14 results in durable disease control in a significant proportion of patients. UM patients were excluded from taking part in Phase III trials, so evidence of efficacy in this setting is limited.
Small studies and retrospective analyses Citation15-17 have shown that partial and even complete responses to ipilimumab in UM are possible, but rare. Median OS of 5.2–9.6 mo, 1 y OS rates of 22–34% and 1 y PFS rates of <11% were comparable to standard chemotherapy Citation10 but lower than in unselected CM cohorts.
One explanation why immunotherapeutics might be less effective in UM compared to CM could be different driving mutations. Citation1,5,18 Furthermore, the overall mutational load is significantly lower Citation5 compared to CM Citation3 and expression of cancer-testis antigens is significantly rarer. Citation19 Consequently, the number and quality of (neo-)antigens presented to the immune system is likely to be different. Additionally, UM may rely on different immune escape mechanisms most clearly evidenced by the observation that higher numbers of infiltrating T-cells in the primary are linked to a worse outcome. Citation20
Very little information exists in the public domain regarding the efficacy of anti-PD1 agents in UM; the largest case series reported consists of data from seven patients who received pembrolizumab. Citation21 We herein report a two center experience of 25 patients who have received pembrolizumab in in the UK EAP.
Patients and treatments
Patient eligibility
Patients treated in the pembrolizumab EAP in our institutions with a diagnosis of metastatic UM were included in this retrospective study. All subjects had received previous ipilimumab and a BRAF inhibitor if eligible. Resolution of AEs due to previous cancer therapy to grade 0 or 1 was required. Additional previous immunotherapies were allowed as long as no severe or life threatening immune-related AEs (“irAEs”) were experienced and there was no ongoing requirement for systemic steroids for the management of irAEs.
Eastern Cooperative Oncology Group performance status (PS) 0/1 was mandated as well as a minimum age of 12. Inclusion criteria included AST and ALT ≤2.5 × upper limit of normal (ULN) or ≤5 × ULN with liver metastases, serum total bilirubin ≤1.5 × ULN or direct bilirubin ≤ULN for patients with total bilirubin level >1.5 ULN. Patients with a history of clinically severe autoimmune diseases, pneumonitis, organ transplant, HIV or active Hepatitis B or C infections and active central nervous system metastases were also excluded. Concomitant systemic antineoplastic therapies were not allowed.
Treatment
Pembrolizumab was administered at 2 mg/kg in 3-weekly intervals until progression by irRC Citation22, complete response, unacceptable toxicity or for up to 2 y. Frequency of radiological tumor assessment was as per standard of care, typically with body CT scans every 2–3 mo and liver MRIs to optimally monitor liver disease. Blood samples were taken before each infusion to allow assessment of renal, liver, thyroid and bone marrow function for safety and toxicity monitoring. AEs were scored using CTCAE version 4.03.
Response evaluation
Tumor response was evaluated using the following radiological scoring systems: RECIST 1.1Citation23 and irRC.Citation22 Best overall response was determined based on irRC criteria where possible to capture delayed antitumor responses.
Data capture and analysis
Patients receiving pembrolizumab were identified from the oncology pharmacy database. Data was collected retrospectively from patients' notes and electronic records and stored into a Microsoft Access database; statistical analysis and graphing was done using GraphPad Prism Version 6.01. Survival curves were calculated using the Kaplan–Meier method. The log-rank test was used to compare curves and determine the p value.
Results
Patient characteristics
Twenty-five patients with metastatic UM were enrolled into the pembrolizumab EAP between the 1/06/2014 and 1/8/2015 at our centers. All patients had systemic disease spread at baseline and had received a median of one previous lines of systemic treatment; 11 patients (44%) had also received a median of two liver directed therapies. All patients had previously completed a course of ipilimumab, none had experienced an objective response though nine patients (36%) had a period of stable disease; baseline patient characteristics are presented in . All seven patients with available cytogenetic results had chromosome three losses and chromosome eight gains in the primary tumors.
Table 1. Baseline patient characteristics.
Response analysis
All patients received at least one cycle of pembrolizumab. At data collection cut-off time a median of six cycles of pembrolizumab had been administered per patient and two patients were continuing on treatment. Radiological assessments took place as clinically indicated, typically every three to four cycles (9–12 weeks). shows a flow diagram outlining treatment course and outcomes.
Figure 1. Disease status of UM patients participating in the Pembrolizumab EAP. Treatment was administered at 3 weekly intervals with tumor assessments performed at baseline and every three cycles of treatment or as clinically indicated.
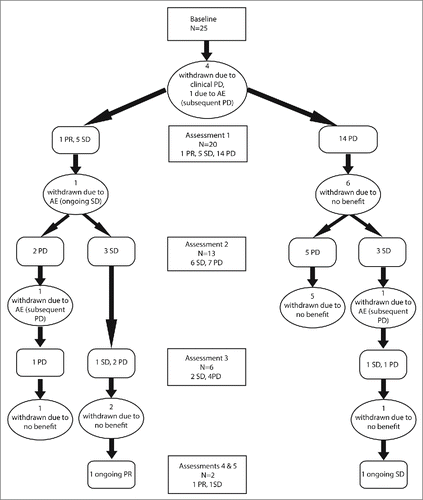
Four subjects deteriorated rapidly due to disease progression and were withdrawn before the first radiological assessment after receiving only one cycle. Eight patients were withdrawn after having only a single radiological assessment demonstrating disease progression due to rapid clinical deterioration. One patient continuing after an initial scan revealing PD exhibited subsequent disease stabilization and remains on treatment. There were two partial responses, one early that was not maintained and one late that remains on treatment. summarizes response types and duration.
Table 2. Best radiological disease response to pembrolizumab; nine patients were alive, one with an ongoing partial response and two with stable disease at the time of writing hence median overall and progression free survival was not reached (NR) for several subgroups.
Survival analysis
After a median follow-up of 225 d at the time of data cut-off median, OS was not reached but will be >225 d with a 1 y survival rate of >28%; median PFS was 91 d (). Eight evaluable patients (32%) achieved disease control (partial response or stable disease) for more than 3 mo and median PFS for this subgroup is projected to be >9.8 mo with a median OS >13.5 mo. A Kaplan–Meier plot of OS and PFS of all patients is presented in , including censored data for patients who are still responding to treatment.
Figure 2. Kaplan–Meier plots of overall and progression free survival of UM patients treated with pembrolizumab at 2mg/kg as part of the expanded access program. (A) Curves for entire group, median OS not reached. (B–H) Curves stratified by best previous response to ipilimumab (B), site of disease progression at baseline (C–D), number of liver directed treatments received prior to enrolling to the EAP (E), number of previous systemic treatments (F), serum LDH (G) and ECOG PS (H).
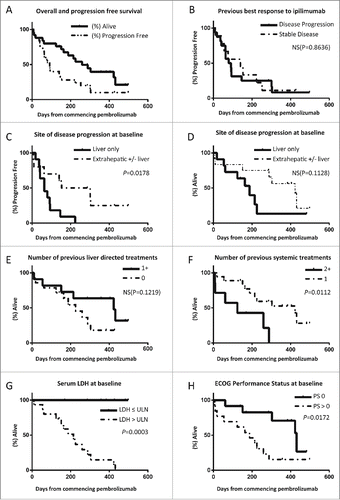
Patients with liver only disease progression at baseline imaging had significantly worse PFS () and on immature data there was a trend for worse OS (). This group had also shorter lead in times from diagnosis of stage IV disease (), as did patients who had no previous liver directed treatments () but in the latter case there was no significant difference in PFS ().
Figure 3. Plots demonstrating lead- in times from diagnosis of UM recurrence to commencing pembrolizumab stratified by (A) location of disease progression at time of commencing pembrolizumab, (B) number of liver directed treatments received prior to enrolling to the EAP and (C) number of previous systemic treatments.
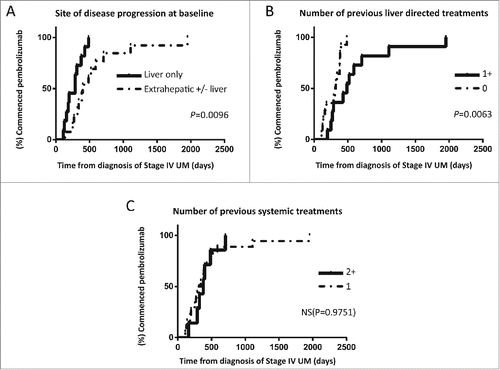
In contrast, PFS and OS was significantly worse in patients who had more than one previous systemic treatment as compared to only one despite similar lead in times (). Previous disease stabilization in response to ipilimumab did not appear to predict response to pembrolizumab ().
In addition both serum lactate dehydrogenase (LDH) and PS at baseline were associated with a worse PFS and OS (); notably all patients with normal LDH at baseline are still alive as opposed to only one with raised LDH.
Safety analysis
Pembrolizumab was tolerated well overall with a frequency and type of AEs commensurate with those reported in larger studies.Citation12 Five patients (20%) experienced at least one grade 3/4 treatment related AE (): one patient experienced grade 4 transaminitis after the first dose and one grade 3 skin rash and pruritus after the second dose; both had to discontinue treatment; two patients experienced grade 3 hypophysitis requiring long term steroid replacement, one of these also had an episode of grade 3 diarrhea that settled spontaneously. Finally, one patient experienced grade 3 fatigue and elected to discontinue treatment.
Table 3. Treatment related adverse events.
Discussion
Anti-PD-1 agents are now approved for use in US and European markets for the treatment of metastatic CM pre and post ipilimumab. As entry criteria of the registration trials excluded UM, data on efficacy of anti-PD-1 based immunotherapy in UM is limited.
In our patients, objective response rates were lower than in CM studies,Citation12 however, PFS rates were comparable and a significant number (>32%) of patients experienced prolonged (>3 mo) periods of disease stabilization with 28% maintaining disease control for >6 mo.
High LDH and poorer PS at baseline—known markers of disease burden and/or aggressiveness—predicted a shorter duration of benefit. While not surprising this provides additional prognostic information. AEs seen were in line with safety analyses from larger studies Citation12 and confirm that pembrolizumab is well tolerated in this patient population.
The most dramatic observation relates to the role of uncontrolled intrahepatic metastases. Patients with liver deposits as the only site of progression had significantly shorter PFS as compared to subjects with extrahepatic sites (median of 63 vs 153 d, ); additionally, the former uniformly went on to develop disease progression in the liver alone.
The reasons behind this behavior are unclear and likely multifactorial. The liver microenvironment is known to facilitate immune escapeCitation24 and the specific mechanisms involved may account both for the predilection of UM for liver metastases and the reduced efficacy of immunotherapeutic agents in patients with liver disease. A related and not mutually exclusive possibility is that extrahepatic sites of disease allow better priming of an anticancer immune response which can then control intrahepatic disease. Finally, the pattern of metastatic spread may reflect underlying biological differences—e.g., mutational load, underlying immune escape mechanisms—that influence the ability of checkpoint inhibitors such as pembrolizumab to drive an effective immune response.
While this is a small study, it has significant implications for the management of UM if the findings are confirmed. First, while pembrolizumab may not reproduce the impressive response rates seen in CM, it nevertheless appears to achieve disease control of clinically meaningful duration for a significant proportion of patients, justifying its use in the single agent setting, particularly in the absence of alternative effective systemic treatment options.
Second, active liver metastases in the absence of extrahepatic disease appear to have major prognostic significance as in those circumstances pembrolizumab appears to be ineffective. This finding—if confirmed—advocates against pembrolizumab single agent use in this setting.
Finally, a multimodality approach could target intrahepatic immune escape, utilizing liver directed treatments both prior to commencing pembrolizumab and/or during treatment in response to liver only progression. There is a growing body of evidence Citation25 suggesting that liver directed treatments such as metastasectomy, hepatic arterial embolization and percutaneous hepatic chemoperfusion can result in clinically meaningful periods of disease control; some approaches may additionally stimulate or boost adaptive immune responses through immunogenic cell death and dysregulation of local immune escape mechanisms. Citation26,27
Conclusion
Pembrolizumab as a single agent can be used in the management of metastatic UM with an acceptable toxicity profile and provides clinically meaningful benefit in a significant proportion of patients. Prospective clinical trials are needed to characterize the magnitude of benefit and whether specific groups would be best served by alternative or combination treatments and determine the optimal modalities and sequencing. Further research on underlying immune escape mechanisms is needed to drive the rational design of future studies for this rare malignancy.
Disclosure of potential conflicts of interest
CO has had consulting/advisory roles and has received funding for travel and speaker's honoraria with Merck Sharp & Dohme. All remaining authors have declared no conflicts of interest.
Funding
This work was supported by funding from Southampton CR-UK Center and NIHR Southampton Experimental Cancer Medicine Center.
References
- Martorano LM, Winkelmann RR, Cebulla CM, Abdel-Rahman MH, Campbell SM. Ocular melanoma and the BAP1 hereditary cancer syndrome: implications for the dermatologist. Int J Dermatol 2014; 53:657-63; PMID:24697775; http://dx.doi.org/10.1111/ijd.12386
- Papadopoulos S, Benter T, Anastassiou G, Pape M, Gerhard S, Bornfeld N, Ludwig W-D, Dörken B. Assessment of genomic instability in breast cancer and uveal melanoma by random amplified polymorphic DNA analysis. Int J Cancer 2002; 99:193-200; PMID:11979433; http://dx.doi.org/10.1002/ijc.10297
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale A-L et al. Signatures of mutational processes in human cancer. Nature 2013; 500:415-21; PMID:23945592; http://dx.doi.org/10.1038/nature12477
- Harbour JW. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res 2012; 25:171-81; PMID:22268848; http://dx.doi.org/10.1111/j.1755-148X.2012.00979.x
- Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res 2015; 43:D805-11; PMID:25355519; http://dx.doi.org/10.1093/nar/gku1075
- Luscan A, Just PA, Briand A, des Roziers CB, Goussard P, Nitschké P, Vidaud M, Avril MF, Terris B, Pasmant E. Uveal melanoma hepatic metastases mutation spectrum analysis using targeted next-generation sequencing of 400 cancer genes. Br J Ophthalmol 2015; 99:437-9; PMID:25361747; http://dx.doi.org/10.1136/bjophthalmol-2014-305371
- Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi I, Jampol LM, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol Chic Ill 1960 2005; 123:1639 -43; PMID: 16344433; http://dx.doi.org/10.1001/archopht.123.12.1639
- Albert DM, Ryan LM, Borden EC. Metastatic ocular and cutaneous melanoma: a comparison of patient characteristics and prognosis. Arch Ophthalmol 1996; 114:107-8; PMID:8540843; http://dx.doi.org/10.1001/archopht.1996.01100130103030
- Flaherty LE, Unger JM, Liu PY, Mertens WC, Sondak VK. Metastatic melanoma from intraocular primary tumors: the Southwest Oncology Group experience in phase II advanced melanoma clinical trials. Am J Clin Oncol 1998; 21:568-72; PMID:9856657; http://dx.doi.org/10.1097/00000421-199812000-00008
- Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, Linette GP, Gajewski TF, Lutzky J, Lawson DH et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014; 311:2397-405; PMID:24938562; http://dx.doi.org/10.1001/jama.2014.6096
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015; 372:2521-32; PMID:25891173; http://dx.doi.org/10.1056/NEJMoa1503093
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Kelderman S, van der Kooij MK, van den Eertwegh AJM, Soetekouw PMMB, Jansen RLH, van den Brom RRH, Hospers GAP, Haanen JBAG, Kapiteijn E, Blank CU. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology (WIN-O). Acta Oncol 2013; 52:1786-8; PMID:23607756; http://dx.doi.org/10.3109/0284186X.2013.786839
- Maio M, Danielli R, Chiarion-Sileni V, Pigozzo J, Parmiani G, Ridolfi R, Rosa FD, Vecchio MD, Guardo LD, Queirolo P et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol 2013; 24:2911-5; PMID:24067719; http://dx.doi.org/10.1093/annonc/mdt376
- Zimmer L, Vaubel J, Mohr P, Hauschild A, Utikal J, Simon J, Garbe C, Herbst R, Enk A, Kämpgen E et al. Phase II DeCOG-Study of Ipilimumab in Pretreated and Treatment-Naïve Patients with Metastatic Uveal Melanoma. PLoS ONE 2015; 10:e0118564; PMID:25761109; http://dx.doi.org/10.1371/journal.pone.0118564
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N et al. Mutations in GNA11 in Uveal Melanoma. N Engl J Med 2010; 363:2191-9; PMID:21083380; http://dx.doi.org/10.1056/NEJMoa1000584
- Errington JA, Conway RM, Walsh-Conway N, Browning J, Freyer C, Cebon J, Madigan MC. Expression of cancer-testis antigens (MAGE-A1, MAGE-A3/6, MAGE-A4, MAGE-C1 and NY-ESO-1) in primary human uveal and conjunctival melanoma. Br J Ophthalmol 2012; 96:451-8; PMID:22190731; http://dx.doi.org/10.1136/bjophthalmol-2011-300432
- De Cruz POL, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer 1990; 65:112-5; PMID:2293857; http://dx.doi.org/10.1002/1097-0142(19900101)65:1%3c112::AID-CNCR2820650123%3e3.0.CO;2-X
- Kottschade LA, McWilliams RR, Markovic S, Block MS, Villasboas Bisneto J, Pham AQ, Dronca RS. The use of pembrolizumab for the treatment of metastatic uveal melanoma. ASCO Meet Abstr 2015; 33:9010
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin Cancer Res 2009; 15:7412-20; PMID:19934295; http://dx.doi.org/10.1158/1078-0432.CCR-09-1624
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990 2009; 45:228 -47; PMID:19097774; http://dx.doi.org/10.1016/j.ejca.2008.10.026
- Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol 2011; 11:876 -8; PMID: 21241810; http://dx.doi.org/10.1016/j.intimp.2010.12.024
- Agarwala SS, Eggermont AMM, O'Day S, Zager JS. Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer 2014; 120:781-9; PMID:24301420; http://dx.doi.org/10.1002/cncr.28480
- den Brok MHMGM, Sutmuller RPM, Nierkens S, Bennink EJ, Frielink C, Toonen LWJ, Boerman OC, Figdor CG, Ruers TJM, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006; 95:896; PMID:16953240; http://dx.doi.org/10.1038/sj.bjc.6603341
- Haen SP, Pereira PL, Salih HR, Rammensee H-G, Gouttefangeas C. More Than Just Tumor Destruction: Immunomodulation by Thermal Ablation of Cancer. Clin Dev Immunol; vol. 2011, Article ID 160250, 19 pages, 2011; PMID: 22242035; http://dx.doi.org/10.1155/2011/160250
