γδ T cells are a subset of CD3+CD4−CD8−lymphocytes expressing a peculiar T-cell receptor (TCR) that recognizes peptide as well as non-peptide antigens in an MHC-unrestricted fashion.Citation1 A priori, the diversity of γδ TCRs would be greater than that of conventional αβ TCRs and B-cell receptors (BCRs) combined, in both mice and humans. However, such a potential diversity is never fully realized. Indeed, γδ T cells develop under a very strict endogenous and microenvironmental control, which allows for the emergence of TCRs that are encoded by a single Vγ and Vδ gene and often exhibit limited, if any, junctional diversity.Citation1 Thus, at least in some aspects, γδ T cells are more similar to cells of the innate immune system like natural killer (NK) cells than to αβ T lymphocytes and B cells. Moreover, γδ T cells are much less abundant in the peripheral blood than αβ T cells, but are particularly enriched in epithelial tissues like the skin and intestinal tract, where they can account of up to 50% of CD3+ cells.Citation1
Classically, γδ T cells were regarded as immune effector cells responding to a few specific signals of viral or endogenous origin, encompassing (but not limited to) NKG2D ligands that are expressed by malignant precursors as a result of DNA damage or oncogenic stress.Citation2 Upon TCR ligation, some subsets of γδ T cells mediate cytotoxic effects through perforin 1 (PRF1) and granzyme B (GRZB), and support TH1 helper responses by secreting interferon gamma (IFNγ) and tumor necrosis factor (TNF).Citation1,3 In specific preclinical models, interleukin 17 (IL-17)-producing γδ T cells also contribute to tumor-targeting immune responses elicited by chemotherapy or specific radiotherapeutic regimens.Citation4-6 These considerations have fostered the development of multiple strategies to promote tumor recognition and elimination by γδ T cells,Citation7 including bispecific antibodies simultaneously targeting one Vγ chain and one tumor-associated antigen.Citation8,9 To the best of our knowledge, however, γδ T cell-activating approaches achieved limited, if any, clinical success so far.Citation1,10 Accumulating evidence suggests indeed that multiple γδ T-cell subsets favor, rather than counteract, tumor progression, mainly as they secrete potentially immunosuppressive cytokines like interleukin-10 (IL-10) or IL-17.Citation11,12 Recent data from George Miller's laboratory (from New York University School of Medicine, New York, US) lend further support to this notion as they demonstrate that γδ T cells foster pancreatic carcinogenesis by engaging immunological checkpoints on αβ T cells.Citation13
Daley and collaborators observed that γδ T cells are virtually absent from the normal human pancreas but account for up to 75% CD3+ T lymphocytes infiltrating human pancreatic ductal adenocarcinomas (PDAs). γδ T cells outnumbered CD8+ αβ T cells in the microenvironment of human PDAs as they exhibited a considerable enrichment in effector memory (CD27−CD45RA−) activated (CD62L−) components. Moreover, Vγ9+ γδ T cells, which are known to mediate tumoricidal functions, were poorly represented among PDA-infiltrating γδ T cells, whereas they were abundant among peripheral blood mononuclear cell (PBMC)-derived CD3+ cells.Citation13 Along similar lines, γδ T cells abundantly infiltrated mouse PDAs generated by the orthotopic implantation of malignant cells from Pdx1Cre;KrasG12D;Tp53R172H(KPC) mice (which spontaneously develop invasive PDAs by 8 weeks of age) into syngeneic immunocompetent hosts. In this system, γδ T cells exhibited a peculiar phenotype (as compared to splenic γδ T cells), as they were characterized by the upregulation of FAS ligand (FASLG), killer cell lectin-like receptor subfamily B member 1C (KLRB1C, best known as NK1.1), ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39), junction adhesion molecule like (JAML), and TNF receptor superfamily member 4 (TNFRSF4, best known as OX40), as well as by enriched FOXP3+ and Vγ4+ fractions. Moreover, PDA-infiltrating γδ T cells differed from their splenic counterparts relative to the upregulation of IL-10, interleukin 17A (IL-17A), NKG2D, multiple Toll-like receptors (namely, TLR4, TLR7, and TLR9), and chemokine (C–C motif) receptors (namely, CCR2, CCR5, and CCR6). Similar observations were obtained in 6-month-old Ptf1aCre;KrasG12D (KC) mice, a model of pre-invasive PDA.Citation13 Of note, KPC-derived cells orthotopically implanted into Ccr2−/−, Ccr5−/−, or Ccr6−/− mice recruited limited amounts of γδ T cells as compared to KPC-derived PDAs established in wild-type (WT) mice, demonstrating the importance of chemokine signaling in this setting.Citation13
To test whether γδ T cells would play an etiologic role in pancreatic carcinogenesis, Daley and collaborators crossed KC mice with Tcrd−/−mice (which lack γδ T cells but contain normal amounts of αβ T lymphocytes). Tumor progression and cancer-associated fibrosis were considerably retarded in the pancreata of Ptf1aCre;KrasG12D;Tcrd−/−mice as compared with the pancreata of KC mice, and the Tcrd−/−genotype was sufficient to confer a 1-year survival extension to KC mice. Along similar lines, 6-week-old KC mice receiving a γδ T cell-depleting antibody for eight consecutive weeks exhibited retarded tumor progression and limited fibrosis as compared to KC mice treated with a control antibody. Moreover, KPC-derived aggressive PDAs orthotopically established in Tcrd−/−mice were unable to progress as rapidly as KPC-derived PDAs implanted into WT hosts. In this setting, the Tcrd−/−genotype as well as the administration of a γδ T cell-depleting antibody considerably extended the survival of tumor-bearing mice.Citation13
Upon excluding the possibility that γδ T cells would promote pancreatic carcinogenesis by a direct effect on malignant cells, the authors postulated that PDA-infiltrating γδ T cells might resemble CD4+CD25+FOXP3+ TREG cells in their ability to mediate robust immunosuppressive effects. Indeed, KPC-derived PDAs established in Tcrd−/−mice recruited superior amounts of CD4+ and CD8+αβ T cells as compared to KPC-derived PDAs growing in WT hosts, and PDA-infiltrating αβ T cells exhibited signs of activation and TH1 polarization, including increased expression of IFNG, TNF, T-box 21 (TBX21, best known as T-bet), CD44, inducible T cell co-stimulator (ICOS), cytotoxic T lymphocyte associated protein 4 (CTLA), GRZB, OX40, and programmed cell death 1 (PDCD1, best known as PD-1), as well as CD62L downregulation. Similar results were obtained by comparing PDAs developing in Ptf1aCre;KrasG12D;Tcrd−/−versus KC mice. Moreover, the co-depletion of CD4+ and CD8+ T lymphocytes with specific antibodies abolished the beneficial effects of the Tcrd−/−genotype on pancreatic carcinogenesis, which de facto ascribed the ability of γδ T cells to foster tumor progression (at least in the pancreas) to the inhibition of αβ T cells.Citation13
After excluding the possibility that such an activity would rely on soluble mediators, Daley and colleagues investigated the expression of immunosuppressive molecules on PDA-associated versus splenic γδ T cells, finding increased levels of galectin 9 (LGALS9) and CD274 (best known as PD-L1) on γδ T cells infiltrating invasive as well as pre-invasive PDAs. Notably, PDA-infiltrating γδ T cells expressed higher levels of LGALS9 and PD-L1 than PDA cells themselves, although LGALS9 and PD-L1 expression was comparable in PDA-associated γδ T cells, macrophages, and myeloid-derived suppressor cells (MDSCs). CCR2, CCR5, and CCR6 signaling was required not only for the recruitment of γδ T cells to developing PDAs, but also for normal LGALS9 and PD-L1 expression. These preclinical findings matched the observation that PDA patients had increased levels of LGALS9+ and PD-L1+ γδ T cells in the circulation and even more so in the tumor microenvironment as compared to healthy subjects.Citation13
The PD-L1/PD-1 axis is well known for its prominent immunosuppressive effects, and no less than three PD-L1/PD-1 targeting monoclonal antibodies (namely, atezolizumab, pembrolizumab and nivolumab) are currently approved by the US Food and Drug Administration (FDA) and equivalent agencies worldwide for use in cancer patients.Citation14 Corroborating a mechanistic involvement of the PD-L1/PD-1 in the ability of γδ T cells to foster pancreatic carcinogenesis, a PD-L1-targeting antibody prevented γδ T cells from inhibiting the acquisition of activation markers by αβ T cells in co-culture experiments. Moreover, KPC-derived PDAs implanted orthotopically in WT mice were sensitive to treatment with either a PD-L1-targeting or a LGALS9-targeting antibody as tumors exhibited increased infiltration by activated (CD44+CD62L−) αβ T cells, while neither of these two immunotherapeutic regimens incremented the beneficial effects on tumor progression provided by the Tcrd−/−genotype.Citation13
In summary, the findings by Daley and collaborators delineate a molecular mechanism through which PDA-infiltrating γδ T cells suppress anticancer immunosurveillance (). This said, whether tumor-infiltrating γδ T cells mediate similar effects in other settings remains to be determined. Indeed, a large meta-analysis of gene expression data from approximately 18,000 human tumors identified a γδ T cell-related genetic signature as the most consistent indicator of improved disease outcome across all types of cancer confounded.Citation15 Thus, although the parallelism with CD4+CD25+FOXP3+ TREG cells leaps to the eye, it may be too early to talk about γδ TREG cells.
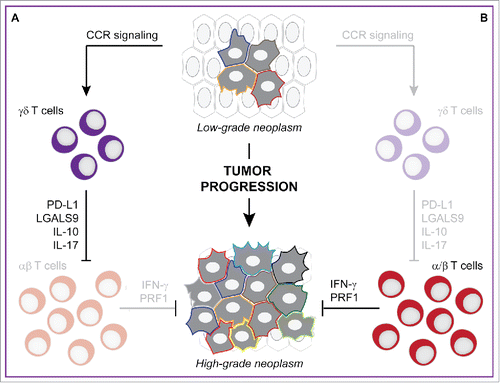
Figure 1. γδ T cells foster pancreatic cancer progression by engaging immunological checkpoints on αβ T cells. Developing pancreatic ductal adenocarcinomas (PDAs) recruit high levels of γδ T cells by secreting ligands for several chemokine (C–C motif) receptors, namely CCR2, CCR5 and CCR6. PDA-infiltrating γδ T cells not only release interleukin 10 (IL-10) and interleukin 17 (IL-17), but also express galectin 9 (LGALS9) and CD274 (best known as PD-L1), hence establishing a robust immunosuppressive microenvironment that foster disease progression (A). In the absence of γδ T cells, developing PDAs recruit increased amounts of αβ T cells that are not subjected to local inhibition via PD-1 and hence efficiently limit disease progression (B).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
ORCID
Laura Mondragón http://orcid.org/0000-0002-3257-045X
References
- Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol 2015; 15:683-91; PMID:26449179; http://dx.doi.org/10.1038/nri3904
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279:1737-40; PMID:9497295; http://dx.doi.org/10.1126/science.279.5357.1737
- Guerville F, Daburon S, Marlin R, Lartigue L, Loizon S, Pitard V, Couzi L, Moreau JF, Dechanet-Merville J, Faustin B. TCR-dependent sensitization of human gammadelta T cells to non-myeloid IL-18 in cytomegalovirus and tumor stress surveillance. Oncoimmunology 2015; 4:e1003011; PMID:26155394; http://dx.doi.org/10.1080/2162402X.2014.1003011
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015; 28:690-714; PMID:26678337; http://dx.doi.org/10.1016/j.ccell.2015.10.012
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491-503; PMID:21383056; http://dx.doi.org/10.1084/jem.20100269
- Galluzzi L, Buqué L, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2016; In press; PMID:27748397; http://dx.doi.org/10.1038/nri.2016.107.
- Bhat J, Kabelitz D. gammadelta T cells and epigenetic drugs: A useful merger in cancer immunotherapy? Oncoimmunology 2015; 4:e1006088; PMID:26155411; http://dx.doi.org/10.1080/2162402X.2015.1006088
- Oberg HH, Peipp M, Kellner C, Sebens S, Krause S, Petrick D, Adam-Klages S, Rocken C, Becker T, Vogel I et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res 2014; 74:1349-60; PMID:24448235; http://dx.doi.org/10.1158/0008-5472.CAN-13-0675
- Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology 2014; 3:e27297; PMID:24701370; http://dx.doi.org/10.4161/onci.27297
- Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. gammadelta T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology 2014; 3:e27572; PMID:24734216; http://dx.doi.org/10.4161/onci.27572
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522:345-8; PMID:25822788; http://dx.doi.org/10.1038/nature14282
- Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol 2010; 40:1927-37; PMID:20397212; http://dx.doi.org/10.1002/eji.200940157
- Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VR et al. gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activation. Cell 2016; 166:1485-99 e15; PMID:27569912; http://dx.doi.org/10.1016/j.cell.2016.07.046
- Buque A, Bloy N, Aranda F, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Marabelle A et al. Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology 2015; 4:e1008814; PMID:26137403; http://dx.doi.org/10.1080/2162402X.2015.1008814
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015; 21:938-45; PMID:26193342; http://dx.doi.org/10.1038/nm.3909
