ABSTRACT
Recently, the monoclonal antibody daratumumab was approved as a single agent for the treatment of patients with relapsed/refractory Multiple Myeloma (MM). Daratumumab is an antibody targeting surface molecule CD38 on myeloma cells and the agent is already widely being used based on its good tolerability and proven efficacy. We believe, however, that the efficacy of this drug and other anti-CD38 monoclonal antibodies can be further improved by combining it with other types of immunotherapies. Furthermore, surface molecule CD38 can be used as a target for immunotherapies other than just naked monoclonal antibodies. In this report, we review the expression pattern of CD38 among normal tissues and in different types of plasma cell dyscrasias including their progenitor cells, minimal residual disease, and circulating tumor cells. We summarize the physiological role of CD38 as well as its role in the pathophysiology of MM and we present the most recent clinical trials using CD38 as a target. In addition, we highlight possible combination immunotherapies incorporating anti-CD38 monoclonal antibodies and we demonstrate alternative immunotherapeutic approaches targeting the same antigen such as CD38-specific chimeric antigen receptor (CAR) T cells.
Introduction
Multiple Myeloma (MM) is an incurable plasma cell malignancy which develops in the patient's bone marrow (BM) and causes renal failure, immunosuppression with repeated infections, anemia, hypercalcemia, and bone lesions.Citation1 In the past 15 y, we have experienced a significant improvement in the patients' overall survival, however, most patients will still develop refractory disease and eventually suffer a fatal relapse. The treatment of patients with high-risk MM in particular has been disappointing and so far we have not been able to identify a convincing way of dealing with this more therapy-resistant and aggressive subtype of myeloma. Immunotherapies targeting surface antigens such as CD38 carry the potential to overcome high relapse rates and the more aggressive clinical course of MM with high-risk cytogenetics because these novel approaches potentially target the tumor cell irrespective of its biologic characteristics.
The first step in designing a novel immunotherapeutic approach is always to identify a promising target antigen. We have defined a few characteristics, which can help to determine the most promising targets expressed by myeloma cells. For example, we believe that an ideal myeloma-associated antigen for antibody-based approaches
| (1) | must be expressed on the surface of myeloma cells, | ||||
| (2) | should be expressed by as few normal tissues as possible, | ||||
| (3) | should be expressed by a sufficiently large proportion of myeloma patients, | ||||
| (4) | should homogeneously be expressed by the tumor cells of a given patient, | ||||
| (5) | should have a central function in the biology and/or pathophysiology of myeloma in order to prevent its downregulation under the selection pressure of an effective immunotherapy. | ||||
In this review, we will determine whether the myeloma-associated surface antigen CD38 fulfills the criteria listed, we will summarize the most recent clinical trials using CD38 as a target, and we will delineate ways in which alternative CD38-targeting approaches could be used and how current approaches using CD38-specific antibodies can be optimized.
Target molecule CD38
CD38 was first identified in 1981 as a cell surface antigen structurally related to the human major histocompatibility (HLA) antigen. It was described as a 45 kDa protein associated with a small subunit distinct from β2-microglobulinCitation2,3 and is a single-chain transmembrane type II glycoprotein with a short cytoplasmic tail, a single membrane-spanning region, and a long extracellular C-terminal domain.Citation4 The gene that encodes human CD38 has been mapped to chromosome 4p15.Citation5
CD38 expression in normal and tumor tissues
Among normal cells, CD38 is expressed on approximately 20% of all human BM cellsCitation6,7 with an expression mainly restricted to plasma cells,Citation8,9 myeloidCitation7,10,11 and erythroid,Citation11 precursors, and a small subset of T cells.Citation7 In addition, CD38 is highly expressed on thymocytes.Citation2,6,7,12 In human lymph nodes, CD38 shows a comparably low expression on germinal center B cellsCitation13 and an increased expression on terminally differentiated B cells.Citation14,15 In the peripheral blood, CD38 expression can be found on approximately 90% of normal plasma cells,Citation8,16,17 approximately 60% of natural killer (NK) cells,Citation18 60% of monocytes,Citation18 and a small subset of normal T cells.Citation18 In contrast, CD38 is absent from normal peripheral blood and BM B cells and neutrophils.Citation7,18
A large body of evidence suggests that CD38 expression on normal T cells is dependent on the activation status of the cells. Accordingly, CD38 has been used as an “intermediate” to “late” T cell activation markerCitation19 and an increased expression of CD38 has been observed on T cells of patients after allogeneic stem cell transplantationCitation20 and in patients with autoimmune diseases such as rheumatoid arthritis.Citation21
Although CD38 was first identified as a surface antigen expressed on a human T cell leukemia line, its expression is not restricted to T cells, but it was also detected on all T and B lymphoblastoid cell lines analyzed.Citation2 Accordingly, among malignant cells CD38 has been described to be expressed on cell lines and primary tumor cells from patients with B cell and T cell leukemiasCitation2,6,14 and high-grade B cell lymphomas such as diffuse large cell lymphoma.Citation12,14 In contrast, it is absent from the tumor cells of patients with B cell chronic lymphocytic leukemia (CLL),Citation14 B cell prolymphocytic leukemia (B-PLL),Citation14 and many patients with Waldenstroem's Disease.Citation14,22
Importantly, the strongest and most frequent CD38 expression out of all hematologic malignancies was consistently observed on the malignant plasma cellsCitation9 of patients with plasma cell dyscrasias such as monoclonal gammopathy of undetermined significance (MGUS),Citation23-26 extramedullary plasmacytoma,Citation27,28 multiple myeloma,Citation22,29 plasma cell leukemia,Citation30,31 and even on myeloma cells within malignant effusions based on MM.Citation32 In fact, >90% of the malignant plasma cells from patients with MM show surface expression of CD38.Citation23,33-37 However, while all available studies agree that malignant plasma cells from the vast majority of patients are positive for CD38, one study indicated that expression levels are significantly lower on clonal plasma cells from patients with MGUS compared to normal BM plasma cells and that expression further decreases in MM with the lowest level found in plasma cell leukemia.Citation38 It has also been reported that myeloma cells may downregulate the expression of CD38 throughout the course of the disease, with a complete loss of CD38 in very aggressive forms of the disease with extramedullary MM.Citation39 Accordingly, it was shown that lower surface expression levels of CD38 on the malignant plasma cells of MM patients is associated with a decreased overall survival.Citation40
Expression on circulating tumor cells, minimal residual disease, and myeloma progenitor cells
The question whether CD38 is expressed on the bulk of tumor cells from MM patients is of major relevance, however, it is of equal importance to determine whether the antigen is present on the subpopulation of tumor cells promoting the development, survival, and progression of MM. We consider it very possible that MM needs to be attacked from different biological angles by a variety of modalities, including immunotherapeutic approaches, to destroy the tumor bulk as well as MM-promoting precursors and eventually achieve cures.
For example, the presence of circulating tumor cells (CTCs) has been associated with an increased risk of malignant transformation to symptomatic MM in both MGUSCitation41 and smoldering MM,Citation42 as well as with an inferior survival among symptomatic newly diagnosedCitation43,44 and relapsed/refractory MM.Citation45 Functionally, CTCs seem to be mostly quiescent, these cells are associated with a higher clonogenic potential,Citation45 and they seem to be more chemotherapy-resistant than conventional myeloma cells.Citation46 Importantly, CTCs show a downregulated expression of CD38 when compared to the patients' BM plasma cells.Citation45 It is, therefore, at least possible that an otherwise effective anti-CD38 therapy might select for clones with an increased potential for spreading to other sites in the BM or even peripheral tissues.
It has been suggested that the fact that most myeloma patients will eventually suffer a fatal relapse even after an initially effective therapy is due to the persistence of chemotherapy-resistant precursor cellsCitation47-49 in the BM even after destruction of the bulk of tumor cells.Citation50-53 Accordingly, it has repeatedly been shown that the persistence of minimal residual disease (MRD) with CD38+ aberrant plasma cells in the BM after autologous transplantation results in a reduced progression-free survival in MM patients.Citation54 Since, these remaining tumor cells express CD38 they could be targeted by anti-CD38 immunotherapies, which could potentially result in the eradication of the disease from the BM.
It is an ongoing debate whether myeloma stem cells really exist and what their phenotype looks like.Citation55 However, it was already shown close to 40 y ago using a newly developed colony forming assay that in MM clonogenic cells represented 0.001–0.1% of the total tumor cell number.Citation56 It has repeatedly been suggested that myeloma progenitor cells might be enriched in CD138-negative but CD38-positive plasma cells.Citation57 In addition, it has been shown that CD138-CD19-CD38+ plasma cells are capable of engrafting in immunocompromised mouse models.Citation57 It is, therefore, important to emphasize that the clonogenic myeloma precursors, should they indeed exist, are potentially negative for plasma cell marker CD138 while they are most likely positive for CD38Citation47 and could probably be targeted by anti-CD38 approaches.
Biological function of CD38
It seems that CD38 ligation has stimulatory effects on normal mature lymphocytes.Citation58-60 For example, it has been shown that CD38 ligation by specific monoclonal antibodies (mAb) on normal peripheral blood T cells induces the secretion of different cytokines such as IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-g, and IL-10.Citation61
CD38 ligation on human B cells results in different effects depending on the differentiation stage of the cell. For example, Zupo and coworkers showed that the agonistic anti-CD38 monoclonal antibody IB4 is capable of preventing apoptosis in human tonsillar germinal center (GC) B cells.Citation62 On the other hand, ligation of CD38 inhibits cell growth and induces apoptosis in normal human B cell precursors in the BM environment.Citation63 Accordingly, CD38 ligation inhibits proliferation and induces apoptosis through the activation of the syk tyrosine kinase and the phosphatidylinositol 3-kinase pathway in human immature B cell lines.Citation64
Later, Deaglio et al. showed that CD31, a member of the Ig gene superfamily characterized by six Ig-like domains and by a unique adhesive ability mediated by homo- and heterophilic mechanisms, is the ligand for CD38. Their results also suggested that the interplay between CD38 and its ligand CD31 is an important step in the regulation of cytoplasmic calcium fluxes identical to the synthesis of different cytokines such as IL-6 and IL-10. Importantly, CD31/CD38 interaction probably also regulates the migration of leukocytes and CD38+ cancer cells through the endothelial cell wall.Citation65 Interestingly, it has been shown that the vast majority of patients with MGUS and MM not only express CD38 on their malignant plasma cells but they are also positive for CD31. In contrast, expression of CD31 was only very rarely detected on the tumor cells of patients with plasmablastic MM and plasma cell leukemia.Citation66
Monoclonal antibodies targeting CD38
The first anti-CD38 monoclonal antibody () was presented in 1991 when Stevenson and coworkers published a preclinical study on a chimeric mouse Fab-human Fc monoclonal antibody they had prepared from the diagnostic mouse anti-CD38 antibody OKT10. They showed that, in contrast to the parent antibody, the chimeric molecule mediated antibody-dependent cellular cytotoxicity (ADCC) very efficiently with human blood mononuclear effector cells in vitro. Importantly, they also demonstrated that, even though CD38 is present on NK cells, there was little deleterious action of the antibody on effector cell function. The antibody also did not affect the growth of progenitor cells of the granulocyte/macrophage or erythroid lineages despite the fact that these cells express CD38.Citation35
Figure 1. Different types of CD38-specific immunotherapies. The figure shows different ways to target the CD38 surface molecule expressed on myeloma cells. The myeloma antigen can be targeted by mAb, antibody-drug conjugates (ADC) or bispecific antibodies, CD38-specific T cells recognizing HLA/CD38 peptide complexes, and CD38-specific CAR T cells.
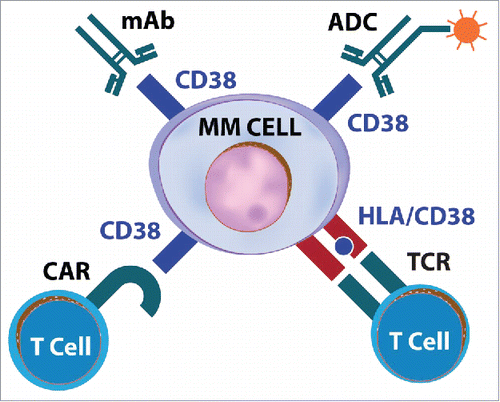
In 1995, Ellis et al. reported on the production of a novel high-affinity monoclonal antibody (AT13/5) against CD38. They prepared two engineered forms of the antibody: a humanized lgG1 and a chimeric mouse Fab/human Fc chimeric antibody. They found both constructs to efficiently direct ADCC against CD38-positive cell lines while complement was activated only poorly. Neither construct caused down-modulation of CD38, nor did they affect the NADase activity of CD38.Citation67
In 2011, de Weers and coworkers first described daratumumab, a novel human IgG1 kappa anti-CD38 monoclonal antibody, which was generated by immunizing human Ig transgenic mice with recombinant CD38 protein and CD38-transfected NIH 3T3 cells.Citation68 Daratumumab was of high affinity and, importantly, was capable of inducing strong ADCC and complement-dependent cytotoxicity (CDC) against myeloma cells in vitro and in a mouse model.Citation68 It was also shown that daratumumab-induced ADCC and CDC were not affected by the presence of BM stromal cells, indicating that daratumumab can effectively kill MM tumor cells in the BM microenvironment. Moreover, no daratumumab-mediated lysis of primary human B and T cells, activated T cells, NK cells, and monocytes was observed, suggesting that daratumumab selectively kills MM tumor cells.Citation68
Accordingly, Nijhof et al. showed that the level of CD38 expression is an important determinant of daratumumab-mediated ADCC and CDC. Importantly, they also demonstrated that all-trans retinoic acid treatment led to an upregulation of CD38 expression and a reduced expression of the complement-inhibitory proteins CD55 and CD59 on MM cells, which improved the efficacy of daratumumab.Citation69 However, it remains to be determined if clinically such an intervention, which is potentially associated with significant toxicity in the form of retinoic acid syndrome, might even be necessary given the relatively consistent CD38 expression levels in MM.
More recently, it was shown that, in addition to exerting CDC and ADCC, daratumumab is capable of efficiently inducing macrophage-mediated phagocytosis. Phagocytosis contributed to the antibody's antitumor activity in a xenograft mouse model and it induced macrophage-mediated phagocytosis of tumor cells isolated from MM patients. These findings indicated that phagocytosis is a clinically relevant mechanism of action that may contribute to the therapeutic activity of daratumumab in myeloma.Citation70
In 2014, Deckert et al. presented their first full-length report on the development of another novel humanized monoclonal antibody named SAR650984.Citation71 The antibody was generated by standard hybridoma technology following immunizations with murine 300-19 cells transfected to express the full-length human CD38 antigen. The authors showed that this antibody was very potent in inducing CDC, ADCC, and antibody-dependent cellular phagocytosis (ADCP) against myeloma and lymphoma cell lines. In addition, they demonstrated that SAR650984 was capable of inducing apoptosis without the addition of an external cross-linking agent and inhibited the ADP-ribosyl cyclase activity of CD38, likely through an allosteric antagonism. Importantly, SAR650984 was also active in B cell lymphoma and MM xenograft mouse models.Citation71 Very recently, it was shown that SAR650984 also decreases MM cell adhesion to BM accessory cells via blockage of CD31–CD38 interaction, rendering the tumor cells more sensitive to antibody-induced cytotoxicity.Citation72
MOR202 (MOR03087) is another fully human anti-CD38 antibody. In addition to ADCC as a potent effector mechanism of MOR202, it was shown that the antibody is capable of inducing killing of MM patient cells via ADCP.Citation73
Van Bueren et al. performed an in vitro comparison of daratumumab with alternative antibodies targeting CD38 including MOR03087, SAR650984, and Ab79.Citation74 They found that daratumumab and the surrogate antibodies showed comparable affinity and induced similar amounts of ADCC. SAR650984 was the only antibody that was able to directly induce apoptosis without Fc crosslinking, a finding which was later confirmed by a different group that showed that SAR650984 can trigger caspase 3/7-dependent apoptosis in MM cells including those with a p53 mutation.Citation75 In addition, SAR650984 also showed the most significant inhibition of the enzymatic activity of CD38. The most striking difference, however, was observed for the ability to induce CDC, the mechanism of action, which seems to be very important for the in vivo anti-myeloma activity of a given antibody. Daratumumab efficiently induced high levels of CDC at low concentrations, whereas the other CD38 mAb were unable or less capable to induce CDC.Citation74
Interestingly, the different monoclonal antibodies seem to be significantly more effective in preclinical models when combined with IMiDs such as lenalidomide. Accordingly, the Utrecht group showed that daratumumab-dependent ADCC using primary multiple myeloma cells was significantly augmented after lenalidomide pre-treatment of the effector cells but not the myeloma cells themselves. They also observed that daratumumab-dependent ADCC was significantly amplified using peripheral blood mononuclear cells from lenalidomide-treated MM patients as effector cells.Citation76
In their recent analysis, Nijhof et al. provided first preclinical evidence for a potential benefit of the daratumumab/lenalidomide combination in lenalidomide- and bortezomib-refractory patients. They showed that daratumumab induced significant lysis of lenalidomide/bortezomib-resistant multiple myeloma cell lines and primary multiple myeloma cells from refractory patients. In these assays, lenalidomide but not bortezomib, synergistically enhanced daratumumab-mediated tumor lysis through activation of NK cells. In an in vivo xenograft model, only the combination of daratumumab with lenalidomide effectively reduced the tumorigenic growth of primary multiple myeloma cells from a lenalidomide- and bortezomib-refractory patient.Citation77
An additive or synergistic effect of the combination of an IMiD with an antiCD38 mAb has not only been demonstrated for daratumumab and lenalidomide. It was shown recently that the next-generation IMiD pomalidomide is capable of enhancing SAR650984-induced direct and indirect killing even in MM cells resistant to pomalidomide and lenalidomide alone.Citation78 The combination with lenalidomide also induced additive to synergistic enhancement of the activity of antibody MOR202, which was mediated by enhanced direct cytotoxicity and increased CD38 expression levels on MM cells.Citation73 Similar effects were observed when MOR202 was combined with the next-generation IMiD pomalidomide.Citation79,80
Finally, it has been shown that anti-CD38 antibodies cannot only effectively be combined with IMiDs. Since antibody-dependent cell-mediated cytotoxicity is an important effector mechanism of daratumumab, Nijhof et al. explored the possibility of improving ADCC by blocking NK cell inhibitory receptors with the human monoclonal anti-KIR antibody IPH2102 in addition to activating NK cells with lenalidomide. They showed that IPH2102 alone significantly augmented daratumumab-induced myeloma cell lysis. A further synergistically improved myeloma cell lysis with the daratumumab-IPH2102 combination was observed by adding lenalidomide,Citation81 which suggests that anti-CD38 strategies can be optimized by combining the respective antibodies with agents that independently modulate NK cell function.
Anti-CD38 immunotoxins and bispecifc antibodies for the treatment of MM
In addition to their application as naked monoclonal antibodies, anti-CD38 approaches have also proven useful in preclinical models when used in the form of immunotoxins or bispecific antibodies (). In 1994, Goldmacher et al. reported the development of an immunotoxin composed of the anti-CD38 antibody HB7 conjugated to a chemically modified ricin molecule. They showed that the conjugated antibody was capable of effectively killing CD38+ human myeloma and lymphoma cell lines as well as primary patient-derived myeloma cells in vitro. Importantly, the antibody did not affect the growth of progenitor cells of the granulocyte/macrophage or erythroid lineages, which to a certain extent also express CD38.Citation82
Later, Mehta and coworkers showed that in vitro treatment with retinoic acid induced high levels of CD38 antigen expression on leukemia cells. As a consequence, the combination of retinoic acid and anti-CD38 gelonin immunotoxin, made of the murine antihuman CD38 mAb IB4, induced a synergistic killing of the tumor cells including multidrug-resistant variants.Citation83 Accordingly, Bolognesi and coworkers showed that an immunotoxin consisting of the same anti-CD38 mAb IB4 and the type 1 ribosome-inactivating protein saporin-S6 was capable of exerting strong and specific cytotoxic effects on selected CD38-positive lymphoma cell lines.Citation84
Recently, the Seattle group presented a preclinical study assessing both conventional radioimmunotherapy (RIT), using a directly radiolabeled antibody, and streptavidin-biotin pretargeted RIT (PRIT). Both approaches were directed against the CD38 antigen and were supposed to deliver radiation doses sufficient for MM cell eradication. Complete remissions were observed in all of the mice treated with anti-CD38 pretargeted 90Y-DOTA-biotin and long-term myeloma-free survival was achieved.Citation85 More recent biodistribution studies demonstrated myeloma-specific targeting by the antibody with minimal levels of yttrium-90 radioactivity detected in normal organs supporting clinical evaluation of bispecific anti-CD38 PRIT in MM.Citation86
Teiluf et al. recently investigated the preclinical efficacy of a different radioimmunoconjugate composed of the α-emitter213Bi coupled to the anti-CD38 monoclonal antibody MOR03087 in MM. They found that 213Bi-anti-CD38 antibody treatment induced DNA damage, which resulted in mitotic arrest and subsequent mitotic catastrophe. The antitumor effect of the immunoconjugate correlated with CD38 expression levels. In myeloma xenografts, treatment with the 213Bi-anti-CD38 antibody conjugate suppressed tumor growth via induction of apoptosis and prolonged survival without significant toxicity.Citation87
Compared to immunotoxins fewer results are available for anti-CD38 bispecific antibodies, however, the findings obtained so far look promising. For example, Flavell and coworkers investigated the effectiveness of two different bispecific antibodies, which were based on the OKT10 anti-CD38 antibody, for delivering the ribosome-inactivating protein (RIP) saporin to human T-ALL cell lines. They found that the administration of the antibodies led to a dramatically increased saporin toxicity in vitro.Citation88 Similarly, French et al. generated a bispecific antibody consisting of the anti-CD38 monoclonal antibody AT13/5 and an antibody against the RIP gelonin. They found that the bispecific antibodies were highly efficient at delivering gelonin to B cell lymphoma cells in vitro and that cytotoxicity correlated closely with the affinity of the respective antibody. Importantly, they also observed that the anti-CD38 treatment was significantly more effective when combined with a monoclonal antibody specific for gelonin and surface molecule CD22.Citation89
It was demonstrated a little later that “true” bispecific antibodies targeting CD38 (mAb AT13/5) as well as CD59, the major membrane-bound complement inhibitor, were capable of lysing B cell lymphoma cell lines in vitro.Citation90 Chu et al. developed humanized and affinity-optimized Anti-CD38 × Anti-CD3 bispecific antibodies which stimulated potent T cell-mediated killing of human myeloma cell lines in a humanized mouse model and CD38+ cells in monkeys.Citation91 Finally, Peng et al. presented a very interesting study where they showed that an oncolytic measles virus genetically modified to display a single-chain antibody (scFV) against CD38 was capable of mediating cytotoxicity against myeloma cell lines in vitro and against CD38-positive tumor cells in a mouse model.Citation92
CD38-specific CAR T cells and TCR-transduced T cells as anti-myeloma approaches
CARs are artificial fusion proteins linking the specificity of an antibody-binding domain to the signaling subunits of activating T cell proteins (). Autologous T cells transduced with these constructs exhibit high and HLA-independent target specificity and cytotoxicity as demonstrated in patients with CD19-positive B cell lymphoma.Citation93 In a recent study, 30 patients with acute lymphoblastic leukemia (ALL) received CD19-specific CAR T cells and 90% achieved durable complete remissions.Citation94 A major obstacle in the development of CAR T cell therapies for indications other than CD19+ B cell lymphoma is their substantial on-target off-tissue toxicity. Unfortunately, virtually no cell surface antigens are exclusively expressed by malignant cells resulting in the undesired targeting of healthy tissues. Therefore, the search for antigens specifically expressed on tumor cells and only non-vital healthy tissues has become a central objective and we believe that CD38 represents one of the most promising targets for CAR T cell treatments in MM.
Mihara and coworkers have provided a first proof-of-principle showing that T cells equipped with an anti-CD38 CAR can be effective in vitro and in vivo against hematologic malignancies such as B cell non-Hodgkin lymphoma (B-NHL)Citation95 even if the tumor cells were highly chemotherapy-resistant.Citation96 The same group later showed that T cells harboring the anti-CD38-CAR were equally efficient in eliminating myeloma cell lines as well as tumor cells from myeloma patients.Citation97
More recently, Drent and colleagues generated retroviral CAR constructs based on human CD38 antibodies as antigen recognition domain and CD3zeta and 41BB (CD137) as signaling domains.Citation98 Their CD38‐CAR T cells effectively proliferated, produced inflammatory cytokines and effectively lysed malignant cell lines and primary malignant cells from multi-drug resistant MM patients in a cell-dose, and CD38 expression‐dependent manner. Importantly, the CAR T cells did not affect the outgrowth of CD34+ cells into various myeloid lineages and were, furthermore, effectively controllable with a caspase-9-based suicide gene. Finally, in a novel in vivo xenotransplant, in which myeloma cells were grown in a human BM-like microenvironment, administration of CD38‐CAR T cells also resulted in significant antitumor efficacy.
Overall, these early results suggest that CD38-specific CAR T cells can be developed into a safe and effective treatment for MM. The advantage of using CD38-specific T cells versus a monoclonal antibody with the same specificity is that such an approach could take full advantage of the specificity of a CD38-targeting antibody combined with the durability and efficacy of a myeloma-targeting memory T cell response.
Unfortunately, clinical results with CAR T cells have thus far been disappointing in tumors other than CD19+ B cell lymphomas, probably reflecting the fact that most cancers do not express highly accessible tumor-specific antigens on their cell surface. Therefore, an alternative approach of engineering tumor-specific autologous T cells is to replace their natural T cell receptor (TCR) with an ectopically expressed TCR specific for the aberrantly expressed antigen (). These TCR-transduced T cells recognize intracellular tumor-specific proteins in a given HLA context. Clinical studies have tested T cells transduced with a TCR specific for an HLA-A2-restricted epitope of the cancer-testis antigen family member NY-ESO-1 in HLA-A2+ patients with advanced melanoma,Citation99,100 synovial cell sarcoma,Citation99,100 and MM.Citation101 The treatment was generally well tolerated and in sarcoma and melanoma patients an impressive overall response rate of 55–60% was reported.Citation100 So far, CD38-specific TCR-transduced T cells () have not been introduced into the clinic, however, this could be developed into a valuable alternative, for example in patients who have lost surface expression of the antigen under the selective pressure of an anti-CD38 antibody therapy but still express the intracellular protein.
Clinical results with anti-CD38 strategies
A number of clinical studies paved the way to the approval of daratumumab, in November 2015, as the first monoclonal antibody directly targeting myeloma cells. The US Food and Drug Administration (FDA) granted accelerated approval to the CD38-targeted monoclonal antibody as a monotherapy for patients with multiple myeloma following at least three prior therapies, based on data from two open-label clinical trials, the MMY2002 study and the GEN501 trial.
The phase II MMY2002 study consisted of two parts. In part one, 34 patients were randomized to daratumumab at 8 mg/kg (n =18) or 16 mg/kg (n = 16). In part 2 of the study, 90 additional patients were treated with daratumumab at the 16 mg/kg dose level. Those 106 patients who had received the 16 mg/kg dose level had a median number of five prior treatment lines, and 95% were refractory to their last PI and IMiD. Adverse events were mostly mild and the overall response rate was 29.2% with a median duration of response of 7.4 mo. The median time to progression was 3.7 mo and the estimated 1-y overall survival rate was 65%.Citation102
The phase I/II GEN501 study also contained two parts. In the first part, 32 patients were treated with daratumumab at increasing doses and in the second phase, 72 patients received daratumumab at either 8 mg/kg or 16 mg/kg. In each group, patients had a median of four prior therapies, 64% had disease refractory to bortezomib and lenalidomide, and 76% had received autologous stem-cell transplants. The majority of patients experienced some sort of infusion-related reactions, which most commonly occurred during the first infusion. However, reactions were generally mild and the treatment was considered safe. In part 2 of the study, the overall response rate was 36% in those 42 patients that received the highest dose level of 16 mg/kg. The median progression-free survival in these patients was 5.6 mo and among those patients who had a response, the estimated median duration of response was 6.9 mo.Citation103
As indicated by preclinical investigations, the combination of the anti-CD38 antibody with an IMiD resulted in an improved efficacy. For example, in a phase I/II dose escalation study daratumumab was combined with lenalidomide. Of the 32 myeloma patients in the expansion cohort that received daratumumab at a dose of 16 mg/kg 34% had prior exposure to lenalidomide. The most common adverse events included neutropenia in 81% of the patients, as well as muscle spasms, cough, diarrhea, fatigue, and hypertension in less than half of the patients. The overall response rate was 88%, with 34% partial responses and 53% ≥ very good partial responses.Citation104
Chari et al. reported on another phase Ib study where daratumumab was given in combination with next-generation IMiD pomalidomide and dexamethasone to patients with relapsed and refractory multiple myeloma. Patients had ≥2 prior lines of therapy including ≥ 2 consecutive cycles of lenalidomide and bortezomib and received daratumumab at 16 mg/kg. A total of 77 patients were enrolled and the median number of prior therapies was 3.5. Eighty-eight percent of the patients were refractory to lenalidomide and 65% to both a PtdIns and an IMiD. There was little additional toxicity when daratumumab was added to pomalidomide/dexamethasone other than daratumumab-specific infusion-related reactions such as chills, cough, and dyspnea. In 53 evaluable patients, the ORR was 58.5% and many responses deepened over time.Citation105
Very recently, Lonial et al. reported results of a multicenter phase II trial where 106 myeloma patients, following a dose-escalation part, received daratumumab at 16 mg/kg. Patients had received a median of five previous lines of therapy, as many as 85% had previously received autologous stem cell transplantation, about 90% were refractory to either lenalidomide or bortezomib, and 83% were double-refractory. In this heavily pretreated and multi-refractory patient population the ORR was still 29.9% with a median duration of the responses of 7.4 mo. As in the other trials with anti-CD38 antibodies, the treatment was well tolerated with 42% of patients experiencing infusion-related reaction, which were generally mild and predominantly occurred during the first infusion.Citation106
There have been a number of clinical trials with alternative anti-CD38 antibodies. In a dose escalation phase I study, the SAR650984 antibody was administered to patients with different CD38+ hematologic malignancies who had progressed on or after standard therapy. A total of 32 patients were treated across all dose levels including 27 patients with MM. The most frequent occurring adverse events were fatigue (46.9%), nausea (31.3%), pyrexia (28.1%), cough (25%), vomiting (21.9%), and hypercalcemia (18.8%). All MM patients had received prior lenalidomide and bortezomib and the median time from diagnosis to first SAR650984 dosing was 6.8 y. Out of all myeloma patients 17 were treated at dose levels between 1 mg/kg and 10 mg/kg. At a dose level of ≥1 mg/kg the overall response rate was 25% and at a dose level of ≥10 mg/kg objective responses were seen in 31% of all myeloma patients.Citation107
The same monoclonal antibody was later combined with lenalidomide in a different phase Ib dose escalation trial. Out of the 31 MM patients included 95% had prior exposure to an IMiD and more than 85% had relapsed after or were refractory to at least one prior IMiD-based therapy. With a median follow-up of 6 mo, the overall response rate was 64.5% including two complete responses and eight very good partial responses. Of note, among the 24 patients who were relapsed and refractory to their last regimen containing lenalidomide, the overall response rate was still 62.5%. Median duration of all responses was 23 weeks.Citation108
Recently, preliminary results were reported on a phase I dose-escalation study with the anti-CD38 antibody MOR202. For the first 38 patients with relapsed myeloma treated, the treatment proved to be safe and well tolerated but no efficacy data were reported at this early stage of the study.Citation109
There is currently no detailed information on the question whether patients can effectively be retreated with an anti-CD38 antibody after an initial response. However, a recent report of two cases suggested that patients who had an initial response to daratumumab can effectively be retreated following a treatment interruption.Citation110 The authors showed that continuous treatment with daratumumab decreases ADCC-mediated antitumor activity using two independent mechanisms, which can both be recovered by treatment interruption. In conclusion, there may be a need for treatment interruption for optimal maintenance of ADCC.
Immunotherapies targeting CD38 – conclusions, possible limitations, and future perspectives
Myeloma therapy using anti-CD38 antibodies has already proven effective, however, there are certain limitations we need to address in order to further increase the efficacy of this approach. For example, as outlined above there seems to be a progressive decrease in expression levels of CD38 from normal plasma cells to MGUS plasma cells, MGUS to MM, and finally MM to plasma cell leukemia. Accordingly, it has been reported that myeloma cells may downregulate the expression of CD38 or even completely lose its expression in very aggressive forms of the disease with extramedullary MM.Citation39 Furthermore, another recent report suggested that myeloma cells can in rare cases lose expression of CD38 at the time of occurrence of relapsed/refractory MM, even without prior treatment with anti-CD38 approaches.Citation111 We, therefore, believe that CD38 expression should always be re-evaluated prior to considering CD38 monoclonal antibody therapy for patients with relapsed and refractory MM, even if their tumors initially expressed CD38.
Along the same lines, the presence of soluble CD38 protein, in particular in patients with a significant tumor burden, could undermine the therapeutic efficacy of anti-CD38 approaches. Funaro et al. demonstrated the existence of a soluble form of human CD38 derived from the membrane-anchored CD38Citation58 and it remains to be seen whether soluble CD38 present in the peripheral blood and BM environment of patients with MM potentially interferes with clinical responses to anti-CD38 approaches.
Accordingly, in their response to a report on the design of an anti-CD38 immunotoxin Vooijs and colleagues considered expression of CD38 by basophils an important drawback. They remarked that a destruction of these cells by an anti-CD38 immunotoxin may result in anaphylactic shock caused by the release of vasoreactive agents. They even concluded that anti-CD38 monoclonal antibodies “are principally not the right candidates for immunotherapy […] in patients with MM”.Citation112 Recent clinical data using anti-CD38 monoclonal antibodies clearly show that this approach is safe and, with the exception of infusion-related reactions with the first application, does not cause any major toxicities. However, unspecific expression of the target antigen could become a significant problem especially with the introduction of more potent approaches such as CD38-specific CAR T cells.
Apart from the assessment of antigen expression on myeloma cells, we currently do not have any biomarkers with a predictive value for anti-CD38 approaches. In a very recent study, serum samples of myeloma patients were analyzed before and during treatment with daratumumab to identify proteins and biological pathways associated with a clinical response to the antibody. Profiling of 1,129 serum proteins was performed and the investigators found 51 proteins to be significantly different between clinical responders and non-responders. Interestingly, a subset of the differentially expressed proteins has previously been associated with an immune response and T cell biology. In addition, proteins related to T-cell activity, immune checkpoints, and immune response (such as CCL5, ICOS, Granzyme B, and PD-L1) also showed changes associated with daratumumab treatment, supporting the idea that daratumumab induces a T cell response in MM patients, which may contribute to clinical responses.Citation113
Overall, we believe that the introduction of novel biomarkers assessing CD38 expression on myeloma cells, normal leukocytes, and immune factors within tumor cells and the BM microenvironment will help to further improve the efficacy of anti-CD38 approaches in MM. Furthermore, combining different types of immunotherapies with CD38-specific approaches as well as the introduction of more potent CD38-targeting modalities, such as bispecific antibodies and CD38 CAR T cells, will lead to deeper and more durable responses in patients with MM.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364(11):1046-60; PMID:21410373; http://dx.doi.org/10.1056/NEJMra1011442
- Cotner T, Mashimo H, Kung PC, Goldstein G, Strominger JL. Human T cell surface antigens bearing a structural relationship to HLA antigens. Proc Natl Acad Sci U S A 1981; 78(6):3858-62; PMID:6167992; http://dx.doi.org/10.1073/pnas.78.6.3858
- Terhorst C, van Agthoven A, LeClair K, Snow P, Reinherz E, Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell 1981; 23(3):771-80; PMID:7013988; http://dx.doi.org/10.1016/0092-8674(81)90441-4
- Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J 1996; 10(12):1408-17; PMID:8903511
- Nakagawara K, Mori M, Takasawa S, Nata K, Takamura T, Berlova A, Tohgo A, Karasawa T, Yonekura H, Takeuchi T et al. Assignment of CD38, the gene encoding human leukocyte antigen CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase), to chromosome 4p15. Cytogenet Cell Genet 1995; 69(1-2):38-9; PMID:7835083; http://dx.doi.org/10.1159/000133933
- Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A 1980; 77(3):1588-92; PMID:6966400; http://dx.doi.org/10.1073/pnas.77.3.1588
- Janossy G, Tidman N, Papageorgiou ES, Kung PC, Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol 1981; 126(4):1608-13; PMID:6970781
- Tominaga N, Katagiri S, Ohnishi M, Nakao H, Oritani K, Yagura H, Tamaki T, Kanayama Y, Yonezawa T, Tarui S. Analysis of surface antigen expression of human immunoglobulin-secreting cells: phenotypic heterogeneity in normal counterparts of myeloma cells. Br J Haematol 1989; 73(3):302-8; PMID:2605119; http://dx.doi.org/10.1111/j.1365-2141.1989.tb07744.x
- Harada H, Kawano MM, Huang N, Harada Y, Iwato K, Tanabe O, Tanaka H, Sakai A, Asaoku H, Kuramoto A. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 1993; 81(10):2658-63; PMID:8490175
- Crawford DH, Francis GE, Wing MA, Edwards AJ, Janossy G, Hoffbrand AV, Prentice HG, Secher D, McConnell I, Kung PC et al. Reactivity of monoclonal antibodies with human myeloid precursor cells. Br J Haematol 1981; 49(2):209-17; PMID:7028080; http://dx.doi.org/10.1111/j.1365-2141.1981.tb07217.x
- Sieff C, Bicknell D, Caine G, Robinson J, Lam G, Greaves MF. Changes in cell surface antigen expression during hemopoietic differentiation. Blood 1982; 60(3):703-13; PMID:6286014
- Aisenberg AC, Wilkes BM. Unusual human lymphoma phenotype defined by monoclonal antibody. J Exp Med 1980; 152(4):1126-31; PMID:6158547; http://dx.doi.org/10.1084/jem.152.4.1126
- Harada Y, Kawano MM, Huang N, Mahmoud MS, Lisukov IA, Mihara K, Tsujimoto T, Kuramoto A. Identification of early plasma cells in peripheral blood and their clinical significance. Br J Haematol 1996; 92(1):184-91; PMID:8562394; http://dx.doi.org/10.1046/j.1365-2141.1996.300835.x
- Gobbi M, Caligaris-Cappio F. Janossy G. Normal equivalent cells of B cell malignancies: analysis with monoclonal antibodies. Br J Haematol 1983; 54(3):393-403; PMID:6344913; http://dx.doi.org/10.1111/j.1365-2141.1983.tb02114.x
- Hsu SM, Jaffe ES. Phenotypic expression of B-lymphocytes. 1. Identification with monoclonal antibodies in normal lymphoid tissues. Am J Pathol 1984; 114(3):387-95; PMID:6421166
- Van Camp B, Thielemans C, Dehou MF, De Mey J, De Waele M. Two monoclonal antibodies (OKIa1 and OKT10) for the study of the final B cell maturation. J Clin Immunol 1982; 2(3 Suppl):67S-74S; PMID:6813349
- Terstappen LW, Johnsen S, Segers-Nolten IM, Loken MR. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood 1990; 76(9):1739-47; PMID:2224123
- Ortaldo JR, Sharrow SO, Timonen T, Herberman RB. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol 1981; 127(6):2401-9; PMID:6975321
- Cotner T, Williams JM, Christenson L, Shapiro HM, Strom TB, Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med 1983; 157(2):461-72; PMID:6296263; http://dx.doi.org/10.1084/jem.157.2.461
- Fox R, McMillan R, Spruce W, Tani P, Mason D. Analysis of T lymphocytes after bone marrow transplantation using monoclonal antibodies. Blood 1982; 60(3):578-82; PMID:6286011
- Fox RI, Fong S, Sabharwal N, Carstens SA, Kung PC, Vaughan JH. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol 1982; 128(1):351-4; PMID:6976376
- San Miguel JF, Caballero MD, Gonzalez M, Zola H, Lopez Borrasca A. Immunological phenotype of neoplasms involving the B cell in the last step of differentiation. Br J Haematol 1986; 62(1):75-83; PMID:3080019; http://dx.doi.org/10.1111/j.1365-2141.1986.tb02902.x
- Leo R, Boeker M, Peest D, Hein R, Bartl R, Gessner JE, Selbach J, Wacker G, Deicher H. Multiparameter analyses of normal and malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann Hematol 1992; 64(3):132-9; PMID:1373957; http://dx.doi.org/10.1007/BF01697400
- Ocqueteau M, Orfao A, Almeida J, Bladé J, González M, García-Sanz R, López-Berges C, Moro MJ, Hernández J, Escribano L et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol 1998; 152(6):1655-65; PMID:9626070
- Zandecki M, Facon T, Bernardi F, Izydorczyk V, Dupond L, François M, Reade R, Iaru T, Bauters F, Cosson A. CD19 and immunophenotype of bone marrow plasma cells in monoclonal gammopathy of undetermined significance. J Clin Pathol 1995; 48(6):548-52; PMID:7545187; http://dx.doi.org/10.1136/jcp.48.6.548
- Olteanu H, Wang HY, Chen W, McKenna RW, Karandikar NJ. Immunophenotypic studies of monoclonal gammopathy of undetermined significance. BMC Clin Pathol 2008; 8:13; PMID:19040735; http://dx.doi.org/10.1186/1472-6890-8-13
- Tamamori T, Nakayama F, Sugimoto H, Fenxiang J, Iwatsuki K, Takigawa M. Extramedullary plasmacytoma: cytological and genotypic studies. Br J Dermatol 1993; 129(4):468-72; PMID:8217765; http://dx.doi.org/10.1111/j.1365-2133.1993.tb03180.x
- Feng PH, Huang CC, Wang CW, Wu YK, Tsai YH. Solitary pleural plasmacytomas manifested as a massive pleural effusion without evidence of monoclonal gammopathy. Respirology 2008; 13(5):751-3; PMID:18713097; http://dx.doi.org/10.1111/j.1440-1843.2008.01319.x
- Katagiri S, Yonezawa T, Tamaki T, Kanayama Y, Kuyama J, Ohnishi M, Tsubakio T, Kurata Y, Tarui S. Surface expression of human myeloma cells: an analysis using a panel of monoclonal antibodies. Acta Haematol 1984; 72(6):372-8; PMID:6442520; http://dx.doi.org/10.1159/000206423
- Parreira A, Robinson DS, Melo JV, Ayliffe M, Ball S, Hegde U, Baughan A, Fairhead S, Talavera JG, Katzmann JA et al. Primary plasma cell leukaemia: immunological and ultrastructural studies in 6 cases. Scand J Haematol 1985; 35(5):570-8; PMID:4089535; http://dx.doi.org/10.1111/j.1600-0609.1985.tb02830.x
- Garcia-Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, Fernández-Calvo J, Sanz MA, Pérez-Simón JA, Rasillo A et al. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood 1999; 93(3):1032-7; PMID:9920853
- Chang H, Chou WC, Lee SY, Huang JY, Hung YH. Myelomatous pleural effusion in a patient with plasmablastic myeloma: a case report. Diagn Cytopathol 2009; 37(3):205-7; PMID:19170175; http://dx.doi.org/10.1002/dc.21004
- Jackson N, Ling NR, Ball J, Bromidge E, Nathan PD, Franklin IM. An analysis of myeloma plasma cell phenotype using antibodies defined at the IIIrd International Workshop on Human Leucocyte Differentiation Antigens. Clin Exp Immunol 1988; 72(3):351-6; PMID:3048803
- Kawano MM, Huang N, Harada H, Harada Y, Sakai A, Tanaka H, Iwato K, Kuramoto A. Identification of immature and mature myeloma cells in the bone marrow of human myelomas. Blood 1993; 82(2):564-70; PMID:8329711
- Stevenson FK, Bell AJ, Cusack R, Hamblin TJ, Slade CJ, Spellerberg MB, Stevenson GT. Preliminary studies for an immunotherapeutic approach to the treatment of human myeloma using chimeric anti-CD38 antibody. Blood 1991; 77(5):1071-9; PMID:1995092
- Ruiz-Arguelles GJ, San Miguel JF. Cell surface markers in multiple myeloma. Mayo Clin Proc 1994; 69(7):684-90; PMID:8015335; http://dx.doi.org/10.1016/S0025-6196(12)61350-0
- San Miguel JF, González M, Gascón A, Moro MJ, Hernández JM, Ortega F, Jimenez R, Guerras L, Romero M, Casanova F et al. Immunophenotypic heterogeneity of multiple myeloma: influence on the biology and clinical course of the disease. Castellano-Leones (Spain) Cooperative Group for the Study of Monoclonal Gammopathies. Br J Haematol 1991; 77(2):185-90; PMID:1706197; http://dx.doi.org/10.1111/j.1365-2141.1991.tb07975.x
- Perez-Andres M, Almeida J, Martín-Ayuso M, Moro MJ, Martín-Nuñez G, Galende J, Borrego D, Rodríguez MJ, Ortega F, Hernandez J et al. Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironment. Leukemia 2005; 19(3):449-55; PMID:15674420; http://dx.doi.org/10.1038/sj.leu.2403647
- Tembhare P, Yuan C, Korde N, Maric I, Landgren O. Antigenic drift in relapsed extramedullary multiple myeloma: plasma cells without CD38 expression. Leuk Lymphoma 2012; 53(4):721-4; PMID:21942285; http://dx.doi.org/10.3109/10428194.2011.623257
- Perez-Andres M, Almeida J, Martin-Ayuso M, De Las Heras N, Moro MJ, Martin-Nuñez G, Galende J, Cuello R, Abuín I, Moreno I et al. Soluble and membrane levels of molecules involved in the interaction between clonal plasma cells and the immunological microenvironment in multiple myeloma and their association with the characteristics of the disease. Int J Cancer 2009; 124(2):367-75; PMID:19003959; http://dx.doi.org/10.1002/ijc.23941
- Kumar S, Rajkumar SV, Kyle RA, Lacy MQ, Dispenzieri A, Fonseca R, Lust JA, Gertz MA, Greipp PR, Witzig TE. Prognostic value of circulating plasma cells in monoclonal gammopathy of undetermined significance. J Clin Oncol 2005; 23(24):5668-74; PMID:16110026; http://dx.doi.org/10.1200/JCO.2005.03.159
- Bianchi G, Kyle RA, Larson DR, Witzig TE, Kumar S, Dispenzieri A, Morice WG, Rajkumar SV. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013; 27(3):680-5; PMID:22902364; http://dx.doi.org/10.1038/leu.2012.237
- Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, Lust JA, Dispenzieri A, Greipp PR, Kyle RA et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005; 106(7):2276-9; PMID:15961515; http://dx.doi.org/10.1182/blood-2005-05-1858
- Vagnoni D, Travaglini F, Pezzoni V, Ruggieri M, Bigazzi C, Dalsass A, Mestichelli F, Troiani E, Falcioni S, Mazzotta S et al. Circulating plasma cells in newly diagnosed symptomatic multiple myeloma as a possible prognostic marker for patients with standard-risk cytogenetics. Br J Haematol 2015; 170(4):523-31; PMID:26010293; http://dx.doi.org/10.1111/bjh.13484
- Peceliunas V, Janiulioniene A, Matuzeviciene R, Zvirblis T, Griskevicius L. Circulating plasma cells predict the outcome of relapsed or refractory multiple myeloma. Leuk Lymphoma 2012; 53(4):641-7; PMID:21955292; http://dx.doi.org/10.3109/10428194.2011.627481
- Bergsagel PL, Smith AM, Szczepek A, Mant MJ, Belch AR, Pilarski LM. In multiple myeloma, clonotypic B lymphocytes are detectable among CD19+ peripheral blood cells expressing CD38, CD56, and monotypic Ig light chain. Blood 1995; 85(2):436-47; PMID:7529064
- Kim D, Park CY, Medeiros BC, Weissman IL. CD19-CD45 low/- CD38 high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia 2012; 26(12):2530-7; PMID:22733078; http://dx.doi.org/10.1038/leu.2012.140
- Chaidos A, Barnes CP, Cowan G, May PC, Melo V, Hatjiharissi E, Papaioannou M, Harrington H, Doolittle H, Terpos E et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013; 121(2):318-28; PMID:23169779; http://dx.doi.org/10.1182/blood-2012-06-436220
- Matsui W, Borrello I, Mitsiades C. Autologous stem cell transplantation and multiple myeloma cancer stem cells. Biol Blood Marrow Transplant 2012; 18(1 Suppl):S27-32; PMID:22226109; http://dx.doi.org/10.1016/j.bbmt.2011.10.036
- Ladetto M, Pagliano G, Ferrero S, Cavallo F, Drandi D, Santo L, Crippa C, De Rosa L, Pregno P, Grasso M et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol 2010; 28(12):2077-84; PMID:20308672; http://dx.doi.org/10.1200/JCO.2009.23.7172
- Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Feyler S et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol 2013; 31(20):2540-7; PMID:23733781; http://dx.doi.org/10.1200/JCO.2012.46.2119
- Paiva B, Vidriales MB, Cerveró J, Mateo G, Pérez JJ, Montalbán MA, Sureda A, Montejano L, Gutiérrez NC, García de Coca A et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood 2008; 112(10):4017-23; PMID:18669875; http://dx.doi.org/10.1182/blood-2008-05-159624
- Ferrero S, Ladetto M, Drandi D, Cavallo F, Genuardi E, Urbano M, Caltagirone S, Grasso M, Rossini F, Guglielmelli T et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics' impact on survival. Leukemia 2014; PMID:25027515; http://dx.doi.org/10.1038/leu.2014.219
- Liu H, Yuan C, Heinerich J, Braylan R, Chang M, Wingard J, Moreb J. Flow cytometric minimal residual disease monitoring in patients with multiple myeloma undergoing autologous stem cell transplantation: a retrospective study. Leuk Lymphoma 2008; 49(2):306-14; PMID:18231918; http://dx.doi.org/10.1080/10428190701813018
- Paino T, Paiva B, San Miguel J. CD20 positive cells are undetectable in the majority of multiple myeloma cell lines and are not associated with a cancer stem cell phenotype. Haematologica 2012; 97(7):1110-4; PMID:22315496; http://dx.doi.org/10.3324/haematol.2011.057372
- Hamburger A, Salmon SE. Primary bioassay of human myeloma stem cells. J Clin Invest 1977; 60(4):846-54; PMID:302265; http://dx.doi.org/10.1172/JCI108839
- Hosen N, Matsuoka Y, Kishida S, Nakata J, Mizutani Y, Hasegawa K, Mugitani A, Ichihara H, Aoyama Y, Nishida S et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia 2012; 26(9):2135-41; PMID:22430638; http://dx.doi.org/10.1038/leu.2012.80
- Funaro A, Horenstein AL, Calosso L, Morra M, Tarocco RP, Franco L, De Flora A, Malavasi F. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int Immunol 1996; 8(11):1643-50; PMID:8943558; http://dx.doi.org/10.1093/intimm/8.11.1643
- Santos-Argumedo L, Teixeira C, Preece G, Kirkham PA, Parkhouse RM. A B lymphocyte surface molecule mediating activation and protection from apoptosis via calcium channels. J Immunol 1993; 151(6):3119-30; PMID:8397252
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993; 262(5136):1056-9; PMID:8235624; http://dx.doi.org/10.1126/science.8235624
- Ausiello CM, la Sala A, Ramoni C, Urbani F, Funaro A, Malavasi F. Secretion of IFN-gamma, IL-6, granulocyte-macrophage colony-stimulating factor and IL-10 cytokines after activation of human purified T lymphocytes upon CD38 ligation. Cell Immunol 1996; 173(2):192-7; PMID:8912876; http://dx.doi.org/10.1006/cimm.1996.0267
- Zupo S, Rugari E, Dono M, Taborelli G, Malavasi F, Ferrarini M. CD38 signaling by agonistic monoclonal antibody prevents apoptosis of human germinal center B cells. Eur J Immunol 1994; 24(5):1218-22; PMID:8181532; http://dx.doi.org/10.1002/eji.1830240532
- Kumagai M, Coustan-Smith E, Murray DJ, Silvennoinen O, Murti KG, Evans WE, Malavasi F, Campana D. Ligation of CD38 suppresses human B lymphopoiesis. J Exp Med 1995; 181(3):1101-10; PMID:7869031; http://dx.doi.org/10.1084/jem.181.3.1101
- Silvennoinen O, Nishigaki H, Kitanaka A, Kumagai M, Ito C, Malavasi F, Lin Q, Conley ME, Campana D. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-gamma and phosphatidylinositol 3-kinase. J Immunol 1996; 156(1):100-7; PMID:8598449
- Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol 1998; 160(1):395-402; PMID:9551996
- Vallario A, Chilosi M, Adami F, Montagna L, Deaglio S, Malavasi F, Caligaris-Cappio F. Human myeloma cells express the CD38 ligand CD31. Br J Haematol 1999; 105(2):441-4; PMID:10233418; http://dx.doi.org/10.1111/j.1365-2141.1999.01321.x
- Ellis JH, Barber KA, Tutt A, Hale C, Lewis AP, Glennie MJ, Stevenson GT, Crowe JS. Engineered anti-CD38 monoclonal antibodies for immunotherapy of multiple myeloma. J Immunol 1995; 155(2):925-37; PMID:7608568
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011; 186(3):1840-8; PMID:21187443; http://dx.doi.org/10.4049/jimmunol.1003032
- Nijhof IS, Groen RW, Lokhorst HM, van Kessel B, Bloem AC, van Velzen J, de Jong-Korlaar R, Yuan H, Noort WA, Klein SK et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015; 29(10):2039-49; PMID:25975191; http://dx.doi.org/10.1038/leu.2015.123
- Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015; 7(2):311-21; PMID:25760767; http://dx.doi.org/10.1080/19420862.2015.1007813
- Deckert J, Wetzel MC, Bartle LM, Skaletskaya A, Goldmacher VS, Vallée F, Zhou-Liu Q, Ferrari P, Pouzieux S, Lahoute C et al. SAR650984, A Novel Humanized CD38-Targeting Antibody, Demonstrates Potent Antitumor Activity in Models of Multiple Myeloma and Other CD38+ Hematologic Malignancies. Clin Cancer Res 2014; 20(17):4574-83; PMID:24987056; http://dx.doi.org/10.1158/1078-0432.CCR-14-0695
- An G, Jiang H, Acharya C, Zhong MY, Cai T, Yang G, Song Z, Theilhaber J, Adrian F, Tai Y et al. SAR 650984, a therapeutic anti-CD38 monoclonal antibody, blocks CD38-CD31 interaction in Multiple Myeloma. Blood 2014; 124:4729; http://dx.doi.org/10.1182/blood-2014-08-596353
- Endell J, Boxhammer R, Wurzenberger C, Ness D, Steidl S et al. The activity of MOR202, a fully Human antiCD38 antibody, is complemented by ADCP and Is synergistically enhanced by lenalidomide in vitro and in vivo. Blood 2012; 120:4018; http://dx.doi.org/10.1182/blood-2011-10-384685
- van Bueren JL, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, Voorhorst M, Gresnigt E, Wiegman L, Ortiz Buijsse A et al. Direct in Vitro Comparison of Daratumumab with Surrogate Analogs of CD38 Antibodies MOR03087, SAR650984 and Ab79. Blood 2014; 124(21):3474
- Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, Dasilva N, Song Z, Yang G, Adrian F et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia 2016; 30(2):399-408; PMID:26338273; http://dx.doi.org/10.1038/leu.2015.240
- van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, Lokhorst HM, Mutis T. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica 2011; 96(2):284-90; PMID:21109694; http://dx.doi.org/10.3324/haematol.2010.030759
- Nijhof IS, Groen RW, Noort WA, van Kessel B, de Jong-Korlaar R, Bakker J, van Bueren JJ, Parren PW, Lokhorst HM, van de Donk NW et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res 2015; 21(12):2802-10; PMID:25398450; http://dx.doi.org/10.1158/1078-0432.CCR-14-1813
- Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, Dasilva N, Song Z, Yang G, Adrian F et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia 2015; PMID:26338273; http://dx.doi.org/10.1038/leu.2015.240
- Endell J, Boxhammer R, Steidl S. Synergistic in vitro activity of MOR202, a human CD38 antibody, in combination with pomalidomide. Blood 2014; 124:5712
- Boxhammer R, Steidl S, Endell J. Effect of IMiD compounds on CD38 expression on multiple myeloma cells: MOR202, a human CD38 antibody in combination with pomalidomide. J Clin Oncol 2015; 33:8588
- Nijhof IS, Lammerts van Bueren JJ, van Kessel B, Andre P, Morel Y, Lokhorst HM, van de Donk NW, Parren PW, Mutis T. Daratumumab-mediated lysis of primary multiple myeloma cells is enhanced in combination with the human anti-KIR antibody IPH2102 and lenalidomide. Haematologica 2015; 100(2):263-8; PMID:25510242; http://dx.doi.org/10.3324/haematol.2014.117531
- Goldmacher VS, Bourret LA, Levine BA, Rasmussen RA, Pourshadi M, Lambert JM, Anderson KC. Anti-CD38-blocked ricin: an immunotoxin for the treatment of multiple myeloma. Blood 1994; 84(9):3017-25; PMID:7524764
- Mehta K, Ocanas L, Malavasi F, Marks JW, Rosenblum MG. Retinoic acid-induced CD38 antigen as a target for immunotoxin-mediated killing of leukemia cells. Mol Cancer Ther 2004; 3(3):345-52; PMID:15026555
- Bolognesi A, Polito L, Farini V, Bortolotti M, Tazzari PL, Ratta M, Ravaioli A, Horenstein AL, Stirpe F, Battelli MG. CD38 as a target of IB4 mAb carrying saporin-S6: design of an immunotoxin for ex vivo depletion of hematological CD38+ neoplasia. J Biol Regul Homeost Agents 2005; 19(3-4):145-52; PMID:16602630
- Green DJ, Orgun NN, Jones JC, Hylarides MD, Pagel JM, Hamlin DK, Wilbur DS, Lin Y, Fisher DR, Kenoyer AL et al. A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer Res 2014; 74(4):1179-89; PMID:24371230; http://dx.doi.org/10.1158/0008-5472.CAN-13-1589
- Green DJ, Jones JC, Lin Y, Frayo SL, Kenoyer AL, O'Steen S, Peters A, Frost SHL, Orozco JJ, Gopal AK et al. Novel Bispecific CD38 Antibody Eradicates Multiple Myeloma in a Mouse Model Following Yttrium-90-DOTA Capture. Blood 2015; 126(23):118
- Teiluf K, Seidl C, Blechert B, Gaertner FC, Gilbertz KP, Fernandez V, Bassermann F, Endell J, Boxhammer R, Leclair S et al. alpha-Radioimmunotherapy with (2)(1)(3)Bi-anti-CD38 immunoconjugates is effective in a mouse model of human multiple myeloma. Oncotarget 2015; 6(7):4692-703; PMID:25576914; http://dx.doi.org/10.18632/oncotarget.2986
- Flavell DJ, Cooper S, Morland B, French R, Flavell SU. Effectiveness of combinations of bispecific antibodies for delivering saporin to human acute T-cell lymphoblastic leukaemia cell lines via CD7 and CD38 as cellular target molecules. Br J Cancer 1992; 65(4):545-51; PMID:1373293; http://dx.doi.org/10.1038/bjc.1992.112
- French RR, Penney CA, Browning AC, Stirpe F, George AJ, Glennie MJ. Delivery of the ribosome-inactivating protein, gelonin, to lymphoma cells via CD22 and CD38 using bispecific antibodies. Br J Cancer 1995; 71(5):986-94; PMID:7734325; http://dx.doi.org/10.1038/bjc.1995.190
- Harris CL, Kan KS, Stevenson GT, Morgan BP. Tumour cell killing using chemically engineered antibody constructs specific for tumour cells and the complement inhibitor CD59. Clin Exp Immunol 1997; 107(2):364-71; PMID:9030877; http://dx.doi.org/10.1111/j.1365-2249.1997.265-ce1156.x
- Chu SY, Miranda Y, Phung S, Chen H, Rashid R, Endo NA, Chan EW, Pong E, Bonzon C, Muchhal US et al. Immunotherapy with long-lived anti-CD38 × anti-CD3 bispecific antibodies stimulates potent T cell-mediated killing of human myeloma cell lines and CD38+ cells in monkeys: a potential therapy for Multiple Myeloma. Blood 2014; 124:2316; PMID:25301330; http://dx.doi.org/10.1182/blood-2014-05-576900
- Peng KW, Donovan KA, Schneider U, Cattaneo R, Lust JA, Russell SJ. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood 2003; 101(7):2557-62; PMID:12433686; http://dx.doi.org/10.1182/blood-2002-07-2195
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119(12):2709-20; PMID:22160384; http://dx.doi.org/10.1182/blood-2011-10-384388
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371(16):1507-17; PMID:25317870; http://dx.doi.org/10.1056/NEJMoa1407222
- Mihara K, Yanagihara K, Takigahira M, Imai C, Kitanaka A, Takihara Y, Kimura A. Activated T-cell-mediated immunotherapy with a chimeric receptor against CD38 in B-cell non-Hodgkin lymphoma. J Immunother 2009; 32(7):737-43; PMID:19561535; http://dx.doi.org/10.1097/CJI.0b013e3181adaff1
- Bhattacharyya J, Mihara K, Kitanaka A, Yanagihara K, Kubo T, Takei Y, Kimura A, Takihara Y. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eradicating chemotherapy-resistant B-cell lymphoma cells overexpressing survivin induced by BMI-1. Blood Cancer J 2012; 2(6):e75; PMID:22829977; http://dx.doi.org/10.1038/bcj.2012.21
- Mihara K, Bhattacharyya J, Kitanaka A, Yanagihara K, Kubo T, Takei Y, Asaoku H, Takihara Y, Kimura A. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia 2012; 26(2):365-7; PMID:21836610; http://dx.doi.org/10.1038/leu.2011.205
- Drent E, Groen RW, Noort WA, Themeli M, Lammerts van Bueren JJ, Parren PW, Kuball J, Sebestyen Z, Yuan H, de Bruijn J et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica 2016; 101(5):616-25; PMID:26858358; http://dx.doi.org/10.3324/haematol.2015.137620
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29(7):917-24; PMID:21282551; http://dx.doi.org/10.1200/JCO.2010.32.2537
- Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015; 21(5):1019-27; PMID:25538264; http://dx.doi.org/10.1158/1078-0432.CCR-14-2708
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015; 21(8):914-21; PMID:26193344; http://dx.doi.org/10.1038/nm.3910
- Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, Belch A, Krishnan AY, Vescio RA, Mateos M et al. Phase II study of daratumumab (DARA) monotherapy in patients with ≥ 3 lines of prior therapy or double refractory multiple myeloma (MM): 54767414MMY2002 (Sirius). J Clin Oncol 2015; 33: abstr LBA8512
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373(13):1207-19; PMID:26308596; http://dx.doi.org/10.1056/NEJMoa1506348
- Plesner T, Arkenau H, Gimsing P, Krejcik J, Lemech C, Minnema MC, Lassen U, Laubach JP, Palumbo A, Lisby S, et al. Daratumumab in Combination with Lenalidomide and Dexamethasone in Patients with Relapsed or Relapsed and Refractory Multiple Myeloma: Updated Results of a Phase 1/2 Study (GEN503). Blood 2015; 126(23):507
- Chari A, Lonial S, Suvannasankha A, Fay JW, Arnulf B, Ifthikharuddin JJ, Qin X, Masterson T, Nottage K, Schecter JM, et al. Open-Label, Multicenter, Phase 1b Study of Daratumumab in Combination with Pomalidomide and Dexamethasone in Patients with at Least 2 Lines of Prior Therapy and Relapsed or Relapsed and Refractory Multiple Myeloma. Blood 2015; 126(23):508
- Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, Belch A, Krishnan A, Vescio RA, Mateos MV et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387:1551-60; PMID:26778538; http://dx.doi.org/10.1016/S0140-6736(15)01120-4
- Martin TG, Strickland SA, Glenn M, Zheng W, Daskalakis N, Mikhael JR. SAR650984, A CD38 Monoclonal Antibody In Patients With Selected CD38+ Hematological Malignancies – Data From A Dose-Escalation Phase I Study. Blood 2013; 122(21):284
- Martin TG, Hsu K, Charpentier E, Vij R, Baz RC, Benson DM, Lendvai N. A Phase Ib Dose Escalation Trial of SAR650984 (Anti‐CD‐38 mAb) in Combination with Lenalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma. Blood 2014; 124(21):83
- Raab MS, Goldschmidt H, Agis H, Blau I, Einsele H, Engelhardt MM, Ferstl B, Gramatzki M, Röllig C, Weisel K, et al. A phase I/IIa study of the human anti-CD38 antibody MOR202 (MOR03087) in relapsed or refractory multiple myeloma (rrMM). J Clin Oncol 2015; 33:8574
- Alici E, Chrobok M, Lund J, Ahmadi T, Khan I, Duru AD, Nahi H. Re-challenging with anti-CD38 monotherapy in triple-refractory multiple myeloma patients is a feasible and safe approach. Br J Haematol 2015
- Ise M, Matsubayashi K, Tsujimura H, Kumagai K. Loss of CD38 expression in relapsed refractory multiple myeloma. Clin Lymphoma Myeloma Leuk 2016; 16(5):e59-64; PMID:26997107; http://dx.doi.org/10.1016/j.clml.2016.02.037
- Vooijs WC, Schuurman HJ, Bast EJ, de Gast GC. Evaluation of CD38 as target for immunotherapy in multiple myeloma. Blood 1995; 85(8):2282-4; PMID:7718903
- Casneuf T, Lysaght A, LeFave C, Bald J, Weiss B, van de Donk NWCJ, Lokhorst HM, Ahmadi T, Sasser AK. Serum Proteomic Analysis of Multiple Myeloma Subjects Treated with Daratumumab Monotherapy. Blood 2015; 126:1837
