abstract
The ecto-5'-nucleotidase/CD73 enzyme plays a pivotal role in generating an adenosine-enriched immunosuppressed and pro-angiogenic niche supporting cancer development. The targeting of CD73 leads to reorganization of tumor microenvironment, shaping the phenotype of the infiltrating T cells. The development of CD73 monoclonal antibodies offers a promising new avenue for antineoplastic treatment.
It is increasingly recognized that neoplastic cells interact closely with the immune system, blood vessels, mesenchymal cells, and extracellular matrix to generate a dynamic immunosuppressive and pro-angiogenic niche promoting cancer onset and progression.Citation1
The innate and adaptive immune responses that target and eliminate abnormal cells are usually defused within the tumor milieu, resulting in a state of anergy. There are two main causes: (i) accumulation of immune regulatory/suppressor cells of both lymphoid and myeloid origin (i.e., Treg cells, macrophages, dendritic cells, ανδ myeloid-derived suppressor cells) within the tumor microenvironment, and (ii) release of high concentration of immune inhibitory mediators in cancer milieu.Citation2 A number of studies conducted over the past two decades have tried to tease apart the complex interplay between cancer and immune cells. A significant outcome has been the development of novel strategies for triggering immune responses. The introduction of monoclonal antibody (mAb)-based therapies, targeting cytotoxic T-lymphocyte antigen 4 (CTLA4; ipilimumab and tremelimumab), programmed cell death 1 (PD-1; nivolumab, pembrolizumab and pidilizumab), and programmed cell death ligand 1 (PD-L1; atezolizumab, MEDI4736 and MSB0010718C), has revolutionized completely the clinical management of neoplasia, providing novel therapeutic weapons to fight cancer. The development of drugs acting as immune-checkpoint blockers represents a milestone in the field of cancer immunotherapy. However, although these drugs can be effective for some patients, they are ineffective for others. For this reason, we need for identifying other pathways exploited by cancer cells to evade immune surveillance, so we can design novel pharmacological agents that will be synergistic with immune checkpoint inhibitors.
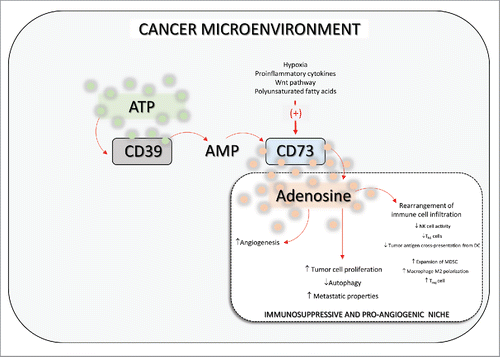
At the end of the 1990s, Blay et al.Citation3 provided the first evidence that adenosine levels are increased in the cancer microenvironment. This sparked the interest of the scientific community in the role played by this nucleoside in tumor onset and progression. It was found that elevated adenosine concentrations within neoplastic milieu exert powerful immunosuppressant activity and attenuate several effector T cell functions, mainly by activation of adenosine A2A receptors.Citation3 It was subsequently observed that purine metabolism is altered within the cancer niche, which is characterized by the increased expression and activity of the adenosine-generating enzyme ecto-5′-nucleotidase, also designated CD73.Citation4 This catabolic enzyme, highly expressed in various types of neoplasia, quickly dephosphorylates extracellular AMP, leading to the generation of a strong immunosuppressive and pro-angiogenic adenosine halo, that facilitates cancer onset and progressionCitation4 (). Consequent to these studies, the CD73-adenosine axis has emerged as one of the most promising pathways in immune-oncology,Citation5 and great effort is being made to design and develop anti-CD73 mAbs, as tentative therapeutic approaches aimed at inducing antitumor immune responses. On the other hand, as pointed out by Allard et al.,Citation5 distinct anti-CD73 mAbs targeting different epitopes could exert variable effects; therefore, deciphering which mAb neutralizing pattern is best for optimizing anticancer activity will be the key to success.
Figure 1. Scheme showing the role played by CD73 in the tumor microenvironment. CD73-derived adenosine shapes the cancer milieu, leading to the generation of a marked immunosuppressive and pro-angiogenic environment that paves the way to neoplasia development. ATP: adenosine triphosphate; AMP: adenosine monophosphate; DC: dendritic cell; MSDC: myeloid-derived suppressor cells; NK cell: natural killer cell; Treg cell: regulatory T cell. ↑: increases; ↓: decreases.
Recently, Geoghegan et al.Citation6 developed a novel human, non-competitive, high-affinity antagonistic antibody against CD73, designated as MEDI9447. This antibody hinders the conversion of both membrane-bound and soluble CD73 from the inactive open conformer to the catalytically active close state, through the interaction with a binding site within the CD73 N-terminal domain.Citation6 The authors reported that the mechanism of action of MEDI9447 is therapeutically advantageous, since the lack of competition with the AMP binding site on CD73 makes it unlikely to have potential cross-reactions with other nucleotide/side binding proteins endowed with structurally conserved catalytic sites.Citation6 In addition, the efficacy of MEDI9447 in inhibiting CD73 does not require the blockade of multiple substrates at level of the active site.Citation6
The study by Hay et al.,Citation7 published in the present issue of OncoImmunology, points out the wide-ranging role of CD73-derived adenosine on the phenotype of both lymphoid and myeloid-derived cells, shaping both the innate and adaptive arms of antitumor immunity. In this study, in vitro experiments documented the efficacy of MEDI9447 in reversing adenosine-mediated CD4+ T cell suppression. Interestingly, the pharmacological blockade of CD73 with MEDI9447 was associated with increased antigen presentation and enhanced lymphocyte activation, resulting in a greater release of proinflammatory Th1 cytokines (IFNγ, IL-1β, and TNF).Citation7 Moreover, when testing the in vivo effects of MEDI9447, the authors observed an inhibition of syngeneic tumor growth.Citation7 At odds with the other cancer immunotherapies, such as checkpoint inhibitors or T cell agonists, MEDI9447 was found to shape the composition of both myeloid and lymphoid infiltrating leukocyte populations within the tumor microenvironment.Citation7 In particular, the blockade of CD73 with MEDI9447 increased the infiltration of several immune cell populations, such as CD8+ effector cells and activated macrophages, into the cancer niche.Citation7 In parallel, MEDI9447 revealed a synergic activity upon its combined administration with anti-PD-1 antibodies, further supporting the potential value of relieving adenosine-mediated immunosuppression.Citation7
Over the years, epidemiological observations have indicated that overfeeding and obesity represent negative prognostic factors with regard to the incidence, progression, and therapeutic response of several neoplasias.Citation8 Indeed, several energy-balance-related host factors are known to affect cancer treatments.Citation9 Recently, Pietrocola et al.Citation8 showed that the condition of starvation, induced artificially by means of pharmacological tools (i.e., caloric restriction mimetics), can improve the immune-dependent antineoplastic effects of chemotherapy, by boosting the autophagy. Of note, starvation has been shown to improve the efficacy of anticancer chemotherapy via both a reduction of tumor infiltration by Tregcells and an immunostimulating effect mediated by the rejuvenation of immune-relevant haematopoietic stem cells.Citation10 In this context, Pietrocola et al.Citation8 highlighted the relevance of the CD39–CD73 axis. In particular, they observed that the combined administration of mitoxantrone (a conventional anticancer drug) with the caloric restriction mimetic agent hydroxycitrate elicited a high rate of autophagy driven by ATP.Citation8 The decrease in ATP concentration, via induction of CD39 expression in engineered cancer cells, determined a failure of mitoxantrone–hydroxycitrate combination in promoting tumor autophagy, thus, highlighting the relevance of ATP in driving this process.Citation8 Of note, the pharmacological blockade of CD73 via a specific mAb improved the efficacy of mitoxantrone as much as hydroxycitrate did without an additive effect, suggesting that hydroxycitrate acts through the modulation of extracellular ATP metabolism.Citation8
At present, increasing effort is being made to improve the efficacy of immunotherapies either by concomitantly stimulating immune functions or by targeting other immunoregulatory mechanisms implicated in shielding cancer cells against immunosurveillance. Several combinatorial approaches, based on the association of immunotherapies with other therapeutic options (including immunostimulant cytokines, inhibitors of indole 2,3-dioxygenase, antitumor vaccines, targeted therapies, oncolytic virotherapy, conventional chemotherapies, and radiation therapy), are currently under clinical evaluation. It has been found that CD73 exerts multifaceted actions within the cancer microenvironment. Through the generation of adenosine, it plays a pivotal role in fine tuning of immune cell functions, as well as in promoting angiogenesis, and the proliferation and spread of tumor cells. The pharmacological modulation of CD73 is proving to have significant potential in terms of antineoplastic efficacy, via a marked rearrangement of tumor milieu, characterized by a restoration of immunosurveillance against cancer cells and shaping the phenotype of immune cells infiltrating the neoplasia. Overall, the rearrangement of the neoplastic microenvironment by means of anti-CD73 therapy could be synergistic with immune checkpoint inhibitors or conventional cancer treatments, thus, counteracting a further source of immunosuppression.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013; 13:842-57; PMID:24226193; http://dx.doi.org/10.1038/nrc3613
- Tsai MJ, Chang WA, Huang MS, Kuo PL. Tumor microenvironment: a new treatment target for cancer. ISRN Biochem 2014; 2014:351959; PMID:25937967; http://dx.doi.org/10.1155/2014/351959
- Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 1997; 57:2602-5; PMID:9205063
- Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Hasko G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2016; 2:95-109; PMID:27014745; http://dx.doi.org/10.1016/j.trecan.2016.01.003
- Allard D, Allard B, Gaudreau PO, Chrobak P, Stagg J. CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy 2016; 8:145-63; PMID:26808918; http://dx.doi.org/10.2217/imt.15.106
- Geoghegan JC, Diedrich G, Lu X, Rosenthal K, Sachsenmeier KF, Wu H, Dall'Acqua WF, Damschroder MM. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. MABs 2016; 8:454-67; PMID:26854859; http://dx.doi.org/10.1080/19420862.2016.1143182
- Hay CM SE, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology 2016; http://dx.doi.org/10.1080/2162402X.2016.1208875
- Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 2016; 30:147-60; PMID:27411589; http://dx.doi.org/10.1016/j.ccell.2016.05.016
- Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M, Kroemer G et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 2016; 44:1255-69; PMID:27332730; http://dx.doi.org/10.1016/j.immuni.2016.06.001
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014; 14:810-23; PMID:24905167; http://dx.doi.org/10.1016/j.stem.2014.04.014
