ABSTRACT
T-cell receptor alternate reading frame protein (TARP) is a 58-residue protein over-expressed in prostate and breast cancer. We investigated TARP peptide vaccination's impact on the rise in PSA (expressed as Slope Log(PSA) or PSA Doubling Time (PSADT)), validated tumor growth measures, and tumor growth rate in men with Stage D0 prostate cancer. HLA-A*0201 positive men were randomized to receive epitope-enhanced (29-37-9V) and wild-type (27-35) TARP peptides administered as a Montanide/GM-CSF peptide emulsion or as an autologous peptide-pulsed dendritic cell vaccine every 3 weeks for a total of five vaccinations with an optional 6th dose of vaccine at 36 weeks based on immune response or PSADT criteria with a booster dose of vaccine for all patients at 48 and 96 weeks. 41 patients enrolled with median on-study duration of 75 weeks at the time of this analysis. Seventy-two percent of patients reaching 24 weeks and 74% reaching 48 weeks had a decreased Slope Log(PSA) compared to their pre-vaccination baseline (p = 0.0012 and p = 0.0004 for comparison of overall changes in Slope Log(PSA), respectively). TARP vaccination also resulted in a 50% decrease in median tumor growth rate (g): pre-vaccine g = 0.0042/day, post-vaccine g = 0.0021/day (p = 0.003). 80% of subjects exhibited new vaccine-induced TARP-specific IFNγ ELISPOT responses but they did not correlate with decreases in Slope Log(PSA). Thus, vaccination with TARP peptides resulted in significant slowing in PSA velocity and reduction in tumor growth rate in a majority of patients with PSA biochemical recurrence.
Abbreviations
| ADT | = | androgen deprivation therapy |
| EE | = | epitope-enhanced |
| ICS | = | intracellular cytokine staining |
| IVS | = | in vitro stimulation |
| PSA | = | prostate-specific antigen |
| PSADT | = | PSA doubling time |
| TARP | = | T-cell receptor alternate reading frame protein |
| WT | = | wild type |
Introduction
Prostate cancer is the leading cause of new cancer cases and the second leading cause of cancer-related death in US men.Citation1 Challenges to treatment include the highly variable outcomes associated with local, locally advanced, or distant metastatic disease despite standard histopathologic grading i.e., Gleason scoreCitation2 and clinical staging.Citation3
Historically, patients with prostate-specific antigen (PSA) biochemical recurrence or persistence following definitive primary therapy with either radical prostatectomy or radiation were often treated with ablative hormone therapy to induce chemical castration. However, this approach in asymptomatic men with a rising PSA has not been shown to be associated with improvements in either progression free or overall survival (OS) and ultimately leads to the development of castration-resistant tumor. While there are ever-expanding therapeutic options available to men with metastatic castrate-resistant prostate cancer (mCRPC), treatment options for men with micrometastatic Stage D0 disease (PSA biochemical recurrence without evidence of measurable local disease or visceral or bony metastases) remain limited. Novel therapeutic interventions are clearly needed to allow control of disease while delaying initiation of androgen deprivation therapy (ADT) until absolutely clinically necessary.
TARP is a novel, immunogenic 58 amino acid residue protein initially described by Pastan and colleaguesCitation4 and reported to be expressed in ∼85–95% of prostate cancer specimens. Transcribed from an alternate reading frame, the protein sequence is unrelated to T-cell receptors and was shown to originate from prostate epithelial cells and not from infiltrating T lymphocytes, to be expressed in normal prostate epithelium and over-expressed in prostate cancer, and subsequently documented by the same group to also be expressed in breast cancer cell lines and tissues.Citation5 Its expression is upregulated by testosterone in cells with a functional androgen receptor, suggesting that TARP has a potentially important role in regulating prostate cancer cell growth and gene expression.Citation6 Its expression has also been associated with conventional markers of unfavorable and more aggressive tumor behavior.Citation7 Additional work by others has shown that TARP is highly expressed in primary as well as metastatic prostate cancer,Citation8 in prostate cancers with all Gleason patterns,Citation9 and in both hormone-sensitive as well as castrate-resistant prostate cancer,Citation10 making it an ideal tumor antigen for use in a therapeutic cancer vaccine for any stage of prostate cancer. Further, a clinical trial of prostate cancer cellular vaccine found that antibodies to TARP correlated with increase in median survival time and in PSA Doubling Time (PSADT), suggesting relevance to that vaccine therapy.Citation11
Our group determined in mice that the immunogenicity of HLA-A*0201 binding TARP peptides could be improved through epitope-enhancement achieved by single amino acid substitutions to increase HLA binding affinity.Citation12 This strategy of epitope enhancementCitation13-16 for tumor antigens has also been advanced by the observation from the Kosmatopoulos lab that subdominant tumor epitopes could be more effective than dominant ones because the former should not have induced tolerance but could be made more immunogenic by sequence modification.Citation17,18 Our studies of these peptides-utilizing human cells confirmed the ability of human T cells to recognize not only HLA-A2 positive cells pulsed with TARP wild type and enhanced peptides but also more importantly HLA-A2 positive tumors naturally expressing TARP, confirming that TARP was endogenously processed and presented in human tumor cells.Citation12 Some of the same and other TARP epitopes for CD8+ and CD4+ T cells have been mapped by others.Citation19,20 To confirm the immunogenicity of wild-type (WT) and epitope-enhanced (EE) TARP peptides, we conducted a first-in-human pilot study to determine the safety and immunogenicity of therapeutic TARP peptide vaccination and its impact on the velocity of PSA rise (expressed as Slope Log(PSA) or PSADTCitation21) and tumor growth rate in men with Stage D0 prostate cancer. In Stage D0 prostate cancer characterized by PSA biochemical recurrence in which no tumor is detectable radiographically, RECIST criteria cannot be used. PSA velocity (in contrast to absolute PSA value) has been demonstrated in retrospective studies to be a significant predictive correlate of clinical outcome including metastasis-free survival and response to therapy,Citation22-27 and is thus the only available reliable measure of tumor progression and clinical outcome in Stage D0 disease.
Results
Baseline patient characteristics
Forty–one HLA-A*0201 patients with hormone-sensitive Stage D0 prostate cancer were enrolled in this trial between September 2009 and December 2011 at the NIH Clinical Center in Bethesda, MD. Patient characteristics at study entry are summarized in . Patients were randomized 1:1 between two arms, A and B (), with different delivery strategies for the TARP peptides because it was not clear a priori which method would be more likely to induce an effective immune response. Arm A patients received 1 mg of each peptide emulsified together with GM-CSF in Montanide ISA51 subcutaneously, whereas Arm B patients received autologous dendritic cells (DCs) pulsed with each peptide (at a concentration of 30 micromolar) plus keyhole limpet hemocyanin (KLH) as a source of help intradermally. There was no significant difference in baseline characteristics between those assigned to Arm A or Arm B. The median age was 62 y (range, 45 to 82 y), with 31 of 41 participants (76%) status post-radical prostatectomy, 26 of 41 (63%) status post-external beam radiotherapy for PSA biochemical recurrence and 14 of 41 (34%) status post-hormone therapy of limited duration (usually in the neoadjuvant or adjuvant setting). Forty patients received all five scheduled doses of TARP vaccine in the primary immunization series and thirty-nine were evaluable for toxicity, preliminary clinical efficacy, and immune response. One patient was removed at study week 6 following a decline in PSADT to ≤ 3 mo after having received a single dose of vaccine and one patient was excluded from the safety and efficacy analysis because he received mixed vaccines due to an inability to consistently generate an autologous DC vaccine product that met protocol-specified release criteria.
Table 1. Demographics and clinical characteristics by study cohort arm.
Safety
TARP vaccination was very safe and well tolerated with adverse events limited to local injection site reactions ≤ Grade 2 per CTCAE v3.0 criteria that were present in 100% of participants with a median duration of 5 d, lasting up to a maximum of 10 d with no differences in the duration of the reaction observed between arms. There were no acute systemic reactions or constitutional symptoms associated with vaccine administration. No other clinical or laboratory abnormalities attributed as possibly, probably, or definitely related to TARP vaccination were observed.
Clinical activity: Modulation of tumor growth
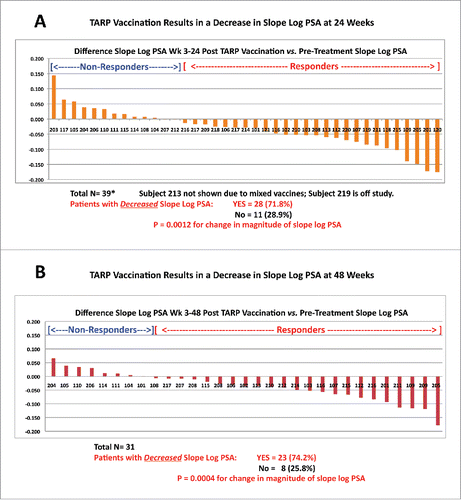
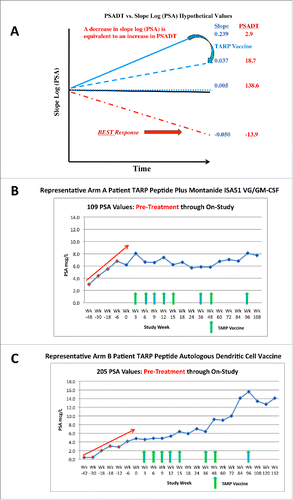
In addition to establishing safety and immunogenicity, a major focus was to determine the impact of TARP vaccination on PSADT, a documented measure of tumor growth in the Stage D0 setting and a marker for predicting clinical outcomes based on retrospective studies in patients with prostate cancer, especially Stage D0 disease.Citation22-27 Although PSADT has not been formally shown prospectively to predict outcome of treatment, if it predicts outcome of disease course it is reasonable to expect it to potentially reflect outcome of treatment that does not directly affect PSA levels themselves. Nomogram-generated calculations of changes in PSA levels over time can be expressed as PSADT or Slope Log (PSA) with both measurements providing insight into the rate of change. As illustrated in (based on hypothetical sample numbers), the PSADT goes to infinity as the slope approaches zero and becomes meaningless when the slope is negative. Consequently, the closely related value (proportional to the inverse of the PSADT), Slope Log (PSA), was utilized instead of PSADT to calculate differences pre- and post-TARP vaccination as it is a continuous variable, whether the slope is positive, zero, or negative. A decrease in Slope Log (PSA) is equivalent to an increase or lengthening in PSADT, and is the desired outcome of vaccination.Citation22-26 Representative examples of patients from both study arms experiencing a slowing in the rate of PSA rise evidenced by a decrease in slope (increase in PSADT) post-TARP vaccination are demonstrated in .
Figure 1. Clinical trial design. HLA-A*0201 positive men with Stage D0 prostate cancer and a PSADT > 3 mo and < 15 mo were randomized 1:1 to receive a primary vaccination series of five TARP peptide vaccines administered q 3 weeks with a conditional booster dose at Week 36 depending on immune reactivity and PSADT at Week 24. Subsequent study amendment allowed booster doses for all patients at Weeks 48 and 96.
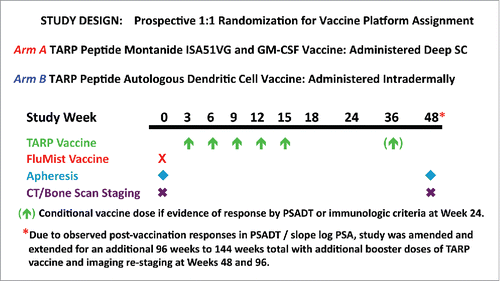
Figure 2. Representative schematic of PSADT relationship to Slope Log(PSA) (A), PSA response to TARP vaccination with Montanide ISA 51-GM-CSF (B) and PSA response to TARP autologous DC vaccination (C). Red arrow in (B) and (C) represents estimated PSA trajectory within the 48 weeks prior to study entry.
When examining differences in Slope Log (PSA) between weeks 3 and 24 or 3 and 48 minus the pre-treatment slope (which was calculated based on ≥ 4 PSA values from the same laboratory drawn over > 3 mo and within 12 mo of study enrollment pre-vaccine), there were no statistically significant differences observed between Arm A and Arm B, resulting in the recommendation to pool all participants from both arms in the formal analysis to increase the statistical power. However, we did note that Arm B (DC vaccine) alone had a statistically significant reduction in slope at week 24 (p = 0.026) and at week 48 (p = 0.0069) compared to baseline slopes by itself, whereas Arm A (peptide in Montanide) did not. With the groups pooled, highlighted in , 71.8% of the 39 patients reaching 24 weeks and 74.2% of the 31 patients reaching 48 weeks had a decrease in Slope Log (PSA) compared to their pre-treatment value, (both highly significant at p = 0.0012 and p = 0.0004 for 24 and 48 weeks, respectively). The effect of decreased slope at weeks 3–24 did not wane over time and was not impacted by an additional vaccine dose at week 36. In addition, compared to the pre-treatment value, the week 48–72 Slope Log(PSA) also showed a statistically significant decline (p = 0.0035, data not shown) although we cannot exclude some bias due to the limited number of patients with data in weeks 48–72 (n = 21). Despite a decrease in the Slope Log(PSA) in the majority of subjects (reflecting an increase in the PSADT), only a minority of patients (6 out of 39, 15.4%) experienced an absolute decrease in PSA values at week 24 with a 95% confidence interval from 5.7% to 29.8%. Nevertheless, even 15% of patients with PSA actually decreasing in absolute magnitude is more than would be expected in untreated Stage D0 patients. There were no correlations or associations with change in Slope Log(PSA) that were strong or statistically significant using any baseline variables including CD4+ and CD8+ T lymphocyte percent/absolute count, 25-OH vitamin D levels, Gleason score, PSA, s/p radical prostatectomy or a baseline PSADT < 6 mo versus ≥ 6 mo.
Growth rate analysis
PSA measurements obtained before and during therapy were used in a two-phase exponential growth and regression mathematical model to estimate concomitant PSA growth and regression rates.Citation28 TARP vaccination resulted in a 50% (2-fold) decrease in the estimated PSA growth rate (g) with pre-vaccine median g = 0.0042/day compared to post-vaccine median g = 0.0021/day, (p = 0.003, ). Analysis of the PSA growth rates calculated for a moving window over time documented a progressive decline in the growth rates and a continued effect of TARP vaccination measured as decline in g out to 651 d on treatment (). Thus, by both slope and growth rate constant calculations, tumor growth rate was significantly decreased in the majority of vaccinated patients.
Figure 4. Changes in tumor growth rate constants (g) calculated from fitting the PSA curves to an exponential tumor growth model. Comparison of the median growth rate constant (the median is the bold horizontal line within the gray shaded box that represents the 25th percentile [lower quartile] to 75th percentile [upper quartile]) of the estimated g of individual subjects pre- (pink dots) and post-TARP vaccination (green dots) (A). For change in tumor growth rate constant over time (B), each black dot at a given time point on treatment is the mean of calculated growth rates to that time point for all patients on study at that time point; the blue vertical lines with horizontal cross hatches represent 95% confidence intervals for each respective mean. Note the marked differences between days 42 and 147/231, despite very little patient attrition, excluding selection of patients with more indolent disease as a reason for the declines.
![Figure 4. Changes in tumor growth rate constants (g) calculated from fitting the PSA curves to an exponential tumor growth model. Comparison of the median growth rate constant (the median is the bold horizontal line within the gray shaded box that represents the 25th percentile [lower quartile] to 75th percentile [upper quartile]) of the estimated g of individual subjects pre- (pink dots) and post-TARP vaccination (green dots) (A). For change in tumor growth rate constant over time (B), each black dot at a given time point on treatment is the mean of calculated growth rates to that time point for all patients on study at that time point; the blue vertical lines with horizontal cross hatches represent 95% confidence intervals for each respective mean. Note the marked differences between days 42 and 147/231, despite very little patient attrition, excluding selection of patients with more indolent disease as a reason for the declines.](/cms/asset/86a553c1-111b-4717-801a-1b810f56c749/koni_a_1197459_f0004_oc.gif)
Immunological response
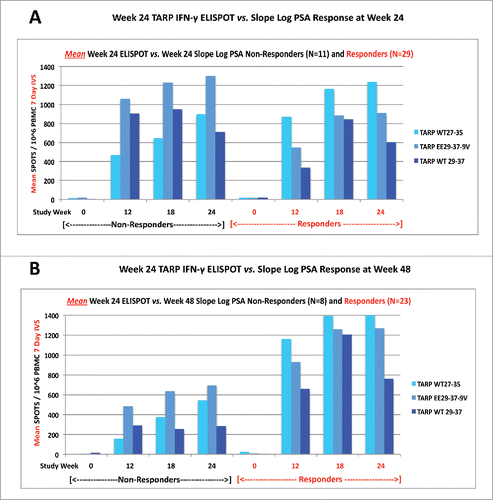
A positive IFNγ ELISPOT response was pre-defined in the study as a 3-fold increase in TARP-specific cells over baseline documented for at least two time points tested between weeks 0 and 24. Direct ex vivo ELISPOT numbers were too small to be useful for analysis. Use of a 7 d in vitro stimulation (IVS) assay as described under methods maximized our ability to detect TARP-specific responses by ELISPOT compared to ex vivo testing of PBMCs, and remained virtually all negative at baseline before vaccination, confirming that the responses observed were due to the vaccine, not a primary response to IVS. Nearly all ELISPOT values increased over baseline at all study time points examined and thus the changes from baseline were clearly large and significant, consistent with a vaccine effect rather than immunity induced by the tumor. The change in IFNγ ELISPOT reactivity to the TARP WT27-35 and EE29-37-9V vaccine platform peptides was highly statistically significant at weeks 12, 18, and 24 (all p values <0.0001, data not shown). Similarly, there was also a highly statistically significant increase in TARP specific reactivity to non-vaccine WT TARP peptide 29–37 at weeks 12, 18, and 24 (all p values < 0.0001, data not shown) that recapitulated observations documented in our pre-clinical animal models of this vaccine platform that the EE peptide-induced human T cells recognized the WT version of that sequence naturally processed and presented on TARP-expressing human tumor cells.Citation12 This finding confirms that the EE peptide TARP EE29-37-9V consistently induces T cells that also recognize the corresponding WT TARP 29-37. Although TARP IFNγ ELISPOT responses were documented at week 24 in 32 of 40 (80.0%) patients tested, there were no differences in ELISPOT reactivity between study arms (p = 0.13, p = 0.33, and p = 0.12 for WT27-35, EE29-37-9V, and WT29-37 peptides, respectively). Furthermore, there were no differences in TARP ELISPOT reactivity between Slope Log(PSA) responders (i.e., equivalent to an increase and lengthening in PSADT) and non-responders at week 24 () or week 48 (): p = 0.74, p = 0.79, and p = 0.07 for week 24, and p =0.61, p = 0.73, and p = 0.66 for week 48, for TARP WT27-35, EE29-37-9V, and WT29-37, respectively. Additional studies examining TARP ELISPOT functional avidity between maximal Slope Log(PSA) responders and maximal non-responders were unrevealing. As would be expected with therapeutic vaccination with short 9-amino acid peptides, there was no evidence of induction of anti-TARP or anti-PSA antibody responses in a subset of 20 patients for whom this was examined. Other qualitative differences in the functional activities of the T cells induced including tetramers and intracellular cytokine staining (ICS) are being examined to detect possible correlates of the effect on PSA velocity.
Figure 5. TARP-specific immunological responses. Shown are the mean spot responses per 106 PBMC following a 7 d in vitro stimulation (IVS) IFN-γ ELISPOT assay to vaccine (WT27-35, EE29-37-9V) and non-vaccine (WT29-37) TARP peptides in subjects with a positive difference in Slope Log(PSA) (non-responders) and a negative difference in Slope Log(PSA) (responders) following TARP vaccination compared to pre-vaccine treatment, at Week 24 (A) and Week 48 (B).
Overall, although the observation that clinical non-responders as well as responders had T cell responses to the vaccine does not allow us yet to define the immune correlate responsible for the vaccine's activity, it does not change the fact that the vaccine met all the primary endpoints of clear safety and strong immunogenicity (80.0% response rate), as well as the secondary endpoint of clinical response as significant slowing of PSA velocity or tumor growth rate would not be expected to happen in nearly three-fourths of Stage D0 patients without intervention. Further, 15% of the patients actually had a declining PSA absolute value, another indication of clinical benefit not expected without intervention.
Discussion
The optimal treatment paradigm for treating asymptomatic men with Stage D0 prostate cancer in which no tumor can be detected or measured radiographically remains a work in progress. Given the undesirable consequences of ADT and the fact that to date clinical benefit has not been documented, the optimal timing for ADT remains unresolved. In this setting, therapeutic cancer vaccines offer an attractive alternative to potentially eradicate micrometastatic disease. In multiple data sets and retrospective analyses, PSADT has been validatedCitation23-27 as a prognostic correlate of time to radiographic evidence of metastases and/or prostate cancer-related death, as well as response to therapy, although it has not yet been evaluated in clinical trials that prospectively confirm these clinical outcomes. In hormone-naive men without radiographically detectable disease for RECIST criteria and with only an abnormal PSA, the PSADT or Slope Log(PSA) is the only validated measure of tumor progression and clinical outcome, including disease recurrence, survival, and response to therapy.Citation23,27 Even though PSADT or Slope Log(PSA) has not been prospectively proven to predict response to therapy, if it predicts clinical outcomes in other settings, changes in this parameter may potentially be an indication of outcome of any therapy that does not directly alter PSA levels themselves. Our TARP vaccine does not contain PSA, does not induce anti-PSA antibodies, and should not directly affect PSA levels except through its effect on tumor growth. TARP vaccination using a peptide emulsion or autologous peptide-pulsed DCs resulted in a statistically significant slowing in the post-vaccination Slope Log(PSA) (equivalent to an increase/lengthening in PSADT), with decreases in over 70% of men in this study at both 24 and 48 weeks, although absolute decreases in serum PSA values were observed only in a minority (15%) of patients. Although this early phase, first-in-human trial naturally had no randomized placebo group, it was of note that in a celecoxib therapy study in a similar population, 80% of the control group failed to meet the criteria for a 2-fold increase in PSADT and about 58% of the placebo control group had an increasing PSA slope. In contrast, only 26% of those vaccinated in our study had an increasing PSA slope, supporting our conclusion that the decreased slope in 74% of subjects that we observed would not be expected in untreated patients.Citation29 The decreased Slope Log(PSA) from weeks 3–24 did not wane significantly over time, was not impacted by an additional vaccine booster dose at week 36 and was persistent beyond 48 weeks: week 48–72 slope minus the pre-vaccination slope still demonstrated a statistically significant decline (p = 0.0035, n = 21 patients, data not shown). These observed responses were accomplished despite using nominal TARP cytotoxic T lymphocyte (CTL) epitopes without inclusion of CD4+ epitopes or toll-like receptor ligands (TLRLs), although the DCs in Arm B were also pulsed with KLH as a source of CD4+ T-cell help. Despite this, the effect of TARP vaccination on increasing the PSADT was sustained, for more than 6 mo after the last vaccine dose in most patients. While other TARP peptides are being investigated in combination with prostate-specific membrane antigen (PSMA) and poly IC-LC adjuvant in a similar population (NCT006945551), our study is the first to document an increase in the PSADT, a widely accepted surrogate for clinical outcomes, in response to TARP vaccination. Of interest, a randomized trial of sipuleucel-T preceded by a limited course of androgen suppression therapy in a similar patient population also documented an increase in PSADT although there was no significant difference in time to PSA biochemical failure.Citation30
As might be expected with peptide vaccines and the use of autologous DCs, TARP vaccination was well tolerated, without acute systemic reactions: adverse events were restricted to minor local injection site reactions of limited duration. Although multiple parameters were examined, there were no correlations or associations observed between any baseline clinical or laboratory parameters and the observed increase in PSADT. Completely re-capitulating observations documented in our pre-clinical animal studies,Citation12 vaccination was highly immunogenic and induced detectable, highly statistically significant increases in TARP-specific IFNγ ELISPOT reactivity to the TARP WT27-35 and EE29-37-9V peptides contained in the vaccine platform as well as the non-vaccine WT29-37 TARP peptide, showing that the response to the EE peptide cross-reacts with the WT version of this sequence. Moreover, we have previously demonstrated that the human T cells induced by these peptides kill human tumor cells naturally expressing TARP, confirming that it is endogenously processed and presented.Citation12 However, as in some other reported cancer immunotherapy trials,Citation31,32 there was no association of the IFNγ-producing T cell responses with clinical outcomes of interest. This may be due to the inherent complexity of the antitumor immune response and the host-related factors involved in its generationCitation33 as well as the parallel complexity of tumor heterogeneity and the immune signature at sites of disease.Citation34,35 The fact that 80.0% of vaccinated subjects generate a new TARP-specific T cell response that was not present at baseline (meeting the primary endpoint for immunogenicity) clearly implies the vaccine is inducing the intended CD8+ T cell response against the epitopes in the vaccine. Therefore, the lack of correlation with clinical response (change in PSA slope) suggests that the T cell response is probably necessary but not sufficient to account for clinical benefit. Other factors in the tumor microenvironment, such as PD-L1, CTLA4, MDSCs, tumor-associated macrophages, and T regulatory cells, are known to affect whether a T cell response can be efficacious against a tumor. We also suspect that there must be heterogeneity in the tumors as well as these other factors that we cannot discern since the disease is micrometastatic at the time of treatment and biopsy is not possible. Thus, future studies will also explore whether the vaccine may synergize with PD-1 or TGF-β blockade or other similar checkpoint inhibition. In addition, it remains unknown which immune response parameters are the most important in mediating clinically relevant responses to cancer immunotherapy. Given the pleiotropism of the immune system, other responses may have also been induced by vaccination that we have not yet been able to characterize that could be contributing toward the improvement in Slope Log(PSA) and clinical benefit. We continue to explore other parameters of TARP-specific reactivity, including assessment of tetramers, functional avidity, ICS, and polyfunctionality, as well as quantitative levels of T regulatory cells and myeloid-derived suppressor cells (MDSCs) in an attempt to identify correlates of response observed in this study.
Novel paradigms are needed for earlier assessment of therapeutic efficacy, and the estimation of tumor growth and regression rate constants from serial PSA measurements, where the exponential growth rate constant, g, derived from a mathematical fit of the PSA curve to an exponential growth model, has been shown to be associated with survival,Citation36 may potentially be a better measure of clinically relevant vaccine activity than immune responses and superior to PSADT in predicting OS.Citation28,37 Indeed, the slowing of tumor growth rates by vaccinesCitation38 could evolve as a novel clinical trial endpoint and surrogate for survival although further validation is required in larger randomized studies. Immune-mediated slowing of tumor growth may have greater impact on OS than transient cytoreduction with conventional therapies that may not affect the growth rates of surviving tumor cells.Citation28,37,39 Here, TARP vaccination was associated with an impressive 50% reduction in the calculated rates of tumor (serum PSA) growth, suggesting that evaluation of this vaccine platform in men with more aggressive D0 disease i.e., a PSADT ≤ 3 mo, may allow objective documentation of this vaccine's specific impact on disease progression and survival within a reasonable time frame.
Because Stage D0 disease is defined as PSA biochemical recurrence without radiographically detectable disease, there is no possibility to use RECIST criteria to measure response. However, in this setting, PSADT is a well-documented measure of tumor growth and a predictor of clinical outcome and response to therapyCitation22-27 and is the only validated surrogate measure available in such patients. Although change in PSA velocity has not been tested prospectively as a measure of treatment response, it stands to reason that if it predicts clinical outcome, it should be a good indicator as long as the treatment does not introduce other direct effects on PSA that could interfere with its use. Although slowing in PSADT and tumor growth rates were observed in the majority of patients, actual declines in absolute PSA values were observed in only 15% of patients, still more than expected without intervention, but suggesting that there is room for further optimization of responses to TARP peptide vaccination, such as combination with a checkpoint inhibitor.
The key limitation is that although the reduction in Slope Log(PSA) was highly statistically significant in nearly 75% of patients at one year on study, a result that would not be expected in the natural course of Stage D0 disease without therapy, this was a phase I study without a placebo control arm. Thus, the result must be confirmed or validated in a randomized placebo-controlled trial. Accordingly, we have opened a prospective, randomized, placebo-controlled study in an identical population of men with Stage D0 disease (NCT02362451). In this study, we are using a second-generation multi-epitope (ME) TARP peptide vaccine platform comprised of the original two WT27-35 and EE29-37-9V peptides plus an additional five overlapping 20-mer peptides that span the entire 58 amino acid TARP protein. Using an identical vaccination regimen to that of Arm B, with autologous DCs, which alone had a more significant effect on Slope Log(PSA) and was more amenable to administration of multiple peptides compared to preparation and separate delivery of multiple, individual TARP peptide Montanide emulsions, this vaccine platform eliminates the need for restricting HLA types and includes all the potential epitopes of the whole protein, including the helper and CTL epitopes recently mapped by other labs,Citation19,20 thereby minimizing the risk of immune escape post-vaccination. In addition, the long synthetic peptides include MHC class II-restricted CD4+ T cell helper epitopes that may allow generation of more optimal CD8+ T cells responses with improved functional avidity and longevityCitation40 as well as anti-TARP antibodies.
Interestingly, in unrelated studies, induction of antibody responses to TARP in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy was associated with an increased median survival time and an increase in PSADT.Citation11 In a patient population identical to ours, use of the tyrosine kinase inhibitor lapatinib that interrupts HER2/neu and epidermal growth factor receptor (EGFR) pathways,Citation41 was also associated with an increase in PSADT.Citation42 These independent observations point to the need for continued clinical studies of novel treatment interventions in prostate cancer patients with Stage D0 disease with a goal of delaying or even eliminating the use of ADT and the development of castration resistant disease. Confirmation of our initial study observations in the randomized placebo-controlled phase II trial could lead to further exploratory studies of TARP vaccination in the neo-adjuvant setting to document pathologic complete response (pCR), to address metastatic disease in more advanced stages of prostate cancer, as well as studies in other solid tumors such as breast cancer and potentially mesothelioma (Brigitte Widemann, NIH, unpublished observations, with permission) where TARP has been shown to be over-expressed.
Patients and methods
Study agents
TARP WT27-35 and EE29-37-9V were manufactured under cGMP conditions (Polypeptide Laboratories, San Diego, CA) and administered as a manually-generated peptide emulsion with Montanide ISA 51VG plus GM-CSF (Arm A) or as autologous DCs pulsed with peptides and KLH (Arm B).
Patient population
HLA-A*0201 positive men with histologically confirmed, hormone-sensitive, Stage D0 prostate cancer (verified by screening imaging), and a PSADT ≥ 3 mo and ≤ 15 mo were eligible for the study. PSADT was calculated using all available PSA values from a single clinical laboratory within 12 mo of study entry utilizing a median of 5 PSA values obtained over a median of 10 mo. Inclusion criteria required complete recovery from all prior definitive therapy, a non-castrate testosterone level ≥ 50 ng/dL (prior ADT allowed but patients were required to be ≥ 6 mo since last ADT dose), ECOG Performance Status 0–2 with life expectancy ≥ 1 y, negative for Hepatitis B and C (unless consistent with prior vaccination or infection and full recovery) and HIV, normal bone marrow, liver, and renal function as defined by Hgb ≥ 10.0 g/dL, WBC ≥ 2,500/mm3, ALC ≥ 500/ mm3, ANC ≥ 1,000/mm3, platelet count ≥ 100,000/mm3, SGPT/SGOT ≤ 2.5X ULN, total bilirubin ≤ 1.5X ULN, creatinine ≤ 1.5X ULN, and estimated GFR (eGFR) ≥ 60 mL/min. Patients were excluded for use of investigational agents or immunosuppressive/immunomodulating agents within 8 weeks of study therapy, receipt of prior prostate cancer vaccines expressing TARP or HLA-A2, concurrent anticancer therapy or alternative medications or agents known to alter PSA.
Study design and procedures
This study was a prospective, open label, single institution trial with patients randomized 1:1 to receive both WT (27-35) and EE (29-37-9V) TARP peptides administered either as a manually-generated peptide emulsion with Montanide ISA51 VG plus GM-CSF (Arm A) or as autologous DCs pulsed with peptides and KLH (Arm B), originally aiming primarily to estimate the immunologic response rates on each arm as the primary endpoint. A Simon two-stage optimal design was used to permit the trial to enroll up to 20 patients per arm in order to rule out a 10% immunologic response rate in favor of a 40% rate on each arm. No formal comparison or selection between the arms was to be done on the basis of immunologic response. Changes in PSADT became the greater focus of the study when a majority of subjects exhibited a slowing in PSA velocity following TARP vaccination.
Patients randomized to autologous DC vaccination underwent a 15-18L apheresis of peripheral blood mononuclear cells followed by counter-flow elutriation for enrichment of autologous monocytes that were aliquoted and cryopreserved for future preparation of DC products. To generate TARP DC vaccines, an enriched monocyte fraction was thawed and cultured over 5 d with GM-CSF, IL-4, and KLH, with the addition of IFNγ and LPS endotoxin on Day 4 to induce DC maturation. The resulting mature DCs were harvested on Day 5 and divided into two equal aliquots: each aliquot was pulsed independently with either TARP 27-35 or TARP EE 29-37-9V for 2 h at 37°C. After removing peptide-pulsing media, DCs were concentrated down in infusion media (Plasma-Lyte A containting 10% autologous heat-inactivated plasma) and administered intradermally. TARP vaccine was delivered at Weeks 3, 6, 9, 12, and 15 for a total of 5 doses, with a conditional dose of vaccine administered at Week 36 if evidence of response was documented by changes in PSADT / PSA or immunologic response criteria at Week 24. Initially 48 weeks in duration, the study was amended and extended to 144 weeks total, with additional booster doses of vaccine and imaging re-staging at Weeks 48 and 96 to allow long-term monitoring of clinical outcomes and post-vaccination changes in slowing of PSA velocity.
Clinical response evaluation
PSA and testosterone levels were collected at every study visit, and testosterone levels were documented to be within normal limits and non-castrate in all patients at all-time points. As previously described, PSA values were available from all patients for the 12 mo prior to enrollment, with ≥ 3 PSA measurements over ≥ 3 mo and the interval between PSA measurements ≥ 4 weeks with all values from the same clinical laboratory for the specified period (median of 5 PSA values per patient over a median duration of 10 mo). Pre-vaccination and post-vaccination (week 3–24 and week 3–48) PSADT /Slope Log(PSA) values were calculated using the Memorial Sloan-Kettering Cancer Center prostate nomogram found at: http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx.
Growth rate constant analysis
The regression-growth equation is based on the assumption that the change of a tumor's quantity during therapy here and throughout, as indicated by the change in the tumor measurement, results from two independent component processes: an exponential (first-order kinetics) decrease/regression and an exponential re-growth of the tumor.Citation28 Given the D0 study population investigated in this study, PSA change with time was used as the measurement to assess this rate of change in tumor quantity.
The equation is: f(t) = exp(−d • t) + exp(g • t) − 1, where exp is the base of the natural logarithm, e = 2.7182…, and f(t) is the PSA measurement at time t in days, normalized to (divided by) the PSA measurement at day 0, the time at which treatment is commenced. Rate constant d (decay, in days−1) represents the exponential decrease/regression rate constant of the PSA signal during therapy. Rate constant g (growth, also in days−1) represents the exponential growth/re-growth rate constant of the tumor during treatment. These rate constants may be expressed in terms of half-lives and doubling times. Thus, d equals In2 (0.693…) divided by the time it takes for the regressing part to shrink by half, whereas g equals In2 divided by the time for the growing component to double.Citation28
Immunological response evaluation
Peptides
TARP WT 27-35
TARP WT 29-37
TARP EE 29-37-9V
HIV-gag
Human T-lymphotropic viruses, type I (HTLV-I) Tax 11–19 (New England Peptide, custom synthesis)
Influenza Matrix Peptide FluM1 58-66 (FMP, American Peptide Company, 72-4-01)
CEF peptide pool: 23 peptides from MHC class I-restricted T-cell epitopes from human CMV, EBV, and influenza virus, designed to stimulate T cells from donors with a variety of HLA types (Mabtech, 3615-1)
Peripheral blood lymphocyte preparation
PBMC were obtained by density gradient centrifugation of whole blood (collected in sodium heparin green top tubes) or a leukapheresis product using Ficoll-Hypaque (GE Healthcare, 17-1440-03). The cells were then placed in freezing media containing 90% Human AB Serum (Gemini, 100-512) and 10% DMSO (Sigma-Aldrich, D2650) before being frozen using a Cryomed-controlled rate freezer and stored in liquid nitrogen. For each patient, PBMC from multiple timepoints were thawed and analyzed in the same assay to avoid inter-assay variability. All cell counts were performed using a Coulter counter and viability was assessed with trypan blue. Median cell yield and viability of PBMC was 70% and 95%, respectively.
Cell culture conditions
Frozen patient PBMC were thawed, re-suspended, and plated into wells of 24-well tissue culture plates (Corning, 3524) at 2-3e6 viable cells/well in CTL media containing a 1:1 ratio of RPMI (Gibco, 21870-084) and Click's Medium (Sigma, C5572), 10% human AB serum (Gemini, 100-512), 1% Pen/Strep-L-glutamine (Gibco, 10378-016), 1% sodium pyruvate (Gibco, 11360-070), 35mM HEPES (Gibco, 15630-080) and 50 uM 2-mercaptoethanol (Sigma, M7522). These PBMC were stimulated with a mixture of TARP WT 27-35, TARP WT 29-37, and TARP EE 29-37 peptides at 10 ug/mL in a 37°C humidified 10% CO2 atmosphere in the presence of 1000 u/mL recombinant human IL-7 (Peprotech, 200-07) on day 0. In addition, patient PBMC and healthy donor controls were stimulated in a similar way with 7.5 ug/mL FMP. Recombinant human IL-2 (Teceleukin; Roche, 23-6019) was added on day 3 at 20 u/mL. On day 5 and 6, half of the culture supernatant was replaced with fresh CTL media without any additional cytokines and then the cells were harvested on day 7 and 8. Median cell yield and viability post-stimulation was 50% and 93%, respectively.
IFNγ enzyme-linked immunosorbent spot (ELISPOT) assay
All ELISPOT assays were validated and standard operating protocols were performed in the Laboratory of Cell-Mediated Immunity at Leidos Biomedical Research, Inc. (formerly SAIC-Frederick, Inc.) which is certified by Clinical Laboratory Improvements Amendments (CLIA). Two frozen healthy donor controls with known response values were run with each assay to assure quality control of the assay results. For all assays, at least one of the two controls was within 2SD of the lab-generated means for FMP and CEF. All assays were performed on 7 and 8 day IVS PBMC (100,000 or 25,000/well) as the effectors and peptide-pulsed autologous day 0 PBMC (100,000 or 25,000/well) as the antigen presenting cells (APC) at a 1:1 ratio in ELISPOT media containing RPMI (Gibco, 21870-084), 5% human AB serum pretested for optimal assay performance (Gemini, 100–512), 1% Pen/Strep-L-glutamine (Gibco, 10378-016), and 2.5% HEPES (Gibco, 15630-080). The APC were pulsed with 10 ug/mL peptide 2 h at 37°C before being plated with the effectors. The response to the TARP peptides, the control peptides HIV-1 p17 Gag (77–85) and HTLV-I (Tax 11–19) plus mitogenic stimulation with PHA (Sigma, L9017) was assessed. In addition to the analysis of IVC cells, all patient PBMC from weeks 0 and 24 and healthy donor control PBMC were analyzed for responses to CEF and PHA and appropriate donors were also analyzed for their responses to FMP and KLH. Briefly, the day before assay setup, 96-well polyvinylidene fluoride (PVDF) membrane, HTS opaque plates (Millipore, MSIPS4W10) were coated overnight with a 1:100 dilution of anti-human IFNγ capture antibody (1 mg/mL, Mabtech 3420-3-1000) in DPBS at room temperature. Antibody-coated plates were washed four times in DPBS the next day and blocked with 5% human AB ELISPOT medium at 37°C for approximately 2 h. The effectors and APC were plated and incubated for 18–20 h at 37°C and 5% CO2. The next day, the plates were manually washed six times with 0.05% Tween 20 in DPBS, followed by a 2-h incubation at room temperature with a 1:2000 dilution of the biotinylated secondary antibody, anti-human IFNγ (1 mg/mL Mabtech, 3420-6-1000) in DPBS/1% bovine serum albumin/0.05% Tween. After incubation and four washes in DPBS to remove excess antibody, a 1:3000 dilution of streptavidin alkaline phosphatase (Mabtech, 3310-10) in DPBS/1% bovine serum albumin, was added to each well for 1 h at room temperature followed by four manual washes in DPBS. Finally, the BCIP/NPT substrate, 100 uL/well, (KPL, 50-81-07) was added for 7–10 min, resulting in the development of spots. The reaction was stopped by washing three times in distilled water. Plates were dried overnight and the spots were visualized and counted using the ImmunoSpot Imaging Analyzer system (Cellular Technology Ltd.)Citation43 and ImmunoSpot software v5.1. All wells were counted with set parameters and then each count was verified to ensure the accuracy of the counting software. ELISPOT results were expressed as the “number of spots per 10Citation6 responder cells” after subtracting background spots obtained in wells of effectors with non-pulsed PBMC.
Statistics
The primary analysis of interest compared post-vaccination Slope Log(PSA) (slope) from 3 to 24 weeks and 3 to 48 weeks to the pre-NIH/pre-vaccination slope (calculated using PSA values within 12 mo of study entry). Evaluations of paired data within an arm (or with all patients pooled together) were performed by a Wilcoxon signed rank test. Comparisons of continuous parameters between arms were performed by an exact Wilcoxon rank sum test. For these two analyses, as well as two primary comparisons between arms, p < 0.025 was required for significance using a Bonferroni criterion. The correlation between the slope at 3 to 24 and 3 to 48 weeks versus the pre-vaccination slope, and versus absolute lymphocyte count, CD4+ and CD8+ T-lymphocyte percent and absolute count, 25-OH vitamin D and PSA levels at week 0 was done using Spearman correlation. A Jonckheere–Terpstra test was used to evaluate differences in slope and Gleason score. Slope differences were compared between those with a pre-NIH PSADT < or ≥ 6 mo or with respect to status post-radical prostatectomy using a Wilcoxon rank sum test.
For ELISPOT response analyses, values of 0 were converted to equal 1.0, since log10 transformation was needed for analysis. Differences between these log 10 values were formed to evaluate changes in ELISPOT values over time, as well as whether changes in ELISPOT values were correlated with post-vaccination slope differences at either 3 to 24 or 3 to 48 weeks. Spearman correlations are interpreted as follows: |r| >0.70 is considered strong; 0.5 < |r| < 0.7, is moderately strong; 0.3 < |r| < 0.5 is weak to moderately strong, while |r| <0.3 is weak. All p values are two-sided.
Study approval
The study protocol (NCT00972309) was reviewed and approved by the NCI Institutional Review Board and the US Food and Drug Administration (BB-IND 13925). Written informed consent was received from all participants prior to inclusion in the study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1197459_s02.pdf
Download PDF (1.8 MB)Acknowledgments
We thank Dr. John Janik, formerly of NCI and now of the Georgia Regents University Cancer Center, for his efforts in the early stages of translating the TARP vaccine from preclinical research to a clinical trial. We thank William Kopp, Ph.D., Kim Dunham, Susan Strobl, Ph.D. and Anatoli Malyguine, Ph.D. of LCMI, Leidos Frederick, M.D., for performance of TARP immune assays, and Fred Aronson, M.D., New England Cancer Specialists and Nicholas J. Vogelzang, M.D., Comprehensive Cancer Centers of Nevada for patient referrals to this study. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Funding
This work was supported by the NCI Intramural Research Program.
References
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. p.2, p.10; http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index
- Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL, Committee IG. 2005 International Society of Urological Pathology (ISUP) Consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol 2005; 29:1228-42; PMID:16096414; http://dx.doi.org/10.1097/01.pas.0000173646.99337.b1
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localized disease. Eur Urol 2011; 59:61-71; PMID:21056534; http://dx.doi.org/10.1016/j.eururo.2010.10.039
- Essand M, Vasmatzis G, Brinkmann U, Duray P, Lee B, Pastan I. High expression of a specific T-cell receptor gamma transcript in epithelial cells of the prostate. Proc Natl Acad Sci U S A 1999; 96:9287-92; PMID:10430935; http://dx.doi.org/10.1073/pnas.96.16.9287
- Wolfgang CD, Essand M, Vincent JJ, Lee B, Pastan I. TARP: a nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor gamma chain locus. Proc Natl Acad Sci U S A 2000; 97:9437-42; PMID:10931945; http://dx.doi.org/10.1073/pnas.160270597
- Wolfgang CD, Essand M, Lee B, Pastan I. T-cell receptor gamma chain alternate reading frame protein (TARP) expression in prostate cancer cells leads to an increased growth rate and induction of caveolins and amphiregulin. Cancer Res 2001; 61:8122-6; PMID:11719440
- Fritzsche FR, Stephan C, Gerhardt J, Lein M, Hofmann I, Jung K, Dietel M, Kristiansen G. Diagnostic and prognostic value of T-cell receptor gamma alternative reading frame protein (TARP) expression in prostate cancer. Histol Histopathol 2010; 25:733-9; PMID:20376779; http://dx.doi.org/10.14670/HH-25.733
- Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005; 8:393-406; PMID:16286247; http://dx.doi.org/10.1016/j.ccr.2005.10.001
- True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci U S A 2006; 103:10991-6; PMID:16829574; http://dx.doi.org/10.1073/pnas.0603678103
- Best CJ, Gillespie JW, Yi Y, Chandramouli GV, Perlmutter MA, Gathright Y, Erickson HS, Georgevich L, Tangrea MA, Duray PH et al. Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res 2005; 11:6823-34; PMID:16203770; http://dx.doi.org/10.1158/1078-0432.CCR-05-0585
- Nguyen MC, Tu GH, Koprivnikar KE, Gonzalez-Edick M, Jooss KU, Harding TC. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol, Immunother 2010; 59:1313-23; PMID:20499060; http://dx.doi.org/10.1007/s00262-010-0858-5
- Oh S, Terabe M, Pendleton CD, Bhattacharyy A, Bera TK, Epel M, Epel M, Reiter Y, Phillips J, Linehan WM et al. Human CTL to wild type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res 2004; 64:2610-8; PMID:15059918; http://dx.doi.org/10.1158/0008-5472.CAN-03-2183
- Berzofsky JA. Epitope selection and design of synthetic vaccines: molecular approaches to enhancing immunogenicity and crossreactivity of engineered vaccines. Ann NY Acad Sci 1993; 690:256-64; PMID:7690214; http://dx.doi.org/10.1111/j.1749-6632.1993.tb44014.x
- Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol 1996; 157:2539-48; PMID:8805655
- Ahlers JD, Takeshita T, Pendleton CD, Berzofsky JA. Enhanced immunogenicity of HIV-1 vaccine construct by modification of the native peptide sequence. Proc Natl Acad Sci USA 1997; 94:10856-61; PMID:9380724; http://dx.doi.org/10.1073/pnas.94.20.10856
- Sarobe P, Pendleton CD, Akatsuka T, Lau D, Engelhard VH, Feinstone SM, Berzofsky JA. Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions. J Clin Invest 1998; 102:1239-48; PMID:9739058; http://dx.doi.org/10.1172/JCI3714
- Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest 2004; 113:425-33; PMID:14755339; http://dx.doi.org/10.1172/JCI200419418
- Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, Lemonnier FA, Kosmatopoulos K. A general strategy to enhance immunogenicity of low-affinity HLA-A2. 1- associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol 2000; 30:3411-21; PMID:11093159; http://dx.doi.org/10.1002/1521-4141(2000012)30:12%3c3411::AID-IMMU3411%3e3.0.CO;2-R
- Carlsson B, Totterman TH, Essand M. Generation of cytotoxic T lymphocytes specific for the prostate and breast tissue antigen TARP. The Prostate 2004; 61:161-70; PMID:15305339; http://dx.doi.org/10.1002/pros.20091
- Kobayashi H, Nagato T, Oikawa K, Sato K, Kimura S, Aoki N, Omiya R, Tateno M, Celis E. Recognition of prostate and breast tumor cells by helper T lymphocytes specific for a prostate and breast tumor-associated antigen, TARP. Clin Cancer Res 2005; 11:3869-78; PMID:15897588; http://dx.doi.org/10.1158/1078-0432.CCR-04-2238
- Arlen PM, Bianco F, Dahut WL, D'Amico A, Figg WD, Freedland SJ, Gulley JL, Kantoff PW, Kattan MW, Lee A et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol 2008; 179:2181-5; discussion 5–6; PMID:18423743; http://dx.doi.org/10.1016/j.juro.2008.01.099
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281:1591-7; PMID:10235151; http://dx.doi.org/10.1001/jama.281.17.1591
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294:433-9; PMID:16046649; http://dx.doi.org/10.1001/jama.294.4.433
- Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res 2005; 11:8669-73; PMID:16361552; http://dx.doi.org/10.1158/1078-0432.CCR-05-1668
- Lee AK, Levy LB, Cheung R, Kuban D. Prostate-specific antigen doubling time predicts clinical outcome and survival in prostate cancer patients treated with combined radiation and hormone therapy. Int J Radiat Oncol Biol Phys 2005; 63:456-62; PMID:15927415; http://dx.doi.org/10.1016/j.ijrobp.2005.03.008
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol 2007; 25:1765-71; PMID:17470867; http://dx.doi.org/10.1200/JCO.2006.08.0572
- Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer 2012; 118:1533-42; PMID:21960118; http://dx.doi.org/10.1002/cncr.26437
- Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, Ning YM, Arlen PM, Price D, Bates SE et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res 2011; 17:907-17; PMID:21106727; http://dx.doi.org/10.1158/1078-0432.CCR-10-1762
- Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol 2006; 24:2723-8; PMID:16782912; http://dx.doi.org/10.1200/JCO.2005.03.7804
- Beer TM, Bernstein GT, Corman JM, Glode LM, Hall SJ, Poll WL, Schellhammer PF, Jones LA, Xu Y, Kylstra JW et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res 2011; 17:4558-67; PMID:21558406; http://dx.doi.org/10.1158/1078-0432.CCR-10-3223
- Lonchay C, van der Bruggen P, Connerotte T, Hanagiri T, Coulie P, Colau D, Lucas S, Van Pel A, Thielemans K, van Baren N et al. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci U S A 2004; 101 Suppl 2:14631-8; PMID:15452345; http://dx.doi.org/10.1073/pnas.0405743101
- Lurquin C, Lethe B, De Plaen E, Corbiere V, Theate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med 2005; 201:249-57; PMID:15657294; http://dx.doi.org/10.1084/jem.20041378
- Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, Atkins MB, Bartunkova J, Bergmann L, Berinstein N et al. Defining the critical hurdles in cancer immunotherapy. J Translat Med 2011; 9:214; PMID:22168571; http://dx.doi.org/10.1186/1479-5876-9-214
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610-8; PMID:21245428; http://dx.doi.org/10.1200/JCO.2010.30.5425
- Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Translat Med 2012; 10:1; PMID:22214470; http://dx.doi.org/10.1186/1479-5876-10-1
- Stein WD, Figg WD, Dahut W, Stein AD, Hoshen MB, Price D, Bates SE, Fojo T. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist 2008; 13:1046-54; PMID:18838440; http://dx.doi.org/10.1634/theoncologist.2008-0075
- Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 2010; 15:969-75; PMID:20798195; http://dx.doi.org/10.1634/theoncologist.2010-0129
- Gulley JL, Madan RA, Stein WD, Wilkerson J, Dahut WL, Heery CR, Schlom J, Wilding G, DiPaola RS. Effect of PSA-tricom, a pox-viral vaccine in prostate cancer (PCa), on tumor growth rates within 80 days after initiation in nonmetastatic PCa. J Clin Oncol 2013; 31(suppl 6); abstr 57
- Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res 2011; 17:3884-91; PMID:21680544; http://dx.doi.org/10.1158/1078-0432.CCR-10-2656
- Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008; 14:178-87; PMID:18172269; http://dx.doi.org/10.1158/1078-0432.CCR-07-1880
- Higa GM, Abraham J. Lapatinib in the treatment of breast cancer. Expert Rev Anticancer Ther 2007; 7:1183-92; PMID:17892419; http://dx.doi.org/10.1586/14737140.7.9.1183
- Liu G, Chen YH, Kolesar J, Huang W, Dipaola R, Pins M, Carducci M, Stein M, Bubley GJ, Wilding G. Eastern Cooperative Oncology Group Phase II Trial of lapatinib in men with biochemically relapsed, androgen dependent prostate cancer. Urol Oncol 2013; 31:211-8; PMID:21784672; http://dx.doi.org/10.1016/j.urolonc.2011.01.002
- Rahma OE, Hamilton JM, Wojtowicz M, Dakheel O, Bernstein S, Liewehr DJ, Steinberg SM, Khleif SN. The immunological and clinical effects of mutated ras peptide vaccine in combination with IL-2, GM-CSF, or both in patients with solid tumors. J Translat Med 2014; 12:55; http://dx.doi.org/10.1186/1479-5876-12-55