 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
MEDI9447 is a human monoclonal antibody that is specific for the ectoenzyme CD73 and currently undergoing Phase I clinical trials. Here we show that MEDI9447 is a potent inhibitor of CD73 ectonucleotidase activity, with wide ranging immune regulatory consequences. MEDI9447 results in relief from adenosine monophosphate (AMP)-mediated lymphocyte suppression in vitro and inhibition of mouse syngeneic tumor growth in vivo. In contrast with other cancer immunotherapy agents such as checkpoint inhibitors or T-cell agonists, MEDI9447 drives changes in both myeloid and lymphoid infiltrating leukocyte populations within the tumor microenvironment of mouse models. Changes include significant alterations in a number of tumor micro-environmental subpopulations including increases in CD8+ effector cells and activated macrophages. Furthermore, these changes correlate directly with responder and non-responder subpopulations within animal studies using syngeneic tumors. Combination data showing additive activity between MEDI9447 and anti-PD-1 antibodies using human cells in vitro and mouse tumor models further demonstrate the potential value of relieving adenosine-mediated immunosuppression. Based on these data, a Phase I study to test the safety, tolerability, and clinical activity of MEDI9447 in cancer patients was initiated (NCT02503774).
Introduction
CD73 (Cluster of Differentiation 73), also known as ecto-5'-nucleotidase (NT5E), is a glycophosphatidylinositol-anchored receptor found on tumor cells as well as on stromal cells such as endothelial cells and certain leukocytes. This enzyme catalyzes the conversion of adenosine monophosphate (AMP) to adenosine and organic phosphate. Binding of adenosine to the extracellular portion of adenosine receptors triggers signaling through cyclic AMP to inhibit T-cell receptor activation (reviewed in refCitation1). CD73 is believed to play a role in mediating the inhibitory function of regulatory B and T lymphocytes Citation2 as well as in maintaining endothelial integrity (reviewed in ref.Citation3).
In addition to their roles in the biology of healthy tissue, CD73 and adenosine each affect tumor biology. The presence of extracellular adenosine within the tumor microenvironment has been described as an immunosuppressive “halo” Citation4 surrounding the tumor and permeating the tumor microenvironment and preventing antitumor immunity. Consistent with this role for adenosine, knockout mice lacking adenosine receptors have been shown to reject tumors more readily than normal mice Citation5 and knockout mice lacking CD73 have increased antitumor immunity Citation6 and show decreased carcinogenesis Citation7 when compared with normal mice. Specifically, extracellular adenosine is believed to mediate the immunosuppressive effects of both regulatory T cells and myeloid-derived suppressor cells (MDSCs), among others (reviewed in ref.Citation8). Recent reports showing tumor growth inhibition with adenosine receptor inhibitors Citation9,10,11,12 further support a role for adenosine in promoting tumor growth. Taken together with other studies showing that molecular inhibition of CD73 with small molecules or antibodies can inhibit tumor formation, growth, and metastasis, it has been hypothesized that tumors use CD73 to generate adenosine and, thereby, suppress antitumor immunity (reviewed in ref.Citation10).
MEDI9447 is a human IgG1λ monoclonal antibody that selectively binds to and inhibits the ectonucleotidase activity of CD73. Experiments described here show the ability of MEDI9447 to reduce immunosuppression in a variety of functional settings. Together, these data offer novel insights into the role of the immune system in the control of tumor growth and further support CD73 as an attractive target for therapeutic intervention. They represent the first functional characterization in vivo of MEDI9447, a fully human antibody that cross reacts with both mouse and human CD73. Furthermore, these results expand upon the number of tumor microenvironment components known to be involved with adenosine-mediated immunosuppression.
Results
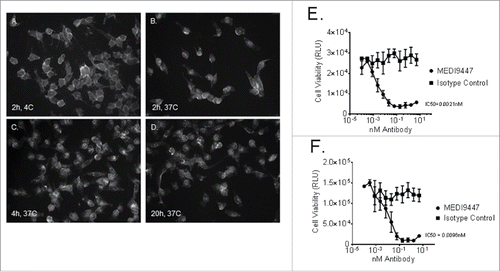
MEDI9447 is a human IgG1λ monoclonal antibody isolated by phage display containing the triple mutation (TM) in the constant region of the heavy chain to reduce IgG effector function.Citation13 MEDI9447 inhibition of CD73 enzymatic activity was tested in a cell-based assay. The ability of cell surface-expressed CD73 to reduce AMP levels in cell culture supernatants showed dose-dependent inhibition with MEDI9447 but not with an irrelevant isotype control antibody as shown in using both human (NCI-H322, Panel A) and mouse (4T1, Panel B) non-small cell lung carcinoma and breast cell lines, respectively. In addition to inhibiting CD73 enzymatic activity, the ability of MEDI9447 to internalize CD73 upon binding was demonstrated by two methods as shown in . Using immunofluorescence, CD73 localization was observed to shift from cell surface staining () to an increasingly intracellular pattern over 20 h () of incubation at 37°C when MEDI9447 was used as the primary antibody. Similarly, internalization of CD73 resulted in cytotoxicity of human MDA-MB-231 () and mouse 4T1 () breast cancer cells when MEDI9447, but not an isotype control antibody, was incubated with these cells in the presence of a toxin-conjugated secondary antibody. Cell killing was both concentration-dependent and specific when compared to cells incubated with an isotype control antibody, R3-47.
Figure 1. CD73-expressing human NCI-H322 (Panel A) or mouse 4T1 (Panel B) cells were incubated with AMP and MEDI9447 or an isotype control antibody at the indicated concentrations. Following incubation, ATP and CellTiter-Glo® were added and light emission inhibition was measured by luminometer. The mean signal of duplicate samples is plotted with error bars representing the standard deviation of the mean. Data are representative of five independent experiments.
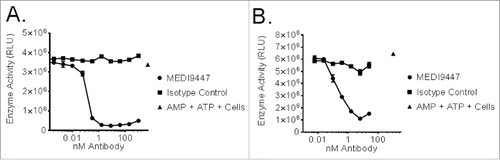
Figure 2. Internalization of CD73 upon binding by MEDI9447 was demonstrated. MDA-MB-231 human breast carcinoma cells were incubated with MEDI9447 and a fluorescently labeled anti-human IgG secondary antibody. Localization of CD73 was imaged after 2 h at 4°C (Panel A) or 37°C and again after 4 (Panel C) and 20 (Panel D) h at 37°C. Internalization of CD73 upon MEDI9447 binding was also demonstrated by measuring MDA-MB-231 (Panel E) and mouse 4T1 (Panel F) breast cancer cell viability following MEDI9447 binding in the presence of a toxin-conjugated secondary antibody. The indicated concentrations of MEDI9447 were incubated with a saporin-conjugated anti-human IgG antibody for 3 d and viability was estimated by measuring ATP levels using the Cell Titer-Glo assay kit. Data are representative of five independent experiments.
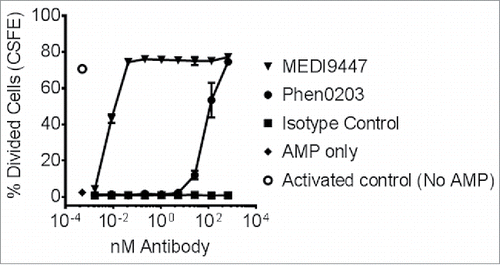
Beyond CD73 enzyme inhibition and internalization, the functional effect upon T-cell proliferation of CD73 inhibition by MEDI9447 was examined. Production of adenosine by CD73 from AMP was modeled in vitro using purified CD4+ T cells activated by TCR-signaling in the presence of AMP. T-cell proliferation was suppressed in the presence of 100 µM of extracellular AMP and this suppression was relieved by addition of MEDI9447 but not an isotype control antibody (). The potency of MEDI9447 was at least two orders of magnitude greater than that of Phen0203, a previously reported Citation14 anti-CD73 antibody—a difference which may reflect the greater than 20-fold difference in affinity for human CD73 between MEDI9447 and Phen0203 (see Fig. S2). These data suggest that binding of an anti-CD73 antibody was able to block or decrease the generation of adenosine from AMP and the subsequent inhibitory effect of adenosine on T-cell function.
Figure 3. CFSE-labeled CD4+ T cells were pre-activated with anti-CD3 and anti-CD28 antibody-coated microbeads and recombinant human IL-2 and then transferred into sterile round-bottomed tissue culture 96 well plates at approximately 50,000 cells per well. T cell proliferation and division was suppressed by the addition of 100-µM AMP. Addition of MEDI9447 and Phen0203 human IgG1 overcame the inhibitory effect of AMP in a concentration-dependent manner. Isotype control antibody R3-47 had no effect. The average of triplicate samples is plotted and error bars represent the standard deviation. Data are representative of three independent experiment.
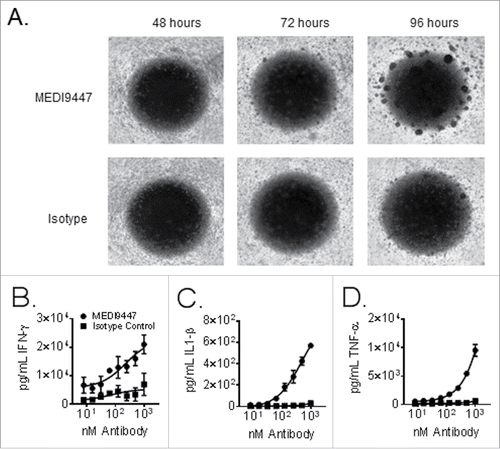
In an effort to examine the role of CD73 inhibition by MEDI9447 beyond that of a purified T lymphocyte system, MEDI9447 was tested in an assay context comprising additional aspects of the human immune response: the two-way mixed leukocyte reaction (MLR). As shown in Panel (A) of , MEDI9447 enhanced both the timing and extent of leukocyte clustering when incubated with equal proportions of peripheral blood mononuclear cells (PBMCs) from two healthy donors in a single microtiter plate well. Consistent with enhanced antigen presentation and lymphocyte activation, levels of Th1 cytokines in the supernatants of MLR wells were increased by MEDI9447. Specifically, levels of interferon-γ (), interleukin 1-β (), and tumor necrosis factor-α () increased with increasing levels of MEDI9447 and showed a relatively smaller increase in response to similar levels of an isotype control antibody. Similar results were observed across 50 different normal donor pairs of normal healthy PBMCs (Fig. S3).
Figure 4. Equal proportions of peripheral blood mononuclear cells from two healthy donors were mixed and incubated in wells of a 96 well plate for 96 h. Panel (A) shows brightfield images were taken at 24 h intervals using a 2.5× objective. Cells were treated with 150 µg/mL of either MEDI9447 (top panel) or and isotype control antibody (bottom panel). Panels (B–D). Equal proportions of peripheral blood mononuclear cells from two healthy donors were mixed incubated in wells of a 96 well plate for 72 h. Cells were treated with the indicated concentrations of either MEDI9447 (circles) or an isotype control antibody (squares). The plate was centrifuged to pellet cells and interferon-γ (Panel B), interleukin-1 β (Panel C), and tumor necrosis factor-α (Panel D) levels in the supernatants were measured by ELISA. Data are representative of experiments involving over 50 different donor-pair combinations.
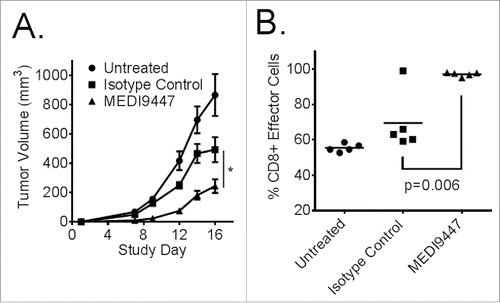
MEDI9447 antitumor activity was tested in vivo using the murine, Balb/c, syngeneic CT26 colon carcinoma tumor model. Test article and control groups were implanted with 5 × 10Citation5 CT26 cells subcutaneously on the right flank and treated intraperitoneally twice weekly for 2 weeks starting 3 d after tumor cell implantation. Data shown in indicate that 10 mg/kg MEDI9447 significantly (p < 0.05) inhibited tumor growth by 50% or greater from study day 7 to study day 16 when compared with isotype control antibody-treated groups, which showed some tumor volume reduction compared with untreated animals. Consistent with reduced tumor growth, MEDI9447-treated tumors showed a significantly (p < 0.05) larger proportion of activated CD8+ lymphocytes () when the phenotypes of tumor-infiltrating leukocytes (TIL) were analyzed by flow cytometry.
Figure 5. (A). Murine CT26 colon carcinoma tumor cells were implanted subcutaneously then treated at 10 mg/kg with MEDI9447, or an isotype control (or untreated), by intraperitoneal injection on days 3, 6, 10, and 13 post-tumor implantation. An untreated control group was also included. The mean tumor volumes of each 10 animal group are plotted with error bars representing the standard error of the mean. Asterisks indicate a statistically significant (*p < 0.05) reduction in tumor volume. (B). Tumor-infiltrating leukocytes were isolated at study day 16 and analyzed by flow cytometry. CD8+ T effector cells were significantly increased in the tumor following treatment with MEDI9447. Data are representative of three independent experiments.
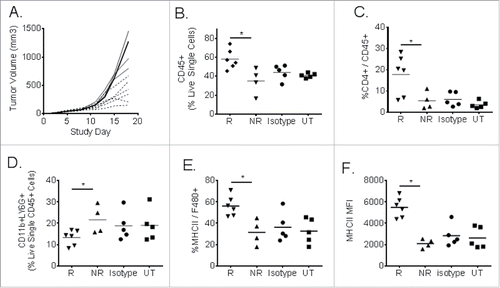
Individual tumors in syngeneic mouse studies showed a range of responses, by volume, to antibody treatment. In order to examine potential pharmacodynamic markers of response to MEDI9447-mediated tumor growth control, TIL were isolated and studied. In an experiment similar to that shown in , syngeneic CT26 tumors were treated with MEDI9447 or isotype control antibodies starting 3 d after tumor cell implantation. As shown in Panel (A) of , individual tumors showed a range of sizes among the 10 animals treated with MEDI9447. Day 18 was chosen for tumor harvest because tumor sizes appeared to divide MEDI9447-treated animals into two subpopulations of “responder” and “non-responder” mice. Analysis of TIL revealed that as many as 70% of the cells in a given tumor treated with MEDI9447 were CD45+ leukocytes, rather than CT26 tumor cells (). Based upon both tumor volume as well as the proportion of non-tumor infiltrate, each of the 10 animals treated with MEDI9447 were grouped into “responder” (six animals) and “non-responder” (four animals) subgroups for subsequent phenotypic analysis. Untreated and isotype antibody-treated tumors did not show the same range of tumor volumes and therefore could not be grouped by response. Cell surface phenotypic data shown in revealed MEDI9447-specific pharmacodynamic changes specific to the “responder” group across both lymphoid and myeloid tumor-infiltrating immune cell subpopulations. Specifically, the proportion of CD4+ () infiltrating lymphocytes was significantly (p < 0.05) higher in the responsive tumors when compared with either non-responsive tumors or tumors in animals treated with an isotype control antibody or left untreated. This response was also seen in the CD8+ TIL fraction (data not shown; see also ). Similarly, the proportion of CD11b, LY6g+ granulocytic monocytes was significantly reduced compared to non-responders and control-treated tumors as shown in Panel (D) of . Finally, both the proportion of MHC class II positive—a marker of enhanced potential for antigen presentation—macrophages () and the absolute mean fluorescent signal () were significantly higher (p < 0.05) in tumors that responded to MEDI9447.
Figure 6. CT26 tumor-infiltrating leukocyte analysis from individual responder (R) and non-responder (NR) mice following MEDI9447 treatment. Murine CT26 colon carcinoma tumors cells were implanted subcutaneously then treated at 10 mg/kg with MEDI9447 by intraperitoneal injection on days 3, 6, 10, and 13 post-tumor implantation. Panel (A). Tumor volumes for individual mice treated with MEDI9447. Whole tumors were removed, dissociated into single cell suspensions, and analyzed by flow cytometry. The fraction of the following subpopulations were determined: total leukocytes (CD45+, Panel B), CD4+ lymphocytes (Panel C), monocyte-derived suppressor cells (Panel D), major histocompatibility (MHCII, Panel E ) expressed on F480+ macrophages as well as the mean fluorescent intensity of MHC2 (Panel F). Each symbol represents the phenotype indicated in the y axis for individual mice. Asterisks indicate significant (p < 0.05) differences between treatment arms. Data are representative of two independent experiments.
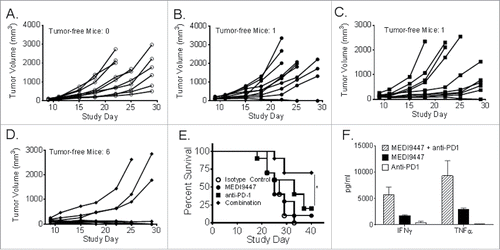
In order to compare the role of anti-CD73 with that of a checkpoint inhibitor antibody, CT26 cells were implanted into syngeneic Balb/c mice and left untreated or treated with an isotype control antibody, anti-CD73 (MEDI9447 mIgG1), anti-PD-1, or a combination of both antibodies. Tumor volumes for individual animals were plotted for individual animals up to study day 29 (). Injection of 10-mg/kg anti-CD73 or anti-PD-1 achieved tumor rejection in only a single animal from each group of 10 mice treated (). In contrast with single antibody injections, six out of ten mice treated with a combination of anti-CD73 and anti-PD-1 rejected their tumors. By study day 40, the proportion of surviving mice was significantly greater for mice treated with the antibody combination when compared with individual antibody treatments (). In similar experiments using “established” tumor sizes of 150 mm3 or greater, enhanced tumor rejection from combined antibody-targeting of CD73 and PD-1 was not consistently observed (Fig. S4). However, even in the absence of additive tumor rejection, an enhanced CD8+ lymphocyte response to the CT26 tumor cell-specific antigen AH1 was observed upon combination with anti-CD73 and anti-PD-1 antibodies (Fig. S4, Panel C). To test whether the combination effects were reflected in human samples, MEDI9447 was combined with anti-PD-1 in MLRs identical to those described in . The combination of MEDI9447 and an anti-PD-1 antibody induced greater than additive levels of IFNγ and TNFα when compared with either antibody alone ().
Figure 7. Murine CT26 colon carcinoma tumor cells were implanted subcutaneously and then treated at 10 mg/kg, by intraperitoneal injection on days 3, 7, 10, and 14 post-tumor implantation, with isotype control antibody (Panel A, open circles), MEDI9447 (Panel A, closed circles), anti-PD-1 (Panel B, squares) antibodies or a combination of MEDI9447 and anti-PD-1 (Panel C, diamonds) antibodies. Tumor volumes for individual animals are plotted until study day 29. The proportion of surviving mice after 40 d (Panel E) and the number of tumor-free mice in each treatment arm are shown. A 72-h mixed leukocyte reaction was performed using human peripheral blood mononuclear cells in the presence of anti-PD-1, MEDI944 or a combination of each antibody. The mean levels of secreted IFNγ and TNFα from duplicate samples are plotted (Panel F). The asterisk indicates a significant (p < 0.05) increase in survival. Data are representative of four independent experiments.
Discussion
These studies contribute new insight into the role of CD73 within the tumor microenvironment and further demonstrate the potential of targeting CD73 for immunotherapy of cancer. As a small diffusible molecule, adenosine is expected to permeate the tumor microenvironment. Therefore, adenosine effects upon tumor infiltrating immune cells are expected to be as pervasive as the expression of adenosine receptors themselves. This suggests that targeting CD73 in the adenosine signaling axis may have a wider-ranging impact upon the tumor microenvironment when compared with various other immune-oncology targets. For example, immune checkpoint inhibitors such as anti-CTLA-4 or anti-PD-1 antibodies stimulate anti-tumor immunity primarily through tumor-specific lymphocytes. Citation15 IDO or TDO inhibitors as well as CD40 agonists are believed to enhance antigen presentation. Citation16-18 Similarly, agonists of the tissue necrosis receptor superfamily such as OX40, CD137, or GITR act primarily through lymphocyte activation and possibly through overcoming suppression by regulatory T cells. Citation19 In contrast to each of these specialized systems, adenosine may sculpt the antitumor immune response by multiple mechanisms. This suggests that even with pharmacologic antagonism or agonism of the various individual specialized immune regulatory or costimulatory pathways, antitumor immune control of tumor growth could still be hampered by the presence of adenosine in the tumor microenvironment. Consistent with this hypothesis, combined use of anti-CD73 Citation20 antibodies or A2AR inhibitors Citation9,10 has been shown to complement antitumor activity of both anti-PD-1 and anti-CTLA-4 antibodies in syngeneic tumor models. Data reported here confirm the wide-ranging role of CD73-derived adenosine on the phenotype of both lymphoid and myeloid-derived cells in shaping both the innate and adaptive arms of antitumor immunity.
The antitumor activity of MEDI9447 can reasonably be linked with its ability to inhibit conversion of AMP to adenosine using in vitro assay systems such as those described in this report: direct cellular enyzme inhibition, removal of CD73 from the cell surface by internalization, and relief from AMP-mediated inhibition of T cell proliferation. However, data showing tumor growth inhibition by MEDI9447 described here do not rule out a role for anti-CD73 in the various ectonucleotidase-independent activities suggested for CD73 in lymphocyte activation Citation21,22,23 or cancer cell adhesion and metastasis. Citation24 In fact, internalization of CD73 as shown here supports both enzyme-dependent and independent mechanisms of action for MEDI9447 since the removal of CD73 from the extracellular tumor microenvironment prohibits CD73 activity by any mechanism in this context. It should be noted that MEDI9447 contains a TM that reduces Fc receptor engagement Citation13, thus de-emphasizing the role of clustering-based lymphocyte activation by anti-CD73 antibodies described by Resta and Thompson. Citation21 Likewise, the version of MEDI9447 used in these mouse studies contains a murine IgG1 Fc domain, which is also expected to show limited Fcγ receptor engagement in mice. Furthermore, these data are not mutually exclusive with similar studies performed with genetic and pharmacologic adenosine receptor inhibition, notwithstanding differences between manipulation of adenosine levels as compared with global receptor inhibition. Citation25,26
To the best of our knowledge, data presented here showing the effects of CD73 inhibition in a two-way MLR are the first of their kind. Although a similar primary lymphocyte reaction system has been reported for studying the immunosuppressive effects adenosine in vitro,Citation27 our studies include a the full array of myeloid and lymphoid cells present in primary PBMC samples. The apparent potency of MEDI9447 in the MLR was less than that observed in those experiments demonstrating relief from AMP-mediated lymphocyte proliferation. Beyond the fundamental differences in readout (CFSE vs. cytokine secretion), differences in potency could have arisen from the heterogeneous mixture of cell types present in the MLR. Changes in an MLR assay are the result of net changes arising from anti-CD73 binding upon each cellular subset present rather than upon purified CD4+ lymphocytes alone measured in the AMP suppression assay. In a mixed setting, it is possible that anti-CD73 could have opposing effects upon different subsets leading to an apparent net reduction in potency. The mechanism of action leading to MEDI9447 activity in the mixed leukocyte assay system could involve simple CD73 enzyme inhibition and/or from removal of the enzyme from the surface of leukocytes through internalization. Attempts to consistently demonstrate trends in leukocyte CD73 internalization were hampered by differences in CD73 expression both within and between MLR cellular subsets. MLR data presented here are important for at least three reasons. First, these data are consistent with the pervasive role believed to be played by adenosine in the immune system. Citation4 For example, the dose-dependent increases in the typically monocyte-derived cytokine IL-1β suggest that CD73 influence extends beyond simple lymphocyte effects shown by increases in interferon γ and tumor necrosis factor α levels. Second, these MLR assays did not require the addition of an exogenous adenosine source as was the case in the lymphocyte suppression assays described in this report. This suggests that the MLR could be used to study the mechanism of anti-CD73 antibody activity in vitro, including antibodies such as MEDI9447 that are not expected to signal through target clustering of CD73 due to their reduced ability to engage Fcγ receptors. Thirdly, combination effects observed in vivo by targeting both the PD-1 and CD73 axes were reflected in the MLR. Finally, the MLR represents a functional assay using primary human blood cells and yielding immunologically relevant readouts from both myeloid and lymphoid compartments that does not require extensive cell subset purification and separation. Thus, it offers an opportunity for relatively high throughput, multiplexed screening of compounds that influence CD73 and other adenosine-linked immunological signaling pathways such T cell checkpoint inhibitors. Furthermore, PBMCs from numerous healthy and cancer patient donors can be surveyed for profiles correlating with drug “responder” and “non-responder” populations.
Data presented in this report complement those of related studies. MEDI9447 inhibition of CD73 enzyme activity in a cell surface format assay was consistent with data reported earlier using purified recombinant CD73. Citation28 Relief from AMP-mediated suppression of T-cell proliferation in vitro by MEDI9447 was similar to that reported by Jin and co-workers Citation29 using ovarian cancer cell line-derived CD73 and either adenosine receptor inhibitor SCH58261 or CD73 antagonist α,β-methylene-ADP. Similar to the MC38OVA model described by Allard et al.,Citation20 CT26 mouse colorectal carcinoma cells express very low levels of surface CD73. Therefore, MEDI9447 effects in the CT26 model emphasize the role of non-tumoral sources of CD73, such as stromal or infiltrating immune cells in suppressing antitumor immunity. Specifically, the role of B cell CD73 was not examined in these studies although it is reasonable to expect CD73 from B cells to influence antitumor immunity. Citation2,30 The demonstrated reduction in cells with an MDSC phenotype within CT26 tumors is consistent with studies indicating CD73 involvement with granulocytic MDSCs. Citation31 These changes as well as the observed increase in MHC class II-expressing infiltrating monocytes are important in the light of other studies showing that extracellular adenosine promotes a “permissive” tumor microenvironment. Citation32 Conversely, the MEDI9447-mediated CT26 tumor-infiltration by CD8+ lymphocytes shown here compares with reports showing enhanced infiltration of solid tumors by functional cytotoxic T cells following CD73 knockdown or adenosine receptor inhibition. Citation12,29 As such, effects upon leukocyte infiltration described in these experiments support a potential role for CD73 and/or adenosine receptor inhibition in successful adoptive cell therapy. Citation33 Together, these data suggest a role for CD73 inhibition in both Type I and Type IV tumors as classified by Teng et al. Citation34
Data reported here are the first to functionally characterize an antibody targeting both human and murine CD73. As such, they offer an opportunity to demonstrate antitumor activity in mouse models as well as their relevant correlates in human primary cancer and immune cells. For example, potentially confounding variables are avoided by using the identical variable regions in both mouse and human model systems. For example, the TY23 rat anti-mouse CD73 antibody has been studied widely in murine xenografts and syngeneic models. As a rat IgG2, the contribution of murine Fcγ receptor engagement by TY23 influences conclusions about antitumor efficacy with this antibody. In studies described here, the use of MEDI9447 human variable domains with the relatively low Fcγ binding capacity of a wild-type mouse IgG1 Fc domain focuses conclusions upon the direct reduction of CD73 enzyme activity rather than upon Fcγ receptor engagement and subsequent target clustering. Differences between epitopes bound by tool antibodies such as mouse-cross-reactive TY23 and human cross-reactive 1E9 Citation35 or 4G4 Citation36 antibodies raise questions when comparing studies targeting mouse and human CD73, respectively. Even when comparing two human-specific antibodies, the functional importance of binding different CD73 epitopes has been demonstrated. Citation37 By using a single antibody capable of binding both human and mouse CD73, the data reported here link human and mouse tumor biology more closely than previous studies.
Finally, these combination data extend understanding of the relative contributions of the PD1 checkpoint axis and adenosine signaling in suppressing antitumor immunity. The experiments using CT26 syngeneic tumors reported here are consistent with the combination activity reported by Allard et al. Citation20 using the MC38OVA and RM-1 models. The current studies build upon various aspects of the previous reports. First, the subcutaneous mouse CT26 syngeneic model system does not make use of the OVA allo-antigen, suggesting that all observed antitumor immunity in these studies arose from endogenous murine or other antigens expressed by non-engineered CT26 cells. The in vivo antitumor immunity demonstrated here with MEDI9447 arises from the identical epitope/variable domain shown to modulate human lymphocyte activation, rather than from a tool antibody that does not bind human CD73. These data support the hypothesis that the adenosine axis may serve as an “escape” pathway for T-cell receptor signal inhibition in tumors failing to respond to PD-1 axis inhibitors as suggested by Lloyd. Citation38 In summary, the data reported here introduce a novel antibody suitable for the study and promotion of therapeutic antitumor immunity based upon CD73 inhibition. Further studies are needed to dissect the intracellular signaling pathways involved with the PD-1 and adenosine signaling axes in different tumor micro-environmental subsets. Taken together, these data form the preclinical basis for ongoing clinical trials (NCT02503774) using MEDI9447.
Materials and methods
Cell lines and antibodies
CT26 mouse colorectal carcinoma (CD73low) cells, 4T1 mouse breast carcinoma cells (CD73high), MDA-MB-231 (CD73 high) human breast carcinoma. and NCI-H322 (CD73high) non-small cell lung carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in 10% RPMI (ThermoFisher Scientific, Waltham, MA) medium with penicillin, streptomycin, and 10% fetal bovine serum.
Cell lines were authenticated by testing for cross-species contamination (rat, human, hamster, and monkey) and identity (allele size) using short tandem repeat DNA profiling (IDEXX Bioresearch). Healthy control subjects supplying PBMCs consisted of healthy MedImmune or AstraZeneca employees who were anonymously enrolled in the MedImmune Research Specimen Collection Program. Donors with HIV infection, hepatitis B or C virus, Human T-lymphotropic virus, or syphilis were excluded. Written consent for blood draws was obtained from the donor. All protocols and informed consent forms were approved by Chesapeake Institutional Review Board. MEDI9447 is a human IgG1λ monoclonal antibody isolated from DP47 library Citation39 by phage display panning on human and mouse recombinant CD73 protein (Pavlik et al., manuscript in preparation). A heavy chain constant region encodes the TM to reduce IgG effector function. Citation40 Mouse anti-human CD73 antibody Phen0203 was discovered at MedImmune and has been described previously. Citation14 Antibodies used in flow cytometry included CD45 (BD Biosciences cat# 561487), CD8+ (Biolegend cat#100747), F4/80 (eBiosciences cat# 45-4801-82), CD11b (BD Biosciences cat# 562287, Gr1 (Biolegend cat# 108422), MHC class II (eBioscience cat# 11-5980-85), and Ly6g (Biolegend cat# 127624). For the MLR, the anti-PD1 antibody used was AMP-514 (internally supplied). For experiments with mouse models, a version of MEDI9447 was used in which a mouse IgG1 Fc domain was substituted for the human IgG1 Fc domain (mIgG1 MEDI9447).
Enzyme inhibition assay
CD73 enzyme activity was measured by AMP (substrate) depletion as described previously. The CD73-mediated decrease in AMP within the assay system resulted in relief from AMP-mediated inhibition of ATP catabolism to AMP and organic by phosphate luciferase. Thus, in this system, luciferase activity is directly proportional to CD73 ectonucleotidase activity. Citation41 Briefly, CD73-expressing NCI-H322 and 4T1 cells were centrifuged for 5 min at 1,500 × g. The supernatant was removed and replaced with serum-free RPMI medium. The cells were then plated into 96-well plates (Falcon 3788) at 10,000 cells per well in 100 µL of RPMI medium without additives. Antibodies were added in duplicate along with AMP (Sigma, 200-µM final concentration) and plates were incubated at 37°C, 5% CO2 for up to 24 h. Plates were then centrifuged for 3 min at 1,500 rpm. Supernatants were collected into a new 96 well plate (Costar #3605) and ATP was added to a final concentration of 100 µM. CellTiter-Glo® reagent (Promega, Madison, WI) was added 1:1 and cellular CD73 enzyme AMP phosphorylase activity was determined by measuring ATP levels by luciferase-emitted light using the Envision luminescence plate reader (Perkin Elmer). Buffer containing only ATP and AMP was used as a negative control.
Immunofluorescence internalization assay
Human MDA-MB-231 breast carcinoma cells were cultured in RPMI/10%FBS on coverslips. MEDI9447 was used as a primary anti-CD73 antibody and added to a concentration of 10 µg/mL and cells were incubated at 4C or 37°C. Cells were washed, fixed, and permeabilized for 20 min (Cytofix/Cytoperm kit, BD Biosciences), and stained with AlexaFluor488-conjugated donkey anti-human IgG antibody (Jackson Immunoresearch) for 30 min. After washing, coverslips were mounted in Vectashield with DAPI (Vector Laboratories) and visualized using a 10× objective.
Cytotoxicity-based internalization assay
Internalization of antibodies into cell lines MDA-MB-231 and 4T1 was measured using the Fab-ZAP saporin conjugate assay. Cells were plated into 96-well plates at 1,000 cells per well in 50-µL RMPI medium supplemented with 10% FBS and incubated overnight at 37°C, 5% CO2. Serial dilutions of MEDI9447 or an isotype control antibody were pre-incubated for 30 min with 80-nM Fab-ZAP reagent (Fab fragment of a polyclonal anti-human IgG antibody conjugated to the cytotoxic protein saporin; Advanced Targeting Systems) in RPMI/10%FBS and 50 µL of this mixture were added to the cell lines. After 3 d in culture, cell proliferation was measured using the CellTiter-Glo assay (Promega) following the manufacturer's instructions.
Lymphocyte stimulation assay
Primary human CD4+ T cells were isolated from the content of leukocyte cones according to IRB approved human samples guidelines. Briefly, the content from a leukocyte cone was diluted in PBS, and then layered over Ficoll-Paque Plus and centrifuged at 400 × g for 40 min with brakes turned off. PBMCs were then isolated from the interface and washed with PBS by centrifugation at 200 × g for 10 min. Supernatant was discarded and cells were suspended in PBS. Viable cells were determined, and then pelleted at 350×g for 5 min and suspended in Robosep buffer at a concentration of 5 × 107 per mL. CD4+ T cells were isolated from PBMCs by negative selection using the EasySep™ human CD4+ T-cell enrichment kit (StemCell, Vancouver, British Columbia, Canada). Isolated CD4+ cells were labeled with 3 µM of CFSE probe using the CellTrace CFSE cell proliferation kit at a cell density of 1 × 106 cells per mL in PBS containing 0.1% BSA with an incubation period of 15 min at 37°C. Cells were washed twice with warm X-Vivo 15 media and suspended at 5 × 105 cells per mL in the same media supplemented with 60 IU/mL of recombinant human IL-2 (Roche Diagnostics Ltd., Burgess Hill, UK). Dynabeads Human T-Activator CD3/CD28 (Life Technologies, Paisley, UK) reagent was added to cell suspension at 1:1 bead-to-cell ratio and incubated for 1 h at 37°C.
Thereafter, 100 µL of pre-activated CD4+ (approximately 50,000) and bead mixture were added to wells of sterile round-bottom 96-well plates. Serial dilutions of the MEDI9447 and the R347 isotype control antibody were performed in X-Vivo 15 media. To the cells in the plate, 50 µL of diluted reagents were added followed by 50 µL of X Vivo 15 containing 400 µM or 800 µM of AMP. Cells in the assay were gently pelleted by centrifugation at 100×g for 2 min and placed in a 37°C humidified tissue culture incubator with 5% CO2 for 72 h. After 72 h of incubation, cells were pelleted by centrifugation at 380 g for 4 min, washed once with 100 µL of FACS buffer (2% fetal bovine serum in PBS) and finally suspended in 100 µL of PBS containing 3.7% of formaldehyde for flow cytometry analysis on a BD FACSCanto II. CFSE+ CD4+ cells with no AMP well were used to identify cells that have undergone cellular division.
Mixed leukocyte reactions
PBMC were isolated fresh from healthy donors or from frozen healthy leukopak aliquots. For fresh PBMCs, blood from healthy normal donors was collected into 8 mL BD Vacutainer® CPT™ Cell preparation tubes then centrifuged for 25 min at 2,700 × g with no break. PBMCs were collected and then washed three times with AIM-V assay medium (Gibco). Red blood cells were lysed using Red Blood Cell Lysing Buffer (Gibco). PBMCs were prepared from leukopaks (All Cells) by collecting into 1-L bottles and dilution with wash buffer (PBS containing 2% fetal bovine serum). The PBMCs were then isolated by layering over LSM Separation Medium (MP Biomedicals) in 50 mL Sepmate Tubes (Stem Cell Technologies). The tubes were centrifuged at 1,200 × g for 10 min. The cells were collected and then washed by centrifugation three times with wash buffer at 300 × g for 8 min each wash. Red blood cells were lysed using Red Blood Cell Lysing Buffer (Gibco). PBMCs were re-suspended in Cell Freezing Medium (Gibco) and frozen at −80°C.
For the MLR, PBMCs were re-suspended in assay medium. PBMCs from two donors were mixed together 1:1 in AIM-V assay medium and plated into 96-well U-bottom plates (Costar) at a density of 200,000 cells per well per donor in 100 µL. MEDI9447 and an isotype control antibody were diluted in serum-free AIM-V medium and then added to the cells for 72–96 h. Brightfield images of the plates were taken daily at 2.5× magnification using the Cellomics ArrayScan instrument (Thermo Fischer). Plates were then centrifuged at 1,200×g for 3 min. Supernatant was harvested and then assayed for cytokine secretion using the human TH1/TH2 multiplex ELISA (Meso Scale Discovery) according to the manufacturer's instructions.
Syngeneic tumor model growth inhibition experiments
Syngeneic tumors were established by subcutaneous injection of 5 × 105 CT26 cells suspended in 0.1 mL of PBS into the right flanks of 4-to-6-week-old female Balb/C mice. Animals were randomized into groups based on body weight. Ten mice per group were used in these studies. For anti-PD-1 dosing, clone RMP1-14 was used (BioXcell, West Lebanon, NH). Antibodies were dosed via intraperitoneal injection at 10 or 20 mg per kg as indicated in the figure legends. Tumor volume was calculated based on the following formula:
All procedures were run in accordance with the Guide for the Care and Use of Laboratory Animals Citation42 and institutional standards in our AAALAC accredited facility and were approved by MedImmune's Institutional Animal Care and Use Committee.
Tumor isolation for flow cytometry
Tumors were dissected from CT26 tumor-bearing mice, cut into small pieces, and digested with collagenase. After a 30 min incubation, the digested sample was passed through a 70-micron filter. Dissociated cells were pelleted at 800 ×g for 5 min at 4°C and were re-suspended in fluorescence-activated cell sorting (FACS) buffer. Cells were counted on Vi-Cell using the default setting. 1 × 106 cells were plated per well. Cells were stained with anti-CD45 (to detect all leukocyte) anti-CD11b with anti-GR1 (a phenotype typically classified as a myeloid derived suppressor cells or “MDSC”) and anti-Ly6g (granulocytic MDSC). Data were acquired on the LSRII flow cytometer.
Statistics
Significant p values were obtained from t-test analysis using Graphpad Prism software for comparisons between tumor volumes, between TIL subpopulations and between secreted cytokine levels in the MLR. For the MLR, paired t-tests were performed for each leukocyte donor pair. Survival fractions were plotted using the Kaplan–Meier method with comparison of survival curves performed using the Log-rank (Mantel-Cox) test in GraphPad Prism.
Disclosure of potential conflicts of interest
C.H., E.S., Q.H., K.M., S.R.F., K.A.M., S.A.M., R.R., J.R., E.P., N.H., N.M.D., C.L., M.D., R.H., R.E.H., and K.F.S. are full-time employees of MedImmune, LLC.
KONI_A_1208875_supplementary_data.zip
Download Zip (230.9 KB)References
- Linden J, Cekic C. Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol 2012; 32:2097-103; PMID:22772752; http://dx.doi.org/10.1161/ATVBAHA.111.226837
- Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 2013; 122:9-18; PMID:23678003; http://dx.doi.org/10.1182/blood-2013-02-482406
- Jalkanen S, Salmi M. VAP-1 and CD73, endothelial cell surface enzymes in leukocyte extravasation. Arterioscler Thromb Vasc Biol 2008; 28:18-26; PMID:17962625; http://dx.doi.org/ATVBAHA.107.153130
- Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19:355-67; PMID:23601906; http://dx.doi.org/10.1016/j.molmed.2013.03.005
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 2006; 103:13132-7; PMID:16916931; http://dx.doi.org/0605251103
- Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother 2012; 61:917-26; PMID:22116345; http://dx.doi.org/10.1007/s00262-011-1155-7
- Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res 2012; 72:2190-6; PMID:22396496; http://dx.doi.org/10.1158/0008-5472.CAN-12-0420
- Antonioli L, Giron MC, Colucci R, Pellegrini C, Sacco D, Caputi V, Orso G, Tuccori M, Scarpignato C, Blandizzi C et al. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PLoS One 2014; 9:e116253; PMID:25549098; http://dx.doi.org/10.1371/journal.pone.0116253
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res 2015; 3:506-17; PMID:25672397; http://dx.doi.org/10.1158/2326-6066.CIR-14-0211
- Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: New therapeutic approaches. Cancer Discov 2014; 4:879-88; PMID:25035124; http://dx.doi.org/10.1158/2159-8290.CD-14-0341
- Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J 2015; 13:265-72; PMID:25941561; http://dx.doi.org/10.1016/j.csbj.2015.03.008
- Clancy-Thompson E, Perekslis TJ, Croteau W, Alexander MP, Chabanet TB, Turk MJ, Huang YH, Mullins DW. Melanoma induces, and adenosine suppresses, CXCR3-cognate chemokine production and T-cell infiltration of lungs bearing metastatic-like disease. Cancer Immunol Res 2015; 3(8):956-67; PMID:26048575; http://dx.doi.org/2326-6066.CIR-15-0015
- Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human FC fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64:700-4; PMID:18560159; http://dx.doi.org/10.1107/S0907444908007877
- Rust S, Guillard S, Sachsenmeier K, Hay C, Davidson M, Karlsson A, Karlsson R, Brand E, Lowne D, Elvin J et al. Combining phenotypic and proteomic approaches to identify membrane targets in a 'triple negative' breast cancer cell type. Mol Cancer 2013; 12:11-4598-12-11; PMID:23406016; http://dx.doi.org/10.1186/1476-4598-12-11
- Lesokhin AM, Callahan MK, Postow MA, Wolchok JD. On being less tolerant: Enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci Transl Med 2015; 7:280sr1; PMID:25810313; http://dx.doi.org/10.1126/scitranslmed.3010274
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res 2013; 19:1035-43; PMID:23460534; http://dx.doi.org/10.1158/1078-0432.CCR-12-2064
- Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol 2015; 5:673; PMID:25628622; http://dx.doi.org/10.3389/fimmu.2014.00673
- Rolinski J, Hus I. Breaking immunotolerance of tumors: A new perspective for dendritic cell therapy. J Immunotoxicol 2014; 11:311-8; PMID:24495309; http://dx.doi.org/10.3109/1547691X.2013.865094
- Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol 2013; 25:230-7; PMID:23414607; http://dx.doi.org/10.1016/j.coi.2013.01.004
- Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013; 19:5626-35; PMID:23983257; http://dx.doi.org/10.1158/1078-0432.CCR-13-0545
- Resta R, Thompson LF. T cell signalling through CD73. Cell Signal 1997; 9:131-9; PMID:9113412; http://dx.doi.org/S0898-6568(96)00132-5
- Mikhailov A, Sokolovskaya A, Yegutkin GG, Amdahl H, West A, Yagita H, Lahesmaa R, Thompson LF, Jalkanen S, Blokhin D et al. CD73 participates in cellular multiresistance program and protects against TRAIL-induced apoptosis. J Immunol 2008; 181:464-75; PMID:18566412; http://dx.doi.org/181/1/464
- Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer 2014; 134:1466-73; PMID:23982901; http://dx.doi.org/10.1002/ijc.28456
- Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. Anti-human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J Immunol 2013; 191:4165-73; PMID:24043904; http://dx.doi.org/10.4049/jimmunol.1301274
- Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res 2014; 74:7239-49; PMID:25341542; http://dx.doi.org/10.1158/0008-5472.CAN-13-3581
- Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J 2015; 13:265-72; PMID:25941561; http://dx.doi.org/10.1016/j.csbj.2015.03.008
- Fedele G, Sanseverino I, D'Agostino K, Schiavoni I, Locht C, Horenstein AL, Malavasi F, Ausiello CM. Unconventional, adenosine-producing suppressor T cells induced by dendritic cells exposed to BPZE1 pertussis vaccine. J Leukoc Biol 2015; 98:631-9; PMID:26089537; http://dx.doi.org/10.1189/jlb.3A0315-101R
- Geoghegan JC, Diedrich G, Lu X, Rosenthal K, Sachsenmeier KF, Wu H, Dall'Acqua WF, Damschroder M. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. MAbs 2016; 8(3):454-67; PMID:26854859; http://dx.doi.org/10.1080/19420862.2016.1143182
- Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res 2010; 70:2245-55; PMID:20179192; http://dx.doi.org/10.1158/0008-5472.CAN-09-3109
- Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol 2012; 189:2226-33; PMID:22826317; http://dx.doi.org/10.4049/jimmunol.1200744
- Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol 2011; 187:6120-9; PMID:22039302; http://dx.doi.org/10.4049/jimmunol.1101225
- Muller-Haegele S, Muller L, Whiteside TL. Immunoregulatory activity of adenosine and its role in human cancer progression. Expert Rev Clin Immunol 2014; 10:897-914; PMID:24871693; http://dx.doi.org/10.1586/1744666X.2014.915739
- Beavis PA, Slaney CY, Kershaw MH, Neeson PJ, Darcy PK. Enhancing the efficacy of adoptive cellular therapy by targeting tumor-induced immunosuppression. Immunotherapy 2015; 7:499-512; PMID:26065476; http://dx.doi.org/10.2217/imt.15.16
- Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75:2139-45; PMID:25977340; http://dx.doi.org/10.1158/0008-5472.CAN-15-0255
- Thomson LF, Ruedi JM, Glass A, Moldenhauer G, Moller P, Low MG, Klemens MR, Massaia M, Lucas AH. Production and characterization of monoclonal antibodies to the glycosyl phosphatidylinositol-anchored lymphocyte differentiation antigen ecto-5'-nucleotidase (CD73). Tissue Antigens 1990; 35:9-19; PMID:2137649
- Airas L, Salmi M, Jalkanen S. Lymphocyte-vascular adhesion protein-2 is a novel 70-kDa molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol 1993; 151:4228-38; PMID:8409398
- Airas L, Niemela J, Salmi M, Puurunen T, Smith DJ, Jalkanen S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol 1997; 136:421-31; PMID:9015312; http://dx.doi.org/10.1083/jcb.136.2.421
- Lloyd A, Vickery ON, Laugel B. Beyond the antigen receptor: Editing the genome of T-cells for cancer adoptive cellular therapies. Front Immunol 2013; 4:221; PMID:23935598; http://dx.doi.org/10.3389/fimmu.2013.00221
- Groves M, Lane S, Douthwaite J, Lowne D, Rees DG, Edwards B, Jackson RH. Affinity maturation of phage display antibody populations using ribosome display. J Immunol Methods 2006; 313:129-39; PMID:16730741; http://dx.doi.org/10.1016/j.jim.2006.04.002
- Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64:700-4; PMID:18560159; http://dx.doi.org/10.1107/S0907444908007877
- Sachsenmeier KF, Hay C, Brand E, Clarke L, Rosenthal K, Guillard S, Rust S, Minter R, Hollingsworth R. Development of a novel ectonucleotidase assay suitable for high-throughput screening. J Biomol Screen 2012; 17:993-8; PMID:22522649; http://dx.doi.org/10.1177/1087057112443987
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011; PMID:21595115; http://dx.doi.org/NBK54050 [bookaccession]
