ABSTRACT
Background: Ipilimumab has proven to be effective in metastatic melanoma patients. The purpose of this study was to determine the efficacy of ipilimumab in advanced melanoma patients who showed progressive disease upon experimental dendritic cell (DC) vaccination. Methods: Retrospective analysis of 48 stage IV melanoma patients treated with ipilimumab after progression upon DC vaccination earlier in their treatment. DC vaccination was given either as adjuvant treatment for stage III disease (n = 18) or for stage IV disease (n = 30). Ipilimumab (3 mg/kg) was administered every 3 weeks for up to 4 cycles. Results: Median time between progression upon DC vaccination and first gift of ipilimumab was 5.4 mo. Progression-free survival (PFS) rates for patients that received ipilimumab after adjuvant DC vaccination, and patients that received DC vaccination for stage IV melanoma, were 35% and 7% at 1 y and 35% and 3% at 2 y, while the median PFS was 2.9 mo and 3.1 mo, respectively. Median overall survival of patients pre-treated with adjuvant DC vaccination for stage III melanoma was not reached versus 8.0 mo (95% CI, 5.2–10.9) in the group pre-treated with DC vaccination for stage IV disease (HR of death, 0.36; p = 0.017). Grade 3 immune-related adverse events occurred in 19% of patients and one death (2%) was related to ipilimumab. Conclusions: Clinical responses to ipilimumab were found in a considerable number of advanced melanoma patients with progression after adjuvant DC vaccination for stage III disease, while the effect was very limited in patients who showed progression after DC vaccination for stage IV disease.
Abbreviations
| CI | = | confidence interval |
| CTCAE | = | Common Terminology Criteria for Adverse Events |
| CTLA-4 | = | cytotoxic T-lymphocyte-associated antigen 4 |
| DC | = | dendritic cell |
| DTH | = | delayed-type hypersensitivity |
| EMA | = | European Medicines Agency |
| FDA | = | Food and Drug Administration |
| HR | = | hazard ratio |
| IL | = | interleukin |
| LDH | = | lactate dehydrogenase |
| MDSC | = | myeloid-derived suppressor cell |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| PD-1 | = | programmed death 1 |
| RECIST | = | Response Evaluation Criteria in Solid Tumors |
| SKIL(s) | = | skin-test infiltrating lymphocyte(s) |
| TAA | = | tumor-associated antigen(s) |
| Th | = | T-helper |
| TIL | = | tumor-infiltrating lymphocytes |
| ULN | = | upper limit of normal |
Introduction
Melanoma is the most deadly form of skin cancer.Citation1 Before 2011, systemic treatment for advanced melanoma consisted only out of chemotherapy (typically dacarbazine) and, in some countries interleukin-2 (IL-2), but both options have a minimal effect on survival.Citation2 However, since 2011, multiple new drugs have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA). These new therapeutics include the checkpoint inhibitors ipilimumab, nivolumab and pembrolizumab, and the targeted agents vemurafenib, cobimetinib, dabrafenib and trametinib.Citation3-9 Furthermore, multiple other treatments, among which dendritic cell (DC) vaccination, are still under investigation.Citation10 With the growing field of treatment options for melanoma, it is of vital importance that patients receive a personalized treatment-schedule, in which action-mechanisms and toxicity-profiles are considered.
Over the past years, we have treated stage III and stage IV melanoma patients with DC vaccination in different trials.Citation11-13 DCs are the most efficient antigen-presenting cells of the immune system due to their capacity to activate and prime naïve T cells, and may therefore play a vital role in anticancer immunotherapy. DCs can be isolated directly from patient's blood, or differentiated ex vivo from precursor cells, activated, loaded with tumor antigens and then injected into patients.Citation14,15 Although tumor-specific immune responses were found in stage IV melanoma patients treated with DC vaccination monotherapy, long-lasting clinical responses were rare.Citation13,14,16 Compared to patients with stage IV disease, tumor-specific immune responses were found in a higher percentage of stage III melanoma patients receiving DC vaccination, and in a retrospective analysis a significant increase in overall survival (OS) was found compared to matched controls receiving only surgery.Citation11 However, despite the presence of tumor-specific T cells after DC vaccination, a portion of stage III patients developed recurrent disease. Different immune-escape mechanisms, like immunosuppressive cytokines, regulatory T cells and myeloid derived suppressor cells (MDSCs) in the tumor microenvironment, may play a role in the occurrence of recurrent or progressive disease despite the presence of tumor-specific CD8+ T cells.Citation17,18 Furthermore, immune checkpoint molecules that downregulate pathways of T cell activation, like cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) may hamper potent tumor eradication by activated T cells.Citation3,6 Ipilimumab, a fully human monoclonal antibody that blocks CTLA-4, has proven to be effective in metastatic melanoma. Pooled analysis of phase 2 and 3 trials recently showed a median OS of 9.5 mo and a plateau at 21% in the survival curve beginning approximately 3 y after start of ipilimumab.Citation19 A considerable portion of patients treated with ipilimumab is affected by immune-related adverse events, but most are reversible when treated appropriately.Citation20
Based on their mechanism of action, DC vaccination and ipilimumab might be complementary to each other, either when given concomitantly, or when ipilimumab is given to patients as a subsequent treatment after progression on DC vaccination. These patients may have developed a tumor-specific T cell response on DC vaccination which was not strong enough for disease control, because of the immunosuppressive mechanisms mentioned before, but which could be enhanced by ipilimumab. Pierret and colleagues were the first to suggest a potential correlation between prior DC vaccination and clinical outcome in advanced melanoma patients treated with ipilimumab in a retrospective analysis of a small patient cohort.Citation21 Yuan and colleagues determined the effect of ipilimumab on antigen-specific responses following DNA or protein vaccination in three melanoma patients. Their patients generated weak to no antigen-specific CD4+ or CD8+ T cell responses following vaccination, but they experienced a clear antigen-specific T cell response after ipilimumab treatment, indicating that ipilimumab might have enhanced T cells induced by vaccination.Citation22
The aim of this retrospective study was to explore the clinical effect of ipilimumab in advanced melanoma patients with progressive disease after DC vaccination.
Results
Patients and treatment
Among 48 melanoma patients who progressed after DC vaccination included in this study, 18 patients were treated with adjuvant DC vaccination after radical lymph node dissection for stage III melanoma and 30 patients received DC vaccination for stage IV melanoma. Patient characteristics are shown in . Median progression-free survival (PFS) of DC vaccination was 14.0 mo for stage III patients and 4.0 mo for patients with stage IV disease. Included among these patients were 4 patients (8%) with M1a disease, 10 patients (21%) with M1b disease and 34 patients (71%) had M1c disease at the start of ipilimumab. The median time between progressive disease on DC vaccination and the first cycle of ipilimumab was 5.4 mo for the whole study population. Half of the stage III patients (n = 9) previously treated with adjuvant DC vaccination received ipilimumab as a first line treatment for advanced melanoma, partly because initially ipilimumab was not yet registered as first line treatment. In the group treated with DC vaccination for stage IV melanoma, ipilimumab was given as second (47%), third (50%) or fourth (3%) line of systemic treatment. A total of 34 patients (71%) completed all four cycles of ipilimumab. The most frequent reason for discontinuation of ipilimumab was disease progression (62%).
Table 1. Patient characteristics. Baseline characteristics were scored at the time of start of ipilimumab.
Clinical efficacy of ipilimumab after progression on DC vaccination
Patients were followed for up to 84.1 mo after start of ipilimumab, with a median follow-up time until death or censoring of 9.2 mo. The median PFS of ipilimumab in all patients was 3.1 mo (95% confidence interval (CI), 2.4–3.7) and it was comparable in stage III patients pre-treated with adjuvant DC vaccination and patients who received prior DC vaccination for stage IV disease (2.9 mo vs. 3.1 mo). The PFS rates for ipilimumab in the group pre-treated with adjuvant DC vaccination for stage III disease, and the group pre-treated with DC vaccination for stage IV melanoma, were 35% and 7% at 1 y and 35% and 3% at 2 y (; p = 0.036), indicating more durable clinical responses in the adjuvant DC vaccination group. The individual treatment-schedules including the PFS of DC vaccination and ipilimumab of each patient are shown in . No correlation could be found between the PFS of DC vaccination and the PFS of ipilimumab in the whole study population (r = 0.077; p = 0.602).
Figure 1. Progression-free survival and overall survival of ipilimumab treatment. Kaplan–Meier curves for progression-free and overall survival of ipilimumab treatment in stage IV patients pre-treated with either adjuvant dendritic cell (DC) vaccination for stage III melanoma or with DC vaccination for stage IV melanoma. Survival was calculated from start of ipilimumab to the date of disease progression (Panel A) or death (Panel B). Patients pre-treated with adjuvant DC vaccination showed a significantly better progression-free survival and overall survival compared to patients treated with DC vaccination for stage IV melanoma.
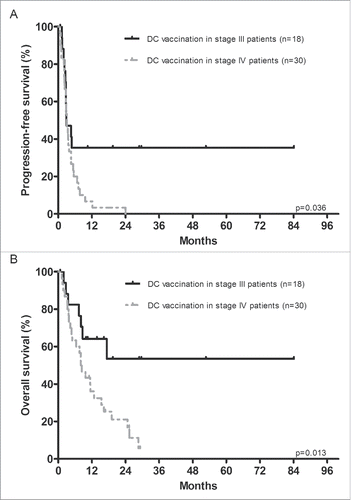
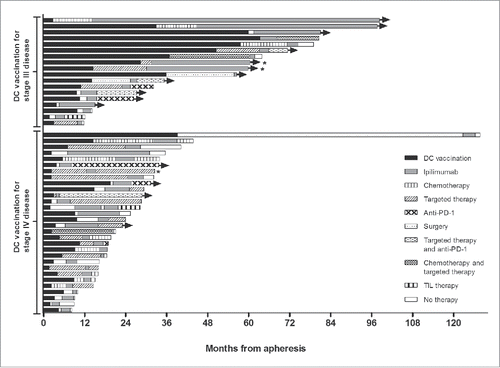
Figure 2. Survival and treatment since start dendritic cell vaccination. Swimmers plot illustrating the progression-free survival of dendritic cell vaccination and ipilimumab, and the different treatments before and after ipilimumab of every individual patient. Progression-free survival of ipilimumab is independent of the progression-free survival of dendritic cell vaccination. Patient alive at last follow-up. *Ipilimumab was started before progression on vemurafenib. Abbreviations: DC, dendritic cell; PD-1, programmed cell death 1; TIL, tumor-infiltrating lymphocytes.
The median OS since start of ipilimumab of the whole study population was 11.4 mo (95% CI, 6.1–16.7). Four baseline characteristics correlated with OS in an univariate Cox proportional-hazards analysis: line of systemic treatment of ipilimumab, baseline serum lactate dehydrogenase (LDH), brain metastases before start of ipilimumab, and the stage of disease in which DC vaccination was administered (). The line of systemic treatment of ipilimumab, baseline LDH, and brain metastases before start of ipilimumab remained to correlate with OS in a multivariate Cox proportional-hazards model (all p < 0.01). Thirty-three patients with normal baseline serum LDH at the start of ipilimumab showed a significant longer OS than 15 patients with LDH levels above the upper limit of normal; median OS 16.3 mo (95% CI, 10.1–22.6) versus 3.3 mo (95% CI, 2.2–4.3; p < 0.001). Furthermore, the median OS of ipilimumab in nine patients with brain metastases before start of ipilimumab was only 3.8 mo (95% CI, 3.4–4.2) compared to 19.1 mo (95% CI, 8.3–29.9) in 29 patients without brain metastases (HR 7.45; 95% CI 2.46–22.61; p < 0.001). Eight of the patients with brain metastases were treated with radiotherapy and/or surgery before or during ipilimumab treatment. In addition, a trend was observed for the stage of disease in which DC vaccination was administered, to be a predictor of OS in the multivariate survival analysis, favoring adjuvant DC vaccination for stage III disease (HR 0.33; 95% CI, 0.10–1.09; p = 0.069). Median OS of patients pre-treated with DC vaccination for stage III melanoma was not reached, as compared to 8.0 mo (95% CI, 5.2–10.9) in the group pre-treated with DC vaccination for stage IV disease. The OS rates for ipilimumab in the group pre-treated with DC vaccination for stage III disease, and the group pre-treated with DC vaccination for stage IV melanoma, were 64% and 36% at 1 y and 53% and 21% at 2 y ().
Table 2. Univariate analysis of overall survival. Univariate analysis of relevant baseline co-variables that might correlate with overall survival following ipilimumab treatment.
The disease control rate (the proportion of patients with a partial response, complete response or stable disease) of the whole study population was 35%. The group pre-treated with adjuvant DC vaccination for stage III disease had a best objective response rate of 22% (three complete responses and one partial response) and a disease control rate of 44% on ipilimumab. The four patients with an objective response showed an ongoing PFS ranging from 10.5 to 84.1 mo. In the group pre-treated with DC vaccination for stage IV melanoma, the best objective response rate was 10% (all partial responses) and the disease control rate was 30%. All stage IV patients with a partial response progressed within 2 y, suggesting a small chance of durable responses in this group of patients.
Effect tumor-specific T cell responses by DC vaccination on ipilimumab treatment
During the DC vaccination trials, delayed-type hypersensitivity (DTH) skin-test biopsies were taken after each cycle of vaccinations. Lymphocytes out of these biopsies (SKILs) of 31 HLA-A*02:01 positive patients were analyzed for antigen-specific T cells by staining with tetrameric-MHC complexes containing the gp100 and tyrosinase epitopes. Furthermore, tumor-associated antigen (TAA) specific functional responses by specific production of T-helper (Th)1 cytokines and no Th2 cytokines were measured in all patients. For the whole study population, tetramer-positive T cells against one or more epitopes, were found in 18 patients (58%) and a functional T cell response was seen in 38% of patients. Patients treated with adjuvant DC vaccination for stage III melanoma showed significantly more tumor-specific T cell responses than patients treated with DC vaccination for stage IV disease, 72% versus 33% (p = 0.009). However, no significant difference in OS since start of ipilimumab was found between patients, with or without, tumor-specific T cells in DTH skin-test biopsy sites; median OS 11.5 mo versus 8.1 mo (p = 0.476) for the whole study population. Additionally, no difference was found when only the patients pre-treated with adjuvant DC vaccination for stage III disease were analyzed (p = 0.883).
Immune-related adverse events of ipilimumab
Immune-related adverse events of any grade occurred in 58% of the patients (). The most common immune-related adverse events were dermatitis (29%) and diarrhea/colitis (27%). The incidence of immune-related grade 3 adverse events was 19%, while no grade 4 events were reported. Immune-related adverse events of any grade that led to discontinuation of ipilimumab occurred in 10% of the patients. One death due to the toxic effects of ipilimumab was reported (bowel perforation caused by inflammatory colitis).
Table 3. Immune-related adverse events ipilimumab. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.02. No grade 4 adverse events were reported. One death was determined by the investigators to be related to ipilimumab (inflammatory colitis with bowel perforation).
Discussion
This retrospective study showed that ipilimumab resulted in effective clinical responses in metastatic melanoma patients who presented with advanced melanoma after previous adjuvant DC vaccination for stage III disease, while ipilimumab had only limited effect on survival in patients with progressive disease on DC vaccination for stage IV disease. The characteristics of the study participants were comparable with those of the landmark studies with ipilimumab, especially with regard to M-stage, LDH level and brain metastases.Citation3,4,23 More than 70% of the patients had M1c disease and more than 30% had an elevated LDH, both of which are associated with poor survival.Citation24,25 The results of this study support the recent findings about the unlikely long-term benefit of ipilimumab treatment for patients with raised baseline serum LDH levels.Citation26,27
Ipilimumab-treated advanced melanoma patients pre-treated with adjuvant DC vaccination for stage III disease showed significantly better PFS and OS rates as compared to ipilimumab-treated patients who progressed on DC vaccination for stage IV melanoma. This might partly be caused by the difference in systemic line of treatment of ipilimumab between both groups. Theoretically, this difference might also be explained by a more effective induction of TAA-specific T cells in patients receiving adjuvant DC vaccination. However, no difference in survival was found between patients with or without TAA-specific T cells after DC vaccination. The time between induction of these T cells and start of ipilimumab might be too long in some patients to benefit from a potential synergy, or the sample size of this study was too small to detect a significant difference. Another reason might be that strengthened T cell responses by ipilimumab were not captured in our method of immunomonitoring. Patients treated with ipilimumab after adjuvant DC vaccination for stage III melanoma showed a best objective response rate of 22%, an estimated 64% of patients were still alive at 1 y, and an estimated 35% of patients were free of progression at 2 y after start of ipilimumab. Despite the small number of patients included in this retrospective analysis, these survival rates are promising in light of the results of phase three trials with ipilimumab, since these studies showed 1-y OS rates of 45.6–47.3%, and 2-y PFS rates of around 12%.Citation3,4 A retrospective study with stage III melanoma patients showed a significantly better OS in patients treated with DC vaccination compared to matched controls who only received surgery.Citation11 Of course, these results have to be confirmed in a prospective randomized clinical trial and compared to the results of trials with adjuvant ipilimumabCitation28 and anti-PD-1 mAb (NCT02388906, NCT02362594). However, the fact that patients with recurrent disease on adjuvant DC vaccination still respond to ipilimumab might be an argument to choose for adjuvant DC vaccination over adjuvant treatment with ipilimumab when comparable clinical outcomes are found in these adjuvant trials, since it increases the treatment-options in case of stage IV disease after adjuvant treatment for stage III disease.
In contrast to patients pre-treated with adjuvant DC vaccination for stage III melanoma, patients who showed progressive disease after DC vaccination for stage IV disease responded very poorly to ipilimumab treatment. Hodi and colleagues found a long-term OS in 20% of pre-treated melanoma patients,Citation3 while all patients pre-treated with DC vaccination for stage IV disease showed progressive disease within 2 y after start of ipilimumab. In theory, this difference might be explained by a selection of patients who are less prone to immunotherapy, since only patients with progressive disease after DC vaccination were included in this study, whereas the patients in the study of Hodi were predominantly pre-treated with standard of care chemotherapy.Citation3 However, long-lasting clinical responses were rare in trials with monotherapy DC vaccination in stage IV melanoma patient,Citation14,29 which makes it unlikely that this selection bias is the sole cause of the limited effect of ipilimumab in these patients. Furthermore, the poor response to ipilimumab may be caused by the fact that more than half of the patients received ipilimumab as third or fourth line therapy. Then again, all patients showed progressive disease on ipilimumab, including the patients who received ipilimumab as second line treatment. So, besides the rare long-lasting clinical responses of DC vaccination in stage IV melanoma, the results of this study point out that monotherapy DC vaccination before ipilimumab is not preferred in stage IV patients. As an alternative, DC vaccination might be a good option as combination-therapy with ipilimumab in stage IV melanoma patients, mainly due to its favorable toxicity profile and the fact that functionality of tumor-specific T cells induced by DC vaccination could be enhanced by blocking the immune checkpoint CTLA-4.Citation30 Murine studies support the concept that anti-CTLA-4 increases the frequency of activated T cells and causes a favorable effector T cell to regulatory T cell ratio.Citation31,32 However, not all early human studies combining anti-CTLA-4 with different forms of vaccines showed positive immunological and clinical results.Citation30 This suggests that potent vaccines are required to benefit from the combination with anti-CTLA-4. DC vaccination has proven to be an effective method of inducing tumor-specific T cell responses.Citation29 Wilgenhof and colleagues recently showed tolerability and encouraging antitumor activity, with an objective response rate of 38%, in a phase 2 study of autologous mRNA electroporated DCs in combination with ipilimumab in patients with pre-treated stage IV melanoma.Citation33 Further clinical investigation with the combination of DC vaccination and immune checkpoint inhibitors is warranted to determine its position in the treatment of advanced melanoma. The choice of antigens to load the DCs will probably become of great importance in these future trials, since recent findings showed that neoantigen-specific T cell reactivity may be critical to the effect of checkpoint blockade.Citation34 Carreno and colleagues showed it was possible to increase the breadth and diversity of melanoma neoantigen-specific T cells in peripheral blood samples of three advanced melanoma patients treated with a DC vaccine with carefully selected patient-specific neoantigens.Citation35
The immune-related adverse event profile of ipilimumab in patients pre-treated with DC vaccination is consistent with that reported in the previous literature; immune-related adverse events of any grade were seen in 58% of patients in this study as compared to 61–70% of patients in phase 2 and 3 trials with ipilimumab.Citation3,36,37 So, prior treatment with DC vaccination did not lead to more or worse immune-related adverse events. Patients with immune-related adverse events were treated by management algorithms with topical or systemic corticosteroids, and if necessary infliximab (antitumor necrosis factor α antibody) was given. Most immune-related adverse events were reversible with this approach, nevertheless one patient died due to a severe colitis with perforation.
In conclusion, this retrospective study showed a good clinical response to ipilimumab among advanced melanoma patients with progressive disease after adjuvant DC vaccination for stage III disease, while the effect was very limited in patients who showed progression after DC vaccination for stage IV disease. However, it is too early to conclude that prior DC vaccination for stage III disease has an additional effect on ipilimumab when given after progression upon DC vaccination, despite the fact that these patients showed promising response rates. Furthermore, pre-treatment with DC vaccination did not seem to cause an aggravation of immune-related adverse events. A prospective randomized clinical trial with DC vaccination has to show at least a comparable clinical outcome with adjuvant ipilimumab to support the future choice of DC vaccination in stage III melanoma patients, preserving ipilimumab in case of progression.
Materials and methods
Patient characteristics
We retrospectively analyzed patients who received ipilimumab for recurrent or progressive disease after pre-treatment in our DC vaccination trials. Ipilimumab was given between October 2008 and February 2015 and these patients were treated in various DC vaccination studies at our institute between February 2002 and September 2014; either as adjuvant therapy within 2 mo after radical lymph node dissection in case of stage III melanoma patients, or for patients with stage IV (advanced) melanoma. Patients treated with ipilimumab for metastatic uveal melanoma were excluded from analysis. All patients treated with DC vaccination followed by ipilimumab at some point in their course of disease, were considered to be eligible for analysis. One patient was treated with four cycles of 10 mg/kg ipilimumab every 3 weeks, followed by 10 mg/kg every 12 weeks as maintenance therapy. All other patients received four cycles of 3 mg/kg ipilimumab every 3 weeks, unless progression or severe side-effects occurred. Patients were staged according to the American Joint Committee on Cancer 2009 criteria.Citation38 All DC vaccination studies were approved by the appropriate Medical Ethical Review Board and written informed consent for these trials was obtained from all patients.
Dendritic cell vaccination
Patients were treated with either monocyte-derived autologous DCs or naturally circulating DCs loaded with TAA of gp100 and tyrosinase according to a schedule of three biweekly vaccinations. DCs were pulsed with 2 gp100-derived peptides (gp100:154–162 and gp100:280-288) and a tyrosinase-derived peptide (tyrosinase:369–377) or electroporated with mRNA encoding gp100 or tyrosinase, as described before.Citation39 Patients received two subsequent cycles of vaccinations at 6-mo intervals in absence of recurrent or progressive disease. For the exact details of the vaccination protocols, we refer to these individual studies (NCT02285413).Citation13,16,29,40-43
A DTH skin-test was performed within 2 weeks after each vaccination cycle as described previously.Citation12 Briefly, DC loaded with either gp100, tyrosinase or both antigens were injected intradermally in the skin of the back of patients at different sites, and after 48 h, punch biopsies (6 mm) were taken. SKILs were analyzed for antigen-specific T cells by staining them with tetrameric-MHC complexes containing the gp100 and tyrosinase epitopes (HLA-A*02:01 positive patients) and TAA-specific functional responses by specific production of Th1 cytokines and no Th2 cytokines to TAA, as described before.Citation12
Response evaluation and toxicity
Patients underwent clinical evaluation at baseline and prior to each ipilimumab infusion. Furthermore, during the treatment with ipilimumab, radiological evaluations (CT or PET/CT scanning) were performed at baseline and week 12, and during follow-up around every 3 mo or when disease progression was clinically suspected. This interval could be prolonged in individual cases with a durable response. Responses were scored by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. PFS (defined as the time from first cycle of ipilimumab to documented disease progression according to RECIST or death from any cause), OS (defined as the time from first cycle of ipilimumab to death from any cause) and the best objective response rate were analyzed.
Safety evaluations were performed in all patients and consisted of immune-related adverse events; they were scored using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.02. Serious adverse events were determined as grade 3 or 4. Patients were treated for immune-related adverse events according to local guidelines.
Statistical analysis
Survival was calculated from the start of ipilimumab to the date of progression (PFS) or death (OS) using the Kaplan–Meier method. Statistical significance was evaluated using a log-rank test. Cox proportional-hazard models were used to perform univariate and multivariate analysis of hazard ratios (HR). Multivariate survival analysis was applied to the significant variables of the univariate analysis. A Pearson correlation coefficient (r) was calculated to measure the relationship between the PFS on DC vaccination and the PFS on ipilimumab. p values less than 0.05 were considered significant. SPSS version 22 software (SPSS Inc., Chicago) and Graphpad Prism 5.03 (GraphPad Software inc, San Diego) were used for statistical analysis.
Disclosure of potential conflicts of interest
RHTK participated in advisory boards of Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, GlaxoSmithKline, Amgen. AJMvdE participated in advisory boards of Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, Novartis, Amgen. JBH participated in advisory boards of Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche, Novartis and Neon Therapeutics. JBH received research grants from Bristol-Myers Squibb, Merck Sharp & Dohme and Novartis. WRG received speakers fees from Astellas, Bayer, Bavarian Nordic, Bristol-Myers Squibb, Janssen-Cilag and ESMO; WRG participated in advisory boards of Amgen, Astellas, Bayer, Bristol-Myers Squibb, Dendreon, Janssen-Cilag, Morphosys, Sanofi and Transgene; WRG participated in ad hoc consultancy for Psioxus Therapeutics, Sotio and Transgene; WRG is founder of Carcinos (global oncology education: immunotherapy of cancer). The other authors declare to have no disclosures.
Acknowledgments
The authors thank the patients who participated in the DC vaccination studies.
Funding
This work is supported by a Radboudumc PhD grant, and Dutch Cancer Society (KWF) grant KWO-2009-4402. IJMdV is recipient of NWO-Vici grant 016.140.655, CGF received an NWO Spinoza award and ERC Advanced Grant PATHFINDER (269019).
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49:1374-403; PMID:23485231; http://dx.doi.org/10.1016/j.ejca.2012.12.027
- Dummer R, Schadendorf D, Ascierto PA, Larkin J, Lebbe C, Hauschild A. Integrating first-line treatment options into clinical practice: what's new in advanced melanoma? Melanoma Res 2015; 25:461-9; PMID:26426764; http://dx.doi.org/10.1097/CMR.0000000000000200
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; http://dx.doi.org/10.1200/JCO.2013.53.0105
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015; 372:2521-32; PMID:25891173; http://dx.doi.org/10.1056/NEJMoa1503093
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507-16; PMID:21639808; http://dx.doi.org/10.1056/NEJMoa1103782
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372:30-9; PMID:25399551; http://dx.doi.org/10.1056/NEJMoa1412690
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas L et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867-76; PMID:25265494; http://dx.doi.org/10.1056/NEJMoa1408868
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-67; PMID:24872109; http://dx.doi.org/10.1016/S1470-2045(13)70585-0
- Bol KF, Aarntzen EHJG, in't Hout FEM, Schreibelt G, Creemers JHA, Lesterhuis WJ, Gerritsen WR, Grunhagen DJ, Verhoef C, Punt CJA et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology 2016; 5:e1057673; PMID:26942068; http://dx.doi.org/10.1080/2162402X.2015.1057673
- Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, Van Rossum MM, Adema GJ, Figdor CG, Punt CJ, De Vries IJ. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res 2012; 72:6102-10; PMID:23010076; http://dx.doi.org/10.1158/0008-5472.CAN-12-2479
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/10.1158/0008-5472.CAN-12-1127
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39:38-48; PMID:23890062; http://dx.doi.org/10.1016/j.immuni.2013.07.004
- Bol KF, Tel J, de Vries IJ, Figdor CG. Naturally circulating dendritic cells to vaccinate cancer patients. Oncoimmunology 2013; 2:e23431; PMID:23802086; http://dx.doi.org/10.4161/onci.23431
- Bol KF, Figdor CG, Aarntzen EH, Welzen ME, van Rossum MM, Blokx WA, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM et al. Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients. Oncoimmunology 2015; 4:e1019197; PMID:26405571; http://dx.doi.org/10.1080/2162402X.2015.1019197
- Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev 2006; 213:131-45; PMID:16972901; http://dx.doi.org/10.1111/j.1600-065X.2006.00442.x
- Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 2012; 72:876-86; PMID:22174368; http://dx.doi.org/10.1158/0008-5472.CAN-11-1792
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015; 33:1889-94; PMID:25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo KM et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015; 33:3193-8; PMID:26282644; http://dx.doi.org/10.1200/JCO.2015.60.8448
- Pierret L, Wilgenhof S, Corthals J, Roelandt T, Thielemans K, Neyns B. Correlation between prior therapeutic dendritic cell vaccination and the outcome of patients with metastatic melanoma treated with ipilimumab. J Clin Oncol 2009; 27; abstr e20006.
- Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K et al. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother 2011; 60:1137-46; PMID:21465316; http://dx.doi.org/10.1007/s00262-011-1011-9
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Weide B, Richter S, Buttner P, Leiter U, Forschner A, Bauer J, Held L, Eigentler TK, Meier F, Garbe C. Serum S100B, lactate dehydrogenase and brain metastasis are prognostic factors in patients with distant melanoma metastasis and systemic therapy. PloS one 2013; 8:e81624; PMID:24312329; http://dx.doi.org/10.1371/journal.pone.0081624
- Bedikian AY, Johnson MM, Warneke CL, Papadopoulos NE, Kim K, Hwu WJ, McIntyre S, Hwu P. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest 2008; 26:624-33; PMID:18584354; http://dx.doi.org/10.1080/07357900802027073
- Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, Kapiteijn EW, de Groot JW, Soetekouw P, Jansen RL et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63:449-58; PMID:24609989; http://dx.doi.org/10.1007/s00262-014-1528-9
- Martens A, Wistuba-Hamprecht K, Geukes Foppen MH, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016; 22(12):2908-18; PMID:26787752; http://dx.doi.org/10.1158/1078-0432.CCR-15-2412
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16:522-30; PMID:25840693; http://dx.doi.org/10.1016/S1470-2045(15)70122-1
- Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, van Rossum MM, Blokx WA, Jacobs JF, Duiveman-de Boer T et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res 2012; 18:5460-70; PMID:22896657; http://dx.doi.org/10.1158/1078-0432.CCR-11-3368
- Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine 2015; 33:7377-85; PMID:26482147; http://dx.doi.org/10.1016/j.vaccine.2015.10.057
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 1999; 190:355-66; PMID:10430624; http://dx.doi.org/10.1084/jem.190.3.355
- Avogadri F, Zappasodi R, Yang A, Budhu S, Malandro N, Hirschhorn-Cymerman D, Tiwari S, Maughan MF, Olmsted R, Wolchok JD et al. Combination of alphavirus replicon particle-based vaccination with immunomodulatory antibodies: therapeutic activity in the B16 melanoma mouse model and immune correlates. Cancer Immunol Res 2014; 2:448-58; PMID:24795357; http://dx.doi.org/10.1158/2326-6066.CIR-13-0220
- Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. J Clin Oncol 2016; 34(12):1330-8; PMID:26926680; http://dx.doi.org/10.1200/JCO.2015.63.4121
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348:69-74; PMID:25838375; http://dx.doi.org/10.1126/science.aaa4971
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015; 348:803-8; PMID:25837513; http://dx.doi.org/10.1126/science.aaa3828
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11:155-64; PMID:20004617; http://dx.doi.org/10.1016/S1470-2045(09)70334-1
- O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol 2010; 21:1712-7; PMID:20147741; http://dx.doi.org/10.1093/annonc/mdq013
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199-206; PMID:19917835; http://dx.doi.org/10.1200/JCO.2009.23.4799
- Schuurhuis DH, Verdijk P, Schreibelt G, Aarntzen EH, Scharenborg N, de Boer A, van de Rakt MW, Kerkhoff M, Gerritsen MJ, Eijckeler F et al. In situ expression of tumor antigens by messenger RNA-electroporated dendritic cells in lymph nodes of melanoma patients. Cancer Res 2009; 69:2927-34; PMID:19318559; http://dx.doi.org/10.1158/0008-5472.CAN-08-3920
- Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, Scharenborg NM, van de Rakt MW, de Boer AJ, Croockewit S et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res 2011; 17:5725-35; PMID:21771874; http://dx.doi.org/10.1158/1078-0432.CCR-11-1261
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013; 73:1063-75; PMID:23345163; http://dx.doi.org/10.1158/0008-5472.CAN-12-2583
- Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res 2015; 22:2155-66; PMID:26712687; http://dx.doi.org/10.1158/1078-0432.CCR-15-2205
- Bol KF, Aarntzen EH, Pots JM, Olde Nordkamp MA, van de Rakt MW, Scharenborg NM, de Boer AJ, van Oorschot TG, Croockewit SA, Blokx WA et al. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer Immunol Immunother 2016; 65:327-39; PMID:26861670; http://dx.doi.org/10.1007/s00262-016-1796-7
