ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease in need of prognostic markers to address therapeutic choices. We have previously shown that alternative splicing of the actin regulator, hMENA, generates hMENA11a, and hMENAΔv6 isoforms with opposite roles in cell invasion. We examined the expression pattern of hMENA isoforms by immunohistochemistry, using anti-pan hMENA and specific anti-hMENA11a antibodies, in 285 PDACs, 15 PanINs, 10 pancreatitis, and normal pancreas. Pan hMENA immunostaining, absent in normal pancreas and low-grade PanINs, was weak in PanIN-3 and had higher levels in virtually all PDACs with 64% of cases showing strong staining. Conversely, the anti-invasive hMENA11a isoform only showed strong staining in 26% of PDAC. The absence of hMENA11a in a subset (34%) of pan-hMENA-positive tumors significantly correlated with poor outcome. The functional effects of hMENA isoforms were analyzed by loss and gain of function experiments in TGF-β1-treated PDAC cell lines. hMENA11a knock-down in PDAC cell lines affected cell–cell adhesion but not invasion. TGF-β1 cooperated with β-catenin signaling to upregulate hMENA and hMENAΔv6 expression but not hMENA11a In the absence of hMENA11a, the hMENA/hMENAΔv6 up-regulation is crucial for SMAD2-mediated TGF-β1 signaling and TGF-β1-induced EMT. Since the hMENA isoform expression pattern correlates with patient outcome, the data suggest that hMENA splicing and related pathways are novel key players in pancreatic tumor microenvironment and may represent promising targets for the development of new prognostic and therapeutic tools in PDAC.
Abbreviations
| AS | = | alternative splicing |
| DAPI | = | 4′6-diamidino-2-phenylindole |
| ECM | = | extracellular matrix |
| EGF | = | Epidermal Growth Factor |
| EMT | = | epithelial to mesenchymal transition |
| ENA/VASP | = | Enabled/Vasodilator-stimulated phosphoprotein |
| ESRP1/2 | = | epithelial splicing regulatory proteins |
| FFPE | = | formalin-fixed and paraffin-embedded |
| GSK-3β | = | glycogen synthase kinase-3 β |
| hMENA | = | Human MENA |
| HPDE | = | normal human pancreatic ductal cell line |
| IF | = | immunofluorescence analysis |
| MMPs | = | matrix metalloproteinases |
| PDAC | = | pancreatic ductal adenocarcinoma |
| PanIN | = | pancreatic intraepithelial neoplasia |
| pSMAD | = | phosphorylated SMAD |
| qPCR | = | quantitative real-time polymerase chain reaction |
| TGF-β1 | = | transforming growth factor β1 |
| TMAs | = | tissue microarrays |
Introduction
Pancreatic ductal adenocarcinoma (PDAC) arises generally from pancreatic intraepithelial neoplasia (PanIN)Citation1 and progresses with cellular and architectural atypia, along with the acquisition of a complex desmoplastic response.Citation2 Dynamic interactions between tumor, stromal cells, and autocrine and paracrine signaling lead to epithelial to mesenchymal transition (EMT), an early process in the natural history of pancreatic cancer.Citation3 Although the perturbation of epithelial cell–cell junctions is a main trait of EMT in PDAC,Citation4 PDAC with metastatic traits do not lose of E-cadherin, suggesting that EMT-related markers are not prognostic markers.Citation5 Cytoskeletal reorganization,Citation6 extracellular matrix (ECM) remodeling, and matrix metalloproteinase (MMPs)Citation7 contribute to PDAC aggressiveness in cooperation with soluble growth factor or cytokines, with TGF-β1 as a crucial player.Citation8 Alternative splicing is known to play a prominent role in tumor progression and the derived isoforms may represent powerful diagnostic and prognostic factors,Citation9 as we have recently shown for hMENA alternative splicing in early stage non-small cell lung cancer (NSCLC).Citation10 hMENA belongs to the Ena/VASP family of actin regulatory proteins, which modulate cell adhesion and migrationCitation11 and have been involved in axon guidance,Citation12 carcinogenesis,Citation13 and tumor invasiveness.Citation14 We have isolated hMENA by serological analysis of recombinant cDNA expression library (SEREX) of a breast tumor with the autologous patient serum.Citation15 Then, we have shown that alternative splicing generates multiple hMENA protein isoforms with hMENA11a and hMENAΔv6 associated with epithelial or mesenchymal-like cellsCitation14 respectively, and opposite roles in cell proliferationCitation16,17 and invasion.Citation14 The inclusion of exon 11a in hMENA is regulated by the epithelial splicing regulatory proteins (ESRP1/2).Citation18 In PDAC cell lines, hMENA11a expression identifies EGFR-dependent cells that are sensitive to the EGFR inhibitor ErlotinibCitation19 and hMENA11a has been suggested to provide survival signals in breast cancer.Citation20 hMENAΔv6, which lacks exon 6, is expressed only in the invasive T4-2 cells of an isogenic model of breast cancer progression and is related to EMT marker expression and cancer cell invasiveness in breast, cervix, and lung cell lines.Citation10, Citation14
Here, we show that the two alternatively expressed hMENA isoforms are differentially regulated by TGF-β1 and that the presence of hMENAΔv6 along with the lack of hMENA11a is crucial in the TGF-β1-mediated EMT process and cell invasiveness. Moreover, differential isoform expression correlates with patient outcome, suggesting that hMENA expression pattern may be a potential prognostic marker and their related pathways may represent novel therapeutic targets.
Results
The pattern of hMENA isoform expression is a novel prognostic marker for pancreatic ductal adenocarcinoma patients
Our discovery that the pattern of hMENA isoforms may be a useful marker for early stage NSCLC,Citation10 suggested that this unique signature might also fill the lack of prognostic marker for pancreatic cancer. We explored the expression of hMENA isoforms in a series of surgically resected PDACs (n = 285), PanINs (n=15), chronic pancreatitis (n = 10), and normal pancreatic tissues from transplant donors (n = 3) (kindly provided by Lorenzo Piemonti, IRCCS San Raffaele Scientific Institute, Milan, Italy). Representative cases are reported in . The hMENA isoforms evaluated were hMENA11a, hereafter named Iso-11a, (90 kDa), hMENA (88 kDa), and hMENAΔv6, hereafter named Iso-Δv6, (80 kDa) () using pan hMENA and hMENA11a antibodies (). No immunoreactivity was observed in ducts or acini of normal pancreas (), whereas in pancreatitis, a low to moderate immunoreactivity was present (). Similarly, PanIN-1 and PanIN-2 were negative for both antibodies (data not shown), whereas three out of five PanIN-3 showed a weak cytoplasmic pan hMENA and Iso-11a staining ().
Figure 1. (A) Pattern of hMENA isoform expression in human pancreatic ductal carcinogenesis. Representative immunohistochemical staining with pan hMENA and (B) Iso-11a in normal pancreas (a), chronic pancreatitis, (b), PanIN-3 (c), and PDAC (d and e). Red arrows indicate strong staining in non-neoplastic pancreatic ducts adjacent to the tumor. Magnification 10X, Scale Bar = 100 µm (a, b, c, d); Magnification 20X, Scale bar = 30 µm (e). (C) Schematic representation of human hMENA transcripts and isoforms evaluated: hMENA and the two alternatively expressed hMENA11a and hMENAΔv6. (D) hMENA antibodies used in the immunohistochemical analysis.
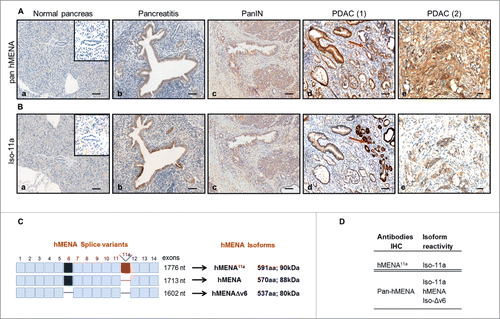
The immunohistostaining was scored according to a four-tiered scale: negative (score 0), weak (score 1), intermediate (score 2), and strong (score 3) (Fig. S1). The Iso-Δv6 antibody was unsuitable for immunohistochemical evaluation (Fig. S3); thus tumors that are pan-hMENA positive/Iso-11a negative would be evaluated as expressing hMENA/Iso-Δv6 as previously reported.Citation14
The characteristics and patterns of expression of hMENA isoforms in PDAC patients are detailed in Table S1. Virtually, all PDACs (233 of 237 evaluable cases, 98%) were positive for pan hMENA, and 152/237 (64%) had score 3 (representative cases shown in ). However, a strong expression (score 3) of Iso-11a was detected only in 26% (51 out of 194) of samples (Table S1), suggesting that a low expression of Iso-11a () may occur in PDAC along with high expression of different hMENA isoforms (i.e., hMENA and Iso-Δv6).
Differently from other cancer types, such as breast and lung,Citation13,10 non-neoplastic pancreatic ducts adjacent to the tumor showed a strong reactivity with both antibodies (). This pattern of hMENA isoform expression was validated also in an independent data set of PDAC whole tissue sections (n=53) available at the Regina Elena National Cancer Institute (data not shown).
When we examined the relationship between hMENA isoform expression and patient clinico-pathological characteristics, pan hMENA expression showed a linear association only with tumor size (T) (p = 0.04), (Fig. S2). As expected, pan hMENA and Iso-11a positivity were significantly correlated (p < 0.0001) (not shown).
To find whether a relationship exists between the pattern of hMENA isoform expression and patient survival, we performed a survival analysis in pan hMENA positive cases. In this group, at a median follow-up of 17 mo (range 1–173), the median overall survival (OS) was 18 mo (CI 95% [16–20]). The following variables were considered at univariate analysis: Sex, Age, Grade, Resection margins, T (size), N, M, Stage, Vascular, Perineural and Fat Invasion, pan hMENA and Iso-11a staining. Tumor grade (3 vs. 1/2) (HR=1.44, CI95% [1.03–2.01], p = 0.03) and stage (IIB/III/IV vs. IA/IIA), (HR=1.58, CI95% [1.02–2.43], p = 0.04) were the only independent prognostic factors at multivariate analysis.
To explore whether different pan hMENA and Iso-11a scores may identify subgroups with different prognosis, we used ROC analysis to determine pan hMENA cutoff able to distinguish cases of Iso-11a positive (score 1–3) and negative (score 0). Results showed that a pan hMENA immunohistochemical score > 2 better discriminated Iso-11a positivity. Thus, two groups were obtained: i) pan hMENA score 3, Iso-11a positive or negative, and ii) pan hMENA scores 1–2, Iso-11a positive or negative. Of note, among cases scored 1–2 for pan hMENA, the patients with 3 y OS were 51% Iso-11a positive vs. 18.2% Iso-11a negative (p = 0.003) (). Interestingly, in this group the expression of Iso-11a was the only significant prognostic indicator at multivariate analysis (HR=3.09, CI95% [1.31–7.25], p = 0.01). No differences in terms of survival exist between Iso-11a positive and negative cases among group scored 3 for pan hMENA (not shown). No statistical significance was observed in tumors with pan hMENA score 3 () and tumors Iso-11a negative ().
Figure 2. Correlation between hMENA isoform expression and patient survival. (A) Kaplan–Meier survival curves in pan hMENA score 1–2 PDAC patients, according to Iso-11a expression status. Among pan hMENA score 1–2 cases, the Iso-11a negative staining was associated with poor overall survival. (B) Kaplan–Meier survival curves for PDAC patients according to pan hMENA immunostaining and (C) to Iso-11a expression.
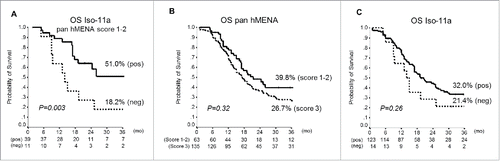
These results clearly indicate that the hMENA isoform expression pattern is clinically relevant in PDACs, and the absence/presence of Iso-11a may be of prognostic value, at least in the subset of patients with pan hMENA 1–2 score.
Silencing of Iso-11a in epithelial PDAC cells disrupts cell junction integrity without inducing cell invasiveness
To understand the mechanisms by which hMENA isoform expression pattern is linked to a different prognosis in PDAC patients we performed molecular, biochemical, and functional experiments using a panel of pancreatic cell lines from non-tumorigenic (HPDE) to highly invasive. The inclusion or skipping of exon 11a and exon 6 was analyzed by semi-quantitative RT-PCR, by using hMENA-specific primers. HPDE and ASPC1 cells showed exon 11a inclusion, whereas PANC1 and C5M2 cells lack exon 11a and express the splice variant with the skipping of exon 6 (). As previously reported in different tumors,Citation10,14,19 WB with available antibodies (Fig. S3B) showed that Iso-11a (90 kDa protein) correlated with E-cadherin expression in HPDE, CFPAC and ASPC1. Conversely, PANC1 and C5M2 cells lacked the Iso-11a isoform but expressed Iso-Δv6 (80 kDa protein) (Fig. S3A), along with vimentin and low levels of E-cadherin. The hMENA isoform (88 KDa) was expressed in all cell lines analyzed.
Figure 3. Silencing of Iso-11a perturbs cell–cell adhesion but does not trigger invasion of PDAC cells. (A) RT-PCR analysis of Iso-11a and Iso-Δv6 expression with primers flanking exon 11a (upper, exon 11a inclusion = □▪□, E11a+; 11a skipping = □□, E11a–) or with primers flanking exon 6 (lower, exon 6 inclusion = □▪□, E6+; exon 6 skipping = □□, E6–), in HPDE, ASPC1, PANC-1 and C5M2 cell lines. (B) Calcium switch assay of HPDE transfected with non-targeting siRNA (si-CNT) or Iso-11a-specific siRNA (si-Iso-11a). Representative IF analysis of cells incubated with calcium supplemented medium (+ Ca2+) for 150 min with Iso-11a (red), β-catenin (green) antibodies. Nuclei were visualized with DAPI (blue). Magnification 63X. Scale bar: 50 µm. (C) Hanging drop aggregation assay in si-CNT and si- Iso-11a HPDE. Cells in drops were classified in small, medium, and large cluster size. Histograms represent the percentage of cells counted in each clusters. (D) qRT-PCR analysis of MMP3 expression of si-CNT and si-Iso-11a HPDE cells. (E) WB analysis of ASPC-1 cells (si-CNT and si-Iso-11a). Representative immunoblot is shown (left panel) and the quantification of E-cadherin and β-catenin expression is reported. (F) Matrigel invasion assay of si-Iso-11a ASPC-1 compared to si-CNT cells. (G) Hanging drop aggregation assay in PANC-1 cells stably transfected with the empty vector (CNT) or with the Iso-11aexpression vector (Iso-11a). The relative WB analysis is shown (right). Data are presented as mean ± SD of three independent experiments; *p ≤ 0.05.
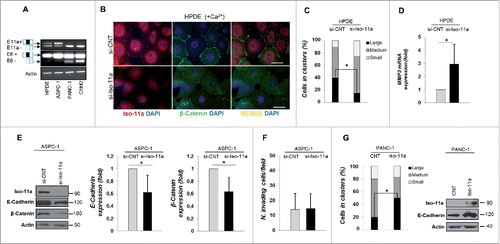
Since our immunostaining results showed that loss of Iso-11a, relative to total hMENA expression, was indicative of a worse prognosis in a subgroup of pancreatic cancers, we analyzed the functional effects of specific Iso-11a silencing in PDAC biology. Considering the role of Iso-11a in cell-cell adhesion as suggested in both murine modelCitation21 and human tissues,Citation14 we evaluated the effect of the depletion of Iso-11a on cell-cell junction formation, using a calcium switch assay. The non-tumorigenic HPDE, a well-established cell line to study adherence junction remodelling in pancreas,Citation22 were transfected with siRNAs directed against Iso-11aCitation17 and non-targeting siRNA (si-Iso-11a and si-CNT, respectively); the knockdown was confirmed by qRT-PCR and WB analyses (Fig. S4 A left and right panels, respectively). Monolayers of si-CNT and si-Iso-11a HPDE cells were placed in low calcium medium to disrupt cell–cell junctions and then switched to high calcium to trigger junction re-assembly. Immunofluorescence analysis of HPDE si-CNT cells showed that 150 min after calcium restoration (+CaCitation2+), Iso-11a was present at cell–cell contacts (, upper panels), and then shuttled to its cytoplasmic localization at 16 h after calcium restoration (not shown). A continuous staining of β-catenin was observed at cell–cell junctions in HPDE si-CNT cells (, upper panels), whereas si-Iso-11a cells showed a reduced and discontinuous β-catenin staining (, lower panels), indicating that Iso-11a downregulation may interfere with the integrity of the cell–cell junction. To confirm the role of Iso-11a in cell–cell adhesion regulation, we carried out a hanging-drop aggregation assay that measures the ability of cells to aggregate by forming cell–cell junctions. After cell–cell junction dissociation, the size of the formed aggregates was evaluated and we found that si-Iso-11a cells were less able to form cell aggregates compared to si-CNT cells. Quantitative analysis of the size of clusters showed that 40.2% of control cells aggregate in large clusters, whereas only 15% of large clusters were observed in Iso-11a-silenced cells, showing that Iso-11a knock-down impairs the formation of cell–cell junctions ().
The weakening of cell–cell adhesion along with the loss of E-cadherin expression has been regarded as a crucial step in the acquisition of a migratory and invasive mesenchymal phenotype.Citation23 To evaluate the functional role of Iso-11a downregulation in tumor cells, we analyzed the effects of Iso-11a silencing in the epithelial ASPC-1 cancer cell line (Fig. S4B), expressing high levels of Iso-11a (Fig. S3A). We found that Iso-11a silencing significantly reduced E-cadherin and β-catenin expression (); similar results were obtained in HPDE cells (not shown). Iso-11a silencing also increased the mRNA expression level of the matrix metalloproteinase 3 (MMP3; ), the overexpression of which in normal breast cells induced a functional alteration in the cell–cell adhesion system,Citation24 further supporting a relevant role of Iso-11a in cell–cell adhesion regulation.
Importantly, however, the silencing of Iso-11a did not induce invasion (), suggesting that reduction of E-cadherin or β-catenin is not sufficient for acquisition of an invasive phenotype. Conversely, the forced expression of Iso-11a in the invasive PANC-1 cells (hMENA/Iso-Δv6 positive) led to enhanced cell–cell aggregation, (50% vs. 20%, respectively) along with a significant increase in E-cadherin expression (). Furthermore, we observed a significant decrease in cell invasiveness when Iso-11a or its splicing regulator, ESRP1, was overexpressed in PANC-1 cells, in agreement with our previous reports on breast and lung cancerCitation14,10 (Fig. S4C and D).
These data show further that Iso-11a when overexpressed, indeed exerts an anti-invasive role also in pancreatic cancer, consistent with clinical follow-up data in PDAC patients. On the other hand, the data above indicate that Iso-11a loss is not sufficient for invasion to proceed. To the best of our knowledge, this is the first time this phenomenon has been reported in the literature and we suggest that the cancer cell invasiveness requires not only the lack of Iso-11a, but also the activation of pathways linked to the expression of Iso-Δv6 isoform.
TGF-β1 induces expression of hMENA and Iso-Δv6 isoforms via β-catenin, a step crucial for TGF-β1-mediated EMT
TGF-β1 is a crucial player in pancreatic cancer.Citation25 We asked whether hMENA expression that is detectable in 98% of PDACs evaluated, may be regulated by TGF-β1. Quantitative RT-PCR (qRT-PCR) analysis of total hMENA mRNAs showed that TGF-β1 treatment induced an increase in total hMENA transcripts. This regulation was evident both in mesenchymal-like cell lines and epithelial cells such as PANC-1 () and ASPC-1 (Fig. S5A), respectively.
Figure 4. hMENA and Iso-Δv6 isoforms are upregulated by TGF-β1 via β-catenin and are required for the TGF-β1-mediated EMT. (A) qRT-PCR analysis of hMENA mRNAs in PANC-1 cells untreated or TGF-β1-treated (24–48 h). (B) Representative WB (left) and quantitative analysis of Iso-Δv6 (middle) and hMENA (right) expression in si-CNT or si-β−catenin PANC-1 cells untreated or TGF-β1-treated (48 h). (C) IF analysis of si-CNT (left) or si-pan hMENA (right) PANC-1 cells, untreated or TGF-β1-treated (48 h). Cells were stained with a pan hMENA antibody (green), F-actin detected by phalloidin (red), nuclei visualized with DAPI (blue). Magnification 63X. Scale bar: 50 µm. hMENA is relocated to actin stress fibers (inset) in TGF-β1-treated cells. Assembly of actin stress fibers (inset) is impaired in si-pan hMENA cells. (D) Quantitative analysis of cell morphology of PANC-1 si-CNT or si-pan hMENA cells, untreated or TGF-β1-treated, evidenced that the morphological index is reduced in si-pan hMENA PANC-1 cells. Data shown are the mean ± SD from two independent experiments. (E) qRT-PCR analysis of Snail1 mRNA expression in si-CNT and si-pan hMENA PANC-1 cells untreated or TGF-β1-treated (24 h). Data are reported as the mean ± SD of three independent experiments; *p ≤ 0.05. (F) WB analysis of vimentin expression in siCNT and si-pan hMENA PANC-1 cells untreated or TGF-β1–treated (48 h).
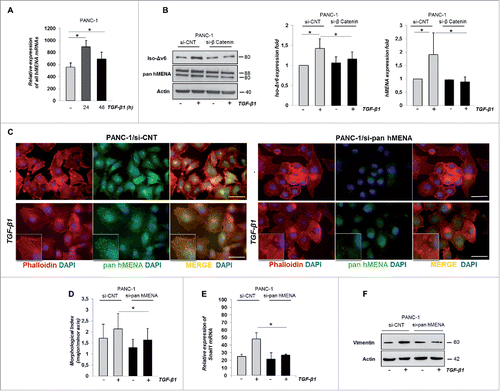
In response to TGF-β1, β-catenin is translocated into the nucleus where it acts as a transcriptional co-factor required for EMT gene transcription.Citation26,27 Indeed, PANC-1 cells treated with TGF-β1 showed a nuclear translocation of β-catenin (Fig. S5B). Since, β -catenin has been reported as a transcriptional regulator of MENA expression Citation(28 and our data presented here) (Fig. S5C), we hypothesized that it may be directly involved in TGF-β1-induced hMENA mRNA upregulation. Thus, depletion of β-catenin expression by siRNA in PANC-1 cells (Fig. S5D) significantly abrogated the TGF-β1-induced hMENA/Iso-Δv6 isoform upregulation (), suggesting that β-catenin is required for TGF-β1-mediated hMENA/Iso-Δv6 overexpression.
Considering the role of EMT process in PDACCitation3 and the major role of TGF-β1 in this process,Citation8 we examined whether hMENA/Iso-Δv6 expression, which correlates with a mesenchymal-like phenotype, is involved also in TGF-β1-mediated EMT. We silenced all hMENA isoforms by siRNA (si-pan hMENA) in PANC-1 cells (Fig. S5E). The silencing was specific for hMENA isoforms, since it did not affect VASP protein expression, the other member of the Ena/VASP family (data not shown). Cells were treated with TGF-β1 and monitored for cell morphology. As expected, upon TGF-β1 treatment, the morphology of si-CNT cells changed significantly from epithelial into a mesenchymal spindle-like phenotype with a consistent rearrangement of actin cytoskeleton and stress fiber formation, as detected by phalloidin staining of F-actin (). Interestingly, in untreated cells, pan hMENA antibody staining was found mainly in structures reminiscent of focal adhesions, whereas after TGF-β1 treatment, hMENA staining was evident in actin stress fibers, suggesting that hMENA/Iso-Δv6 actively participate in TGF-β1-induced EMT.
Immunofluorescence (IF) analysis in si-CNT PANC-1 cells revealed that the actin cytoskeleton was organized in parallel bundles, which were disrupted in si-pan hMENA/PANC-1 cells. Importantly, si-pan hMENA PANC-1 cells did not reach a full morphological mesenchymal transition and were significantly less elongated upon TGF-β1 treatment than si-CNT control cells (). A quantification of the degree of elongated cell morphology, calculated as the ratio of the major axis to the minor axis of cells, is shown in indicating that depletion of hMENA isoforms inhibits the TGF-β-induced morphological changes.
To verify whether this morphological effect observed in pan hMENA depleted PANC-1 cells is accompanied by deregulation of the EMT-related proteins, we measured the level of Snail1 induced by TGF-β1. We found Snail mRNA level to be significantly reduced in si-pan hMENA PANC-1 cells treated with TGF-β1 (). Consistently, we found that downregulation of E-cadherin mRNA expression by TGF-β1 was partially abolished in si-pan hMENA PANC-1 cells (data not shown), along with a reduced TGF-β1-induced Vimentin expression ().
Our findings show that TGF-β1 upregulates hMENA/Iso-Δv6 expression via β-catenin and we demonstrate the crucial role of hMENA/Iso-Δv6 in regulating TGF-β1-mediated EMT in PDAC.
The hMENA/Iso-Δv6 upregulation induced by TGF-β1 is crucial for SMAD2- mediated TGF-β1 signaling and cancer cell invasiveness
We explored whether hMENA depletion (si-pan hMENA) affects TGF-β1-SMAD signaling, by measuring phosphorylation level of SMAD2 in PANC-1, untreated or TGF-β1-treated for 30 and 60 min. Whereas TGF-β1-induced phosphorylation of SMAD2 (S465/467) in control cells (si-CNT) (), hMENA-silenced cells had drastically reduced levels of phosphorylated SMAD2 (p-SMAD2) in response to TGF-β1 (). We obtained similar results by analyzing pSMAD3 expression (data not shown). Since phosphorylation of SMAD2 upon TGF-β1 treatment is required for its nuclear translocation,Citation29 we evaluated whether reduction of p-SMAD2 levels in pan hMENA-silenced cells was accompanied by reduction of nuclear SMAD2 accumulation upon TGF-β1 stimulation. Nuclear extracts of TGF-β1-treated PANC-1 cells showed that p-SMAD2 expression was dramatically reduced in pan hMENA-silenced cells (). Similarly, IF analysis showed that the percentage of cells with a pSMAD2 nuclear positivity was significantly reduced in pan hMENA- silenced cells ().
Figure 5. The expression of hMENA/iso-Δv6 isoforms is crucial for the SMAD2-mediated TGF-β1 signaling and PANC-1 cell invasiveness. (A) Representative WB (left) and quantitative analysis of the ratio of p-SMAD2/total SMAD2 protein levels (right) in si-CNT or si-pan hMENA PANC-1 cells untrated or TGF-β1-treated (30–60 min). (B) WB analysis of pSMAD2 expression in nuclear extracts of si-CNT and si-pan hMENA untreated or TGF-β1-treated PANC-1 cells (24 h). Histone H3 was the control of nuclear fractions. (C) IF analysis of p-SMAD2 (red) and quantification (%) of its nuclear positivity (right) in si-CNT or si-pan hMENA PANC-1 cells, untreated or TGF-β1-treated (24 h). Nuclei were counterstained with DAPI (blue). Magnification 63X. Scale bar: 50 µm. (D) Migration and (E) invasion assays of si-CNT or si-pan hMENA PANC-1 cells pre-treated or not with TGF-β1 (24 h). (F) MMP2 secretion in conditioned medium (CM) from si-CNT and si-pan hMENA PANC-1 cells untreated or TGF-β1-treated (48 h). Data are mean ± SD of three independent experiments. *p ≤ 0.05.
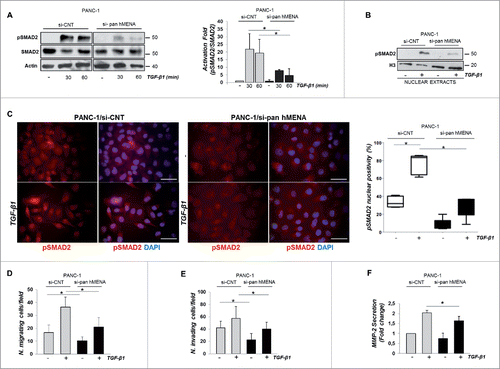
To understand the role of hMENA/Iso-Δv6 in TGF-β1-induced cell migration and invasion, we used a modified Boyden chamber and transwell invasion assay. Si-pan hMENA in PANC-1 cells significantly reduced both migration and invasion ability, in control and TGF-β1-treated cells, indicating that hMENA/Iso-Δv6 expression is crucial in regulation of both phenomena in these cells ().
MMPs were shown to be involved in regulation of PDAC cell invasiveness.Citation7 We evidenced that TGF-β1-induced MMP-2 secretion was significantly reduced in pan hMENA silenced PANC-1 cells ().
Collectively, these data point to the critical role of the hMENA/Iso-Δv6 isoforms in regulating SMAD-mediated TGF-β1 signaling and cell invasiveness in PDAC.
TGF-β1 and β-catenin signaling specifically upregulate hMENA and Iso-Δv6 isoform expression but not Iso-11a
TGF-β1 signaling intersects with other pathways to fine-tune context-dependent biological responsesCitation30 and a co-operation between TGF-β1- and Wnt/β-catenin-pathways occurs.Citation27,31 We, therefore, investigated the effects of LiCl, an activator of Wnt/β−catenin signaling pathwayCitation32 on hMENA expression, alone or in combination with TGF-β1. We treated PANC-1 cells for 24 h with TGF-β and/or with LiCl. The qRT-PCR analysis () showed that LiCl, like TGF-β1 treatment, increased the total levels of hMENA trascripts. Of relevance, the cells treated with both LiCl and TGF-β1 showed a significant increase of hMENA mRNA expression ().
Figure 6. TGF-β1 cooperates with β-catenin signaling to increase hMENA and Iso-Δv6 but not Iso-11a expression. (A) qRT-PCR analysis of mRNA levels of all hMENA isoforms of PANC-1 cells treated with TGF-β1 and/or LiCl (24 h). (B) WB analysis of PANC-1 untreated or TGF-β1 and/or LiCl-treated (48 h). (C) qRT-PCR analysis of mRNA levels of all hMENA isoforms in ASPC-1 untreated or TGF-β1 and/or LiCl-treated (24 h). (D) qRT-PCR analysis of Iso-11a mRNA in ASPC-1 untreated or TGF-β1 and/or LiCl-treated (24 h). (E) WB analysis of ASPC-1 cells treated with TGF-β1 and/or LiCl (48 h). (F) WB analysis of Iso-11a and ESRP1 transfected PANC1 cells, untreated or TGF-β1-treated (48 h). Data are mean ± SD of three independent experiments. *p ≤ 0.05.
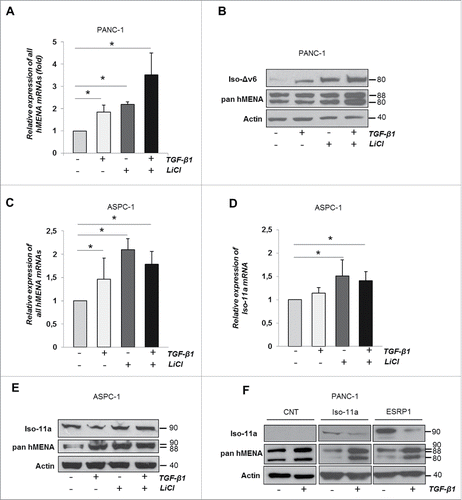
Taking into account that Iso-Δv6 and Iso-11a are expressed in a mesenchymal and an epithelial cell context, respectively, we explored whether TGF-β1 and/or LiCl differentially affect the protein expression of these isoforms. In the context of mesenchymal-like PANC1, either TGF-β1 or LiCl treatments increased both hMENA and Iso-Δv6 expression, with an even greater increase when used in combination (). These data indicate that cooperation between TGF-β and Wnt/β-catenin determine hMENA/Iso-Δv6 overexpression in the mesenchymal PANC-1 cells.
In the epithelial context of Iso-11a expressing ASPC-1 cells, however, a diverse effect of LiCl and TGF-β was observed. The qRT-PCR analysis of Iso-11a mRNA showed that expression of Iso-11a was induced by LiCl treatment, together with an increase in total hMENA transcripts (). TGF-β1 upregulated the total hMENA transcripts (), but not Iso-11a at either mRNA or protein levels, as shown in , respectively. Similar results were obtained in Iso-11a-positive HPDE cells (not shown). These data indicate that TGF-β1 induced total hMENA transcripts and suggest that it specifically downregulates Iso-11a expression at post-transcriptional levels. Indeed TGF-β1 downregulates expression of ESRP-1, the major splicing regulator of Iso-11a (Fig. S6), in agreement with previous published data.Citation33 At protein level, we found that TGF-β1 treatment reduced the exogenously expressed Iso-11a protein, whereas, as expected, hMENA and Iso-Δv6 isoforms were upregulated (). A similar effect was observed in ESRP-1- transduced PANC-1 cells (). Our data indicate that TGF-β1 induces downregulation of the epithelial Iso-11a isoform expression strengthening the hypothesis that the different roles exerted by TGF-β on hMENA isoform expression may represent a critical event for PDAC cells' invasiveness. Our data show further that hMENA overexpression is downstream of TGF-β and Wnt/β−catenin, two major signaling pathways involved in PDAC tumor progression.Citation25,34
The gain of Iso-Δv6 isoform in the absence of Iso-11a increases vimentin expression and is essential for TGF-β1- induced PDAC cell invasiveness
We showed above that hMENA/Iso-Δv6 are essential in regulation of EMT-related proteins, MMP2 activity and cell invasiveness. We overexpressed Iso-Δv6 in PANC-1 cells without affecting the expression of hMENA (data not shown). Interestingly, even in the absence of TGF-β1, the PANC-1 cells overexpressing Iso-Δv6 showed a clear and dramatic increase in vimentin protein expression without a reduction in E-Cadherin levels (). Functionally, we observed an enhanced MMP-2 and MMP-9 activity in PANC1/Iso-Δv6 cells as determined by in-gel zymography assay using gelatine as a substrate (). These data lend strong support to the findings described above that Iso-Δv6 isoform is implicated in regulation of PDAC cell invasion program.
Figure 7. The gain of Iso-Δv6 isoform in the absence of Iso-11a increases vimentin expression and is essential for TGF-β1-induced PDAC cell invasiveness. Representative WB (left) and quantitative analysis of vimentin and E-cadherin expression in PANC-1 cells transfected with hMENAΔv6 compared with control cells (CNT), untreated or TGF-β1-treated (48 h); (B) Gelatine zymography on CM of control and Iso-Δv6 transfected cells, pre-treated or not with TGF-β1 (24 h). MMP2 and MMP9 quantification is shown as the mean ± SD of three independent experiments.*p ≤ 0.05. (C) qRT-PCR analysis of E-cadherin expression in control and Iso-Δv6 transfected PANC-1 cells. (D) Matrigel invasion assays of CNT and Iso-Δv6 PANC-1 cells untreated or TGF-β1-treated (24 h). Histograms represent the increase of TGF-β1-mediated cell invasiveness. (E) Gelatine zymography of CM from PANC-1/CNT and PANC1/ Iso-11a cells, untreated or TGF-β1-treated (24 h). MMP2 and MMP9 quantification is shown. (F) Matrigel invasion assays of PANC-1/CNT and PANC-1/ Iso-11a cells, untreated or TGF-β1-treated (24 h). Histograms represent the increase of TGF-β1-mediated cell invasiveness. Data are mean ± SD of three independent experiments. *p ≤ 0.05.
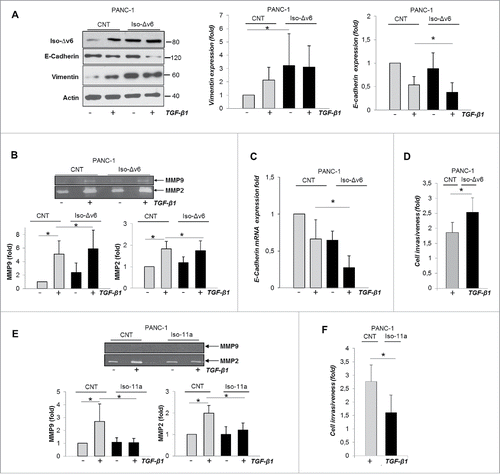
It is important to note that further treatment with TGF-β1 in PANC-1/Iso-Δv6 cells leads to significant E-cadherin downregulation, at both protein () and mRNA levels (), and a consistent increase in TGF-β1-induced cell invasiveness ().
Conversely, the exogenous expression of the epithelial Iso-11a isoform in invasive PANC1 cells dramatically inhibited the TGF-β1 induction of MMP-2 and MMP-9 activities () in parallel with a reduction of TGF-β1-mediated invasiveness (), further supporting its anti-invasive role in PDAC.
In conclusion, these results indicate that the gain of Iso-Δv6 in the absence of Iso-11a expression, a pattern consistent with that found in patients with worse prognosis, is crucial for PDAC cell invasiveness and lends strong support to our contention that this alternative hMENA splicing program and the related pathways may represent promising tools for prognostic and therapeutic targets in PDAC.
Discussion
Herein, we present experimental and clinical evidence supporting the crucial role of the actin regulator hMENA in pancreatic cancer and how the pattern of its isoform expression may represent a diagnostic and prognostic marker in PDAC. These data support our previous findings on the role of hMENA isoforms as a platform for druggable-signaling pathways.Citation17,19,20
Normal pancreas and low grade PanIN (1 and 2) do not express hMENA isoforms, which are expressed in PanIN-3. Nearly all PDAC expressed hMENA isoforms with the anti-invasive Iso-11a detectable at a high level only in 26% of cases. The absence of Iso-11a along with a high level of invasive hMENA/Iso-Δv6 isoforms correlated with a poor outcome, indicating that hMENA isoform pattern identifies subsets of patients with different prognoses, in agreement with our previous data on early NSCLC.Citation10
Our biochemical and functional data show for the first time that hMENA isoforms are crucial in TGF-β1-induced EMT. TGF-β1 downregulates the anti-invasive Iso-11a isoform in parallel with the upregulation of the hMENA/Iso-Δv6 isoforms, a crucial event rendering PDAC cells invasive. The two major signaling pathways involved in PDAC progression, TGF-β1 and Wnt/β−catenin, cooperate in this regulation in line with the broad expression of hMENA isoforms found in PDAC tissues.
During PDAC progression, tumor cells reduce cell–cell adhesion and increase migratory capability,Citation35 and herein, we demonstrate that Iso-11a silencing perturbs cell junction integrity and downregulates E-cadherin and β-catenin protein expression, in agreement with what we have previously reported showing a correlation between Iso-11a and E-cadherin expression in primary breast cancer tissues.Citation14 Since Iso-11a silencing had only a slight effect on the E-cadherin mRNA, we may hypothesize that the regulation may be related to: i) the overexpression of the matrix metalloproteinase MMP3 mRNA, in agreement with the role of this proteinase in triggering E-Cadherin cleavage and degradation;Citation24 ii) the influence of RTKs activation on E-cadherin expression,Citation36 consistent with our recent data that Iso-11a silencing affected both RTK activity and E-cadherin expression.Citation17
However, for the first time we show that the knockdown of Iso-11a does not trigger cell invasiveness, indicating that the exclusion of exon 11a per se is not a feature of invasiveness. Rather we propose that in the absence of Iso-11a, the overexpression of Iso-Δv6 is crucial in EMT and invasiveness, consistent with the increased vimentin expression and MMP activity in Iso-Δv6-transfected cells.
The crucial role of hMENA/Iso-Δv6 was clearly evident at morphological, molecular, and functional levels in TGF-β1-induced EMT. TGF-β1 treatment mobilized hMENA/Iso-Δv6 from focal adhesion to actin stress fibers, fundamental for a mesenchymal spindle-like phenotype and their silencing significantly inhibited this phenotype. These dramatic morphological changes were accompanied by the inhibition of Snail1, the main transcriptional regulator of EMT,Citation37 and at functional level decreased cell migration, invasion, and MMP2 secretion, which were increased in PANC-1-Iso-Δv6 transfected cells.
Actin cytoskeleton also acts as a platform for membrane trafficking and signaling,Citation38 and we observed that hMENA/Iso-Δv6 are crucial for Smad2-mediated TGF-β1 signaling, as evidenced by the strong reduction of nuclear SMAD2 accumulation and phosporylation upon TGF-β1 stimulation in hMENA/Iso-Δv6 depleted cells. The fine-tuning of TGF-β-SMAD signaling is consistent with the effect we have evidenced on Snail1 and E-cadherin regulation.
We hypothesize that, when upregulated by TGF-β1 signaling, hMENA/Iso-Δv6 may act to sustain and amplify TGF-β-signaling branches, such as PI3K/AKT and Rho signaling pathways,Citation39,40 conferring a more aggressive pancreatic tumor-cell phenotype. Notably, we have previously correlated hMENA expression and AKT activation in breast cancer .Citation41 Consistent with the opposite roles of hMENA isoforms in cancer cell invasivenessCitation14 and in patient prognosis, our findings revealed that TGF-β1 upregulates Iso-Δv6 but not Iso-11a, although TGF-β1 increases the expression of hMENA mRNAs both in epithelial and mesenchymal cell lines. This upregulation requires β-catenin expression, given its role as a transcriptional regulator of hMENA.Citation28 This isoform regulation seems to be specific to TGF-β1 and did not occur after the inhibition of GSK3β that participate in the activation of the Wnt/β-Catenin pathway, suggesting that, although the two pathways cooperate in hMENA gene expression, TGF-β1 regulates hMENA isoforms at different levels.
The downregulation of Iso-11a by TGF-β1 occurred in parallel with the downregulation of the splicing regulator ESRP-1, which includes exon 11a,Citation18 in agreement with previous data.Citation33 However, ESRP-1 is not involved in hMENA exon 6 skipping, which takes place through an unidentified mechanism. Our data indicate that TGF-β1 acts not only at post transcriptional, but also at post-translational levels in regulating Iso-11a, as evidenced by Iso-11a protein decrease and Iso-Δv6 increase when Iso-11a was exogenously expressed in PANC-1.
The different effects of TGF-β1 on hMENA isoform expression are consistent with the opposite roles of Iso-11a and Iso-Δv6 in EMT and invasiveness and are supported by our data that PANC-1 Iso-11a engineered cells expressed a higher level of E-cadherin in parallel with a reduced MMP activation and invasive ability in response to TGF-β1. These findings indicate that tumor cells lacking Iso-11a but expressing hMENA and Iso-Δv6, readily undergo EMT, acquiring invasive capability, and imply that the balance between Iso-11a and Iso-Δv6 is important in determining cell fate during cancer progression.
Considering the major role of TGF-β1 as an antagonist of major immune functions,Citation8,42 hMENA and its isoforms may represent fundamental players in the pancreatic tumor immune contexture.
These results provide new insights into molecular pathways involved in PDAC. The pattern of expression of hMENA isoforms may help to tailor therapies and could be used in specific clinical settings for the choice of the most effective treatment.
Materials and methods
Patients and tissues specimens
Ethics
ARC-Net Center: the materials used have been collected under Program 853 protocol 298CE 1502/0215/2002 and Program 1885 protocol 52438 on 2311/1123/2010 and include informed consent of the patient approved by the local ethics committee of the Integrated University Hospital Trust of Verona. Program 853 regarded the collection of pancreas samples for use in molecular research studies. Program 1885 regarded the creation of a coordinated biobank for the collection of samples from all cancer patients. The approved programs include tissue processing and storage methods of formalin-fixed and paraffin-embedded (FFPE) tissues of both neoplastic and normal tissue, including the creation of tissue microarrays. Regina Elena National Cancer Institute: the study and informed consent of patient was reviewed and approved by the local ethics committee (Protocol CE/594/11 on 11/03/2011).
PDAC tissues and TMA construction
A series of 285 FFPE samples from consecutive primary PDACs surgically resected at the University Hospital of Verona from 2004 to 2008 was studied. Thirteen tissue microarrays (TMAs) were assembled with the Manual Tissue Arrayer MTA-1 (Beecher Instruments) and three 1 mm tissue cores obtained from three different tumor areas per single case were included. Twenty additional PDAC-associated normal pancreatic parenchyma cores were used as controls and integrated in the TMAs.
External validation was accomplished using a series of 53 consecutive PDAC patients (median age 62 y; range 39–78 y) who underwent pancreatic resection with curative intent or biopsy at the Regina Elena National Cancer Institute of Rome between 2010 and 2014.
Immunohistochemical analysis
Two consecutive 4 μm sections for each TMA were stained with pan hMENA antibody, (clone 21; BD Bioscience, 610693), that recognizes all hMENA isoforms, including Iso-Δv6, and with a specific monoclonal hMENA11a antibody produced and validated by our group.Citation13,14 Immunostained slides were analyzed and scored independently by four different pathologists, blinded to the clinical data (MF, BR, IC, AS), according to a four-tiered scale as follows: negative (score 0), weak (score 1), intermediate (score 2), and strong (score 3) (Fig. S1).
Cell lines, culture conditions, and reagents
PDAC cell lines CFPAC and PANC1 were kindly provided by Dr. F. Velotti (Tuscia University, Viterbo, Italy), ASPC-1 and C5M2 were a gift from Dr. D. Melisi (University of Verona, Italy). The normal human pancreatic ductal cell line (HPDE) was kindly provided by Dr. P. Allavena (Humanitas Fundation, Milano, Italy). The cell lines were authenticated by chromosomal analysis (BMR Genomics, Italy) and were free of contamination by mycoplasma. The cells were maintained in RPMI 1640 Medium (Euroclone), supplemented with 10% fetal bovine serum (Euroclone), and incubated at 37°C in a 5% CO2 air-humidified atmosphere.
Purified recombinant human TGF-β1 (R&D Systems, 240-B-002) was used at 5 ng/mL in serum-free medium. LiCl (Sigma-Aldrich, 203637) was added to a final concentration of 25 mM.
Transfections, small-interfering RNA treatments, and ESRP-1 infection
Cells in exponential growth phase were plated in six-well plates at a density of 3 × 10Citation5 cells/well and transfected with Iso-11a and Iso-Δv6 expression vectors, or with empty vector,Citation14 using Lipofectamine 2000 reagent (Invitrogen, 11668-019), according to manufacturer's protocol. Iso-11a and relative empty vector stable transfectants were selected with 500 μg/mL of G418 (Invitrogen,10131035). ESRP-1 transduction was performed as previously reported.Citation14
hMENA and β-catenin expression were transiently downregulated using predesigned specific pooled siRNA duplexes (GE-Healthcare, Dharmacon). Specific Iso-11a silencing was performed as recently reported.Citation17 A no targeting siRNA was used as negative control. For TGF-β1 stimulation, 24 h after the transfection, cells were serum starved, treated with 5 ng/mL TGF-β and harvested 24–48 h after the transfection.
Protein extraction and western blot analysis
Protein extraction and Western blot analyses were carried out as previously described.Citation14 Nuclear fractions were prepared using NE-PER Nuclear Cytoplasmic Extraction Reagent kit (Pierce, PI 78833). Primary antibodies used for Western blotting were as follows: Anti-Iso-11a, pan hMENA, and Iso-Δv6 as already described (Citation14 and Fig. S3), anti-E-cadherin (Cell Signaling Technology, 24E10), β−catenin (Cell Signaling Technology, 8480), Vimentin (Cell Signaling Technology, 5741), Snail-1 (Cell Signaling Technology, 3895), phospho-Smad2 (Cell Signaling Technology, 8828), Smad2 (Cell Signaling Technology, 5339), β-actin (Sigma-Aldrich, A4700), HSP70 (Santa Cruz Biotechnology, sc-24), Lamin A/C (Santa Cruz Biotechnology, sc-7292), H3 (Abcam, ab 39655), α-Tubulin (Cell Signaling Technology, 2125). Densitometric quantitation of antibodies immunoreactivity used in WB analysis was determined by Image J 1.49v program (NIH) and normalized in comparison with the β-actin and or HSP70 immunoreactivity.
Hanging drop aggregation assay
Cells were trypsinzed and resuspended at 2.5 × 105 cells/mL in serum free medium. Drops (25 µL) of cell suspension were placed into the inner surface of the lid of a Petri dish and incubated for 150 min. Subsequently, hanging drops were placed into a glass slide and four random fields of cells were photographed for each condition and cells in aggregates (clusters) of different sizes (small: 1–10 cells, medium: 11–50 cells, large: >50 cells) were counted. This experiment was performed three times for each cell line condition. Between 1900 and 2000 cells of each cell type were counted.
Calcium switch assay
siRNA-transfected cells were serum starved and then treated with 4 mM EGTA for 30 min followed by replacement of the fresh medium containing Ca2+ (1.8 mM) to initiate junction reassembly. At the 150 min time point, cells were fixed in 3.7% paraformaldehyde and processed for IF analysis.
Semiquantitative and real-time RT-PCR
5 µg of total mRNA were extracted using Trizol reagent (Invitrogen) to obtain the relative cDNA by first strand cDNA synthesis kit (GE-Healthcare, 27-9261-01) according to the manufacturer's protocol.
Platinum Pfx DNA polymerase (Invitrogen, 11708013) was used for semiquantitative RT-PCR reactions and the inclusion or skipping of exons 11a and 6 was analyzed by using hMENA specific primers, as already reported:Citation14 P7 forward (5′-GAATTGCTGAAAAGGGATCGAATTGCTGAAAAGGGATC-3′) and P8 reverse (5′ CTGTTCCTCTATGCAGTATTTGAC-3′) flanking the exon 11a to detect the inclusion/skipping of exon 11a or with primers MTC1 forward (5′-GCTGGAATGGGAGAGAGAGCGCAGAATATC-3′ and MTC2 reverse (5′-GTTCACACCAATAGCATTCCCTCCACTTG-3′) flanking exon 6. RT-PCR for β-actin was performed as control of normalization and PCR products were run on 1% agarose gels.
Quantitative RT-PCR (qRT-PCR) reactions were carried out in triplicates with KAPA SYBR FAST or KAPA PROBE FAST qPCR Master Mix (KK4601 and KK4702 RESNOVA) on an ABI Prism 7500 Real-time PCR instrument (AB Applied Biosystems, Life Technologies).
The primer sequences used for the qRT-PCR were as follows: human E-cadherin (NM-004360.3) forward 5′-GCCCCGCCTTATGATTCTCTGC-3′, reverse 5′-CTCGCCGCCTCCGTACATGTC-3′; human Vimentin (NM-003380.2) forward 5′-CTCTTCCAAACTTTTCCTCCC-3′, reverse 5′-AGTTTCGTTGATAACCTGTCC-3′; human Snail 1 (NM-005985.3) forward 5′-TTCTTCTGCGCTACTGCTGCG-3′, reverse 5′-GGGCAGGTATGGAGAGGAAGA-3′; human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control.
TaqMan Gene Expression Assay (from Applied Biosystems) were used for amplification and quantification of hMENA (Hs00430216), ESRP-1 (Hs00227840), MMP3 (Hs00968305-m1), and for hypoxanthine phosphoribosyltransferase 1 (4331182) gene, used as an endogenous control. qRT-PCR for Iso-11a mRNA quantification was done using custom designed probe and primers. (probe-CTCCAGACGGGATTCT, Oligo 1-forward 3′-ATGGCAGCAAGTCACCTGTTAT-5′, Oligo 2 reverse 3′-TGTAATGAATCATAGGACCTGTTGTCAAAA-5′).
The comparative Ct method (2−ΔΔ/Ct method) was used to determine changes in relative levels of different genes.Citation43
Immunofluorescence
Cells transfected with siRNAs and/or treated with TGF-β1 were fixed and permeabilized as previously described.Citation14 Cells were stained with anti- Iso-11a, anti-pan hMENA (Sigma, HPA028696), total and/or phosphoSmad2 anti-antibodies (Cell Signaling Technology, 8828, 5339), E-cadherin (BD Biosciences, 610181), and β-catenin (Cell Signaling Technology, 8480).
Alexa Fluor 488–labeled phalloidin (Invitrogen, A12379) was used to stain F-actin and nuclei were stained with DAPI (4′6-diamidino-2-phenylindole) (Invitrogen, 62247). Immunofluorescence was analyzed by Leica DM IRE2 microscopy with Leica FW 4000 software.
Quantification of elongated cell morphology
Measurements of TGF-β–treated PANC-1 control and pan hMENA silenced cells were made using images of cells that were stained for F-actin and nuclei and were acquired using a 40× objective. The lengths of the major and minor cell axes were measured using the Image J 1.49v program (NIH). The ratio of the major axis to the minor axis of cells indicated the degree of elongated cell morphology. For each experiment, between 30 and 40 cells of each cell type were measured.
Cell migration and invasion assays
Twenty-four hours after transfection, cells were pre-treated or not with TGF-β1 for 24 h and migration and invasion assays were performed. The migration assay was carried out using a modified Boyden's chamber assay. Briefly, 28 μL of RPMI with 10% FCS was added to the lower chamber and 50 μL of cell suspension (5 × 105 cells/mL) in a serum free medium was added to the upper chamber. After 4 h of incubation, the cells on the lower side of the membrane were stained, photographed, and counted in eight randomly chosen fields.
For cell invasion assay 5 × 105 cells/mL were seeded in Matrigel invasion chamber (8.0 µm pore size; BD Biosciences, 354480). Top chambers (culture inserts) were filled with serum-free medium and bottom chambers were filled with medium containing 10% FBS. After 24–48 h cells at the bottom surface of the membranes were stained, photographed, and at least 10 fields were counted. Each experiment was performed for three times, in triplicates.
Gelatin zymography
The activity of the matrix metalloproteinase MMP2 and 9 was measured by gelatin zymography. 24 h from transfections, cells were washed, and then TGF-β1 was added at 5 ng/mL concentration in serum-free medium. At 48 h of treatment conditioned medium was collected and centrifuged at 6,000 rpm for 10 min at 4°C. MMP-2 and MMP-9 activation was analyzed by gelatin zymography using 10% polyacrylamide resolving gel containing 1 mg/mL gelatine. After electrophoresis, gels were washed with 50 mM Tris–HCl pH 7.5, 5 mM CaCl2 and 2.5% Triton X-100 and then incubated in 50 mM Tris–HCl pH 7.5, 5 mM CaCl2 at 37°C overnight. Gels were stained with 0.25% Coomassie brilliant blue (R-250) dye in 10% acetic acid and 10% isopropanol. Semi-quantitative densitometry was performed using the Image J 1.49v program (NIH).
ELISA analysis of total MMP2 protein levels
Cells transfected with siRNAs were treated or not treated with TGF-β1 for 48 h. Culture media were collected and MMP2 concentration was determined by ELISA analysis. (ELISA kit, R&D Systems, MMP200).
Statistical analysis
Descriptive statistics were used to summarize pertinent study information. The association between variables was tested by the Pearson Chi-Square test or Fisher's exact test, when appropriate.
To identify the optimum cut off point that would more accurately categorize hMENA expression as high or low risk for negative and positive Iso-11a, a receiver operator characteristic (ROC) analysis was conducted. OS was calculated by the Kaplan–Meier product-limit method from the date of the surgery until death. If a patient had not died, OS was censored at the time of the last visit. The log-rank test was used to assess differences between subgroups. Significance was defined at the p ≤ 0 .05 level. The Hazard risk and the confidence limits were estimated for each variable using the Cox univariate model. All reported p values are based two-sided tests, and a p value less than 0.05 indicates statistical significance. A multivariate Cox proportional hazard model was also developed using stepwise regression (forward selection) with variables that were significant in the univariate analyses. Enter limit and remove limit were p = 0.10 and p = 0.15, respectively. SPSS (SPSS version 21.0, SPSS Inc.) and MedCalc® (14.0) statistical programs were used.
For in vitro experiments, data are expressed as the mean of at least three different experiments ± SD. Comparison between two groups was analyzed using the Student's t-test. Statistical significance was defined as p ≤ 0 .05. Asterisks indicate significant differences between experimental groups.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1221556_s02.docx
Download MS Word (2.9 MB)Acknowledgments
The authors thank Giuliana Falasca, Mariangela Panetta, Barbara Antoniani and Maria Vincenza Sarcone for the technical contribution.
Funding
This work was supported by the Italian Association for Cancer Research AIRC: 5 × 1000, 12182 (P.N., A.S.) and IG 15224 (P.N.).
References
- Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001; 25(5):579-86; PMID:11342768; http://dx.doi.org/10.1097/00000478-200105000-00003
- Neesse A, Algül H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 2015; 64(9):1476-84; PMID:25994217; http://dx.doi.org/10.1136/gutjnl-2015-309304
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 20148(1–2):349-61; PMID:22265420; http://dx.doi.org/10.1016/j.cell.2011.11.025
- Kohler I, Bronsert P, Timme S, Werner M, Brabletz T, Hopt UT, Schilling O, Bausch D, Keck T, Wellner UF. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol 2015; 30 Suppl 1:78-84; PMID:25827809; http://dx.doi.org/10.1111/jgh.12752
- Liu X, Huang H, Remmers N, Hollingsworth M. Loss of E-cadherin and epithelial to mesenchymal transition is not required for cell motility in tissues or for metastasis. Tissue Barriers 2014; 8; 2(4):e969112; PMID:25610757; http://dx.doi.org/10.4161/21688362.2014.969112
- Morris HT, Machesky LM. Actin cytoskeletal control during epithelial to mesenchymal transition: focus on the pancreas and intestinal tract. Br J Cancer 2015; 112(4):613-20; PMID: 25611303; http://dx.doi.org/10.1038/bjc.2014.658
- Mehner C, Miller E, Khauv D, Nassar A, Oberg AL, Bamlet WR, Zhang L, Waldmann J, Radisky ES, Crawford HC et al. Tumor cell-derived MMP3 orchestrates Rac1b and tissue alterations that promote pancreatic adenocarcinoma. Mol Cancer Res 2014; 12(10):1430-9; PMID:24850902; http://dx.doi.org/10.1158/1541-7786.MCR-13-0557-T
- Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 2013; 13(11):788-99; PMID: 24132110; http://dx.doi.org/10.1038/nrc3603
- Pajares MJ, Ezponda T, Catena R, Calvo A, Pio R, Montuenga LM. Alternative splicing: an emerging topic in molecular and clinical oncology. The Lancet Oncology 2007; 8(4):349-357; PMID: 17395108; http://dx.doi.org/10.1016/S1470-2045(07)70104-3
- Bria E, Di Modugno F, Sperduti I, Iapicca P, Visca P, Alessandrini G, Antoniani B, Pilotto S, Ludovini V, Vannucci J et al. Prognostic impact of alternative splicing-derived hMENA isoforms in resected, node-negative, non-small-cell lung cancer. Oncotarget 2014; 5(22):11054-63; PMID:25373410; http://dx.doi.org/10.18632/oncotarget.2609
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol 2003; 19:541-564; PMID:14570581; http://dx.doi.org/10.1146/annurev.cellbio.19.050103.103356
- Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, Kwiatkowski D, Soriano P, Gertler F. Mena is required for neurulation and commissure formation. Neuron 1999; 22(2):313-25; PMID:10069337; http://dx.doi.org/10.1016/S0896-6273(00)81092-2
- Di Modugno F, Mottolese M, Di Benedetto A, Conidi A, Novelli F, Perracchio L, Venturo I, Botti C, Jager E, Santoni A et al. The cytoskeleton regulatory protein hMena (ENAH) is overexpressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor-2-positive/hormonal receptor-negative tumors. Clin Cancer Res 2006; 12(5):1470-8; PMID:16533770; http://dx.doi.org/10.1158/1078-0432.CCR-05-2027
- Di Modugno F, Iapicca P, Boudreau A, Mottolese M, Terrenato I, Perracchio L, Carstens RP, Santoni A, Bissell MJ, Nisticò P. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc Natl Acad Sci U S A 2012; 109(47):19280-5; PMID:23129656; http://dx.doi.org/10.1073/pnas.1214394109
- Di Modugno F, Bronzi G, Scanlan MJ, Del Bello D, Cascioli S, Venturo I, Botti C, Nicotra MR, Mottolese M, Natali PG et al. Human Mena protein, a serex-defined antigen overexpressed in breast cancer eliciting both humoral and CD8+ T-cell immune response. Int J Cancer 2004; 109(6):909-18; PMID:15027125; http://dx.doi.org/10.1002/ijc.20094
- Di Modugno F, DeMonte L, Balsamo M, Bronzi G, Nicotra MR, Alessio M, Jager E, Condeelis JS, Santoni A, Natali PG et al. Molecular cloning of hMena (ENAH) and its splice variant hMena+11a: Epidermal growth factor increases their expression and stimulates hMena+11a phosphorylation in breast cancer cell lines. Cancer Res 2007; 67(6):2657-2665; PMID:17363586; http://dx.doi.org/10.1158/0008-5472.CAN-06-1997
- Trono P, Di Modugno F, Circo R, Spada S, Di Benedetto A, Melchionna R, Palermo B, Matteoni S, Soddu S, Mottolese M et al. hMENA11a contributes to HER3-mediated resistance to PI3K inhibitors in HER2-overexpressing breast cancer cell. Oncogene 2016; 35(7):887-96; PMID:25961924; http://dx.doi.org/10.1038/onc.2015.143
- Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell 2009; 33(5):591-601; PMID:19285943; http://dx.doi.org/10.1016/j.molcel.2009.01.025
- Pino MS, Balsamo M, Di Modugno F, Mottolese M, Alessio M, Melucci E, Milella M, McConkey DJ, Philippar U, Gertler FB et al. Human Mena+11a isoform serves as a marker of epithelial phenotype and sensitivity to epidermal growth factor receptor inhibition in human pancreatic cancer cell lines. Clin Cancer Res 2008; 14(15):4943-50; PMID:18676769; http://dx.doi.org/10.1158/1078-0432.CCR-08-0436
- Trono P, Di Modugno F, Nisticò P. hMENA11a a hMENA isoform sending survival signals. Mol Cell Oncol 2016; 3:2; e1083648; PMID:27308605; http://dx.doi.org/10.1080/23723556.2015.1083648
- Roussos ET, Goswami S, Balsamo M, Wang Y, Stobezki R, Adler E, Robinson BD, Jones JG, Gertler FB, Condeelis JS et al. Mena invasive (Mena(INV)) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin Exp Metastasis 2011; 28(6):515-27; PMID:21484349; http://dx.doi.org/10.1007/s10585-011-9388-6
- Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, Koizumi J, Murata M, Nagayama M, Nobuoka T et al. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol 2010; 177(2):698-712; PMID:20566751; http://dx.doi.org/10.2353/ajpath.2010.091226
- Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 2009; 28(1–2):151-66; PMID:19153669; http://dx.doi.org/10.1007/s10555-008-9179-y
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell M. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 1997; 139(7):1861-72; PMID:9412478; http://dx.doi.org/10.1083/jcb.139.7.1861
- Neuzillet C, de Gramont A, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget 2014; 5(1):78-94; PMID:24393789; http://dx.doi.org/10.18632/oncotarget.1569
- Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-β1-induced epithelial-to-myofibroblast transition: role for β-catenin. Am J Pathol 2004; 165:1955-1967; PMID:15579439; http://dx.doi.org/10.1016/S0002-9440(10)63247-6
- Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS et al. Interactions between β-catenin and transforming growth factor-β signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J Biol Chem 2012; 287(10):7026-38; PMID:22241478; http://111.276311
- Najafov A, Seker T, Even I, Hoxhaj G, Selvi O, Ozel DE, Koman A, Birgül-İyison N. MENA is a transcriptional target of the Wnt/beta-catenin pathway PLoS One 2012; 7(5):e37013; PMID:22615875; http://dx.doi.org/10.1371/journal.pone.0037013
- Lo RS, Massague´ J. Ubiquitin-dependent degradation of TGF-b-activated Smad2. Nature Cell Biol 1999; 1:472-8; PMID:10587642; http://dx.doi.org/10.1038/70258
- Sporn MB. The early history of TGF-beta, and a brief glimpse of its future. Cytokine Growth Factor Rev 2006; 17(1–2):3-7; PMID:16290110; http://dx.doi.org/10.1016/j.cytogfr.2005.09.012
- Tian YC, Phillips AO. Interaction between the transforming growth factor-β type II receptor/Smad pathway and β-catenin during transforming growth factor-β1-mediated adherens junction disassembly. Am. J. Pathol 2002; 160:1619-28; PMID:12000714; http://dx.doi.org/10.1016/S0002-9440(10)61109-1
- Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodeling induced by WNT-7a or lithium. J Cell Sci 1998; 111:1351-61; PMID:9570753
- Horiguchi K, Sakamoto K, Koinuma D, Semba K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K et al. TGF-β drives epithelial-mesenchymal transition through δEF1-mediated downregulation of ESRP. Oncogene 2012; 31(26):3190-201; PMID:22037216; http://dx.doi.org/10.1038/onc.2011.493
- Zhang Y, Morris JP 4th, Yan W, Schofield HK, Gurney A, Simeone DM, Millar SE, Hoey T, Hebrok M, Pasca di Magliano M. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res 2013; 73(15):4909-22; PMID:23761328; http://dx.doi.org/10.1158/0008-5472.CAN-12-4384
- Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology 2014; 146(5):1386-96; PMID:24462734; http://dx.doi.org/10.1053/j.gastro.2014.01.046
- Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol 2014; 31:56-66; PMID:25240174; http://dx.doi.org/10.1016/j.ceb.2014.09.001
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2(2):84-9; PMID:10655587; http://dx.doi.org/10.1038/35000034
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol 2010; 11(5):353-65; PMID:20414257; http://dx.doi.org/10.1038/nrm2890
- Shi YG, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113(6):685-700; PMID:12809600; http://dx.doi.org/10.1016/S0092-8674(03)00432-X
- Fleming YM, Ferguson GJ, Spender LC, Larsson J, Karlsson S, Ozanne BW, Grosse R, Inman GJ. TGF-beta-mediated activation of RhoA signalling is required for efficient (V12)HaRas and (V600E)BRAF transformation. Oncogene 2009; 28(7):983-93; PMID:19079344; http://dx.doi.org/10.1038/onc.2008.449
- Di Modugno F, Mottolese M, De Monte L, Trono P, Balsamo M, Conidi A, Melucci E, Terrenato I, Belleudi F, Torrisi MR et al. The cooperation between hMena overexpression and HER2 signalling in breast cancer. PLoS One 2010; 5(12):e15852; PMID:21209853; http://dx.doi.org/10.1371/journal.pone.0015852
- Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013; 2(8):e25961; PMID:24083084; http://dx.doi.org/10.4161/onci.25961
- Vandesompele J, De Preter K, Pattyn F et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 18; 3(7):RESEARCH0034; PMID:12184808; http://dx.doi.org/10.1186/gb-2002-3-7-research0034.
