ABSTRACT
The humanized immunocytokine, hu14.18-IL2 (ICp), leads to the immune cell-mediated destruction of GD2-expressing tumors in mouse models, resulting in potent antitumor effects with negligible IL2-related toxicity. In contrast, when ICp is used clinically, antitumor activity is accompanied by dose-limiting IL2-related toxicities. These species-specific differences in ICp toxicity may be linked to differential binding to mouse vs. human IL2 receptors (IL2Rs). We evaluated immunocytokines genetically engineered to preferentially bind either high-affinity αβγ-IL2Rs or intermediate-affinity βγ-IL2Rs. These ICs have the IL2 fused to the C-terminus of the IgG light chains rather than the heavy chains. We found that IC35, containing intact huIL2, maintained activation of human and mouse αβγ-IL2Rs but exhibited a 20-fold reduction in the ability to stimulate human βγ-IL2Rs, with no activation of mouse βγ-IL2Rs at the concentrations tested. The reduced ability of IC35 to stimulate human βγ-IL2Rs (associated with IL2-toxicities) makes it a potential candidate for clinical trials where higher clinical IC doses might enable better tumor targeting and increased antitumor effects with less toxicity. Contrastingly, ICSK (IC with an IL2 mutein that has enhanced binding to the IL2R β-chain) showed increased activation over ICp on mouse βγ-IL2Rs, with a dose-response curve similar to that seen with IC35 on human βγ-IL2Rs. Our data suggest that ICSK might be used in mouse models to simulate the anticipated effects of IC35 in clinical testing. Understanding the differences in species-dependent IL2R activation should facilitate the design of reagents and mouse models that better simulate the potential activity of IL2-based immunotherapy in patients.
Abbreviations
| αβγ-IL2R | = | high affinity IL2 receptor |
| βγ-IL2R | = | intermediate affinity IL2 receptor |
| GD2 | = | disialoganglioside expressed on tumors of neuroectodermal origin |
| Hu | = | human |
| ICp | = | hu14.18-IL2 (humanized immunocytokine with IL2 fused to the heavy chains) |
| IL2R | = | interleukin-2 receptor |
| IC | = | immunocytokine (fusion protein combining tumor reactive antibody with an immune activating cytokine) |
| Mu | = | mouse |
| SK | = | superkine (mutated IL2 molecule with enhanced binding to the IL2R β-chain) |
Introduction
Therapy with an anti-disialoganglioside (GD2) reactive mAb, in combination with IL2 and GM-CSF, provides clinical benefit to some patients with GD2+ tumors.Citation1 The hu14.18-IL2 immunocytokine (ICp) is a fusion protein that combines a human IL2 molecule to each of the heavy chains of the intact humanized form of an anti-GD2 monoclonal antibody (hu14.18 mAb).Citation2 Throughout this manuscript, hu14.18-IL2 is referred to as the parent immunocytokine (ICp), since this is the original immunocytokine construct that has been modified to give rise to the novel immunocytokines studied in this report. ICp was created with two major goals: 1) to selectively target malignancies through the recognition of GD2 expressed on the surface of tumor cells, leading to the recruitment and activation of immune cells via engagement of Fc and/or IL2 receptors (IL2Rs); and 2) to reduce systemic IL2 effects by concentrating IL2 at the tumor site and better activating tumor-infiltrating immune cells.Citation3 When tested in mice, ICp demonstrated superior antitumor effects compared to equivalent amounts of the hu14.18 mAb and IL2 administered simultaneously as separate agents.Citation4-7 Interestingly, mice treated with ICp did not exhibit IL2-related toxicity, providing promising results for translation into clinical use. However, ICp therapy in patients was associated with dose-limiting effects related to IL2 toxicities, suggesting that there are differences in the ICp dose-related toxicity relationships with mouse vs. human IL2Rs.Citation8-11
The IL2R family is made up of three separate subunits: α-, β-, and γ-chains, which non-covalently associate to form IL2Rs of varying affinity. When all three chains associate, they form the high-affinity receptor (αβγ-IL2R); the intermediate affinity receptor (βγ-IL2R) is composed of just the β- and γ-chains.Citation12 Evidence suggests that the mechanisms of toxicity and efficacy achieved with IL2 can be therapeutically separated by selectively activating immune cells through their high-affinity IL2Rs, while reducing the activation of intermediate-affinity IL2Rs.Citation13-15 Engineered IL2 muteins with enhanced selectivity for cells expressing αβγ-IL2Rs (vs. cells with βγ-IL2Rs) have been shown to induce comparable antitumor effects to wild-type human IL2 but with dramatically less IL2 toxicity in mice, monkeys, and chimpanzees.Citation16,17 A recent phase I dose-escalation study with an IC bearing an IL2 mutein with high specificity for cells with αβγ-IL2Rs (initially developed to be combined with chemotherapy and/or radiation) demonstrated an improved tolerance and overall favorable safety profile in patients with advanced solid tumors.Citation18 Nevertheless, patients treated with this IC specific for αβγ-IL2Rs showed long-term stable disease as best response but did not show any objective tumor responses by RECIST criteria,Citation18 suggesting that some level of βγ-IL2R activation may be required to mount an effective antitumor immune response using monotherapy.
In this study, we investigated the differences between human (hu) and mouse (mu) IL2R activation using novel ICs designed to exhibit varying degrees of selectivity for αβγ-IL2R and βγ-IL2Rs. These new generation ICs have IL2 fused to the C-terminus of the light chains of the ch14.18 mAb rather than on the heavy chains.Citation19 These novel ICs were constructed using the ch14.18 (chimeric anti-GD2 mAb) rather than hu14.18 (humanized anti-GD2 mAb) due to patent limitations on the humanized mAb. Importantly, the chimeric mouse framework regions on ch14.18 do not change the function of the mAb and both the chimeric and humanized mAbs bind GD2 with the same affinity. Three new constructs were used in this study: IC35 with intact IL2 molecules, IC45 with a truncated form of IL2 (missing five amino acids), and ICSK with a mutated IL2 “superkine” (SK) proteinCitation20 with enhanced affinity toward the IL2R β-chain ( and Table S1). In the three-dimensional conformation of these molecules (Fig. S1), the IL2 molecules within the ICp located at the end of the IgG heavy chains are exposed and available for interaction with IL2Rs. In contrast, for these new ICs with the IL2 molecules located at the end of the IgG light chains, the N-terminus of IL2 is adjacent to the C-terminal cysteine of the light chain which, in turn, is covalently linked to the hinge region of the IgG heavy chain. Therefore, the IL2 molecules are somewhat “tucked in” to the three-dimensional structure and thus are not easily accessible for interaction with IL2Rs. This modification creates a sequestering effect in IC35 and IC45 that results in less efficient binding of the IL2 to βγ-IL2Rs, limiting access to a critical contact residue of IL2 by the β-chain, while maintaining binding and activation of the αβγ-IL2R.Citation19 On the other hand, ICSK, containing the mutated IL2-SK molecule,Citation20 which has enhanced binding to IL2Rβ, is able to overcome the sequestering effect and rescues the ability of ICSK to activate βγ-IL2Rs despite the IL2-SK being fused to the light chains of the mAb.
Figure 1. Schematic representation of hu14.18-IL2 immunocytokines. (A) Parental immunocytokine (ICp) with original intact huIL2 bound to the H-chains of the hu14.18 mAb. (B) IC35, with huIL2 bound to the L-chains of the ch14.18 mAb. (C) IC45, with a huIL2 variant missing 5 a.a. bound to the L-chains of the ch14.18 mAb. (D) ICSK, with the IL2 superkine mutein exhibiting enhanced binding to IL2Rβ bound to the L-chains of the ch14.18 mAb. Dark gray portion represents the 98% human portion of the hu14.18 mAb that recognizes GD2. Light gray portion represents the mouse framework in the chimeric mAb. Red portion represents the 2% mouse protein remaining in the antigen-binding region.
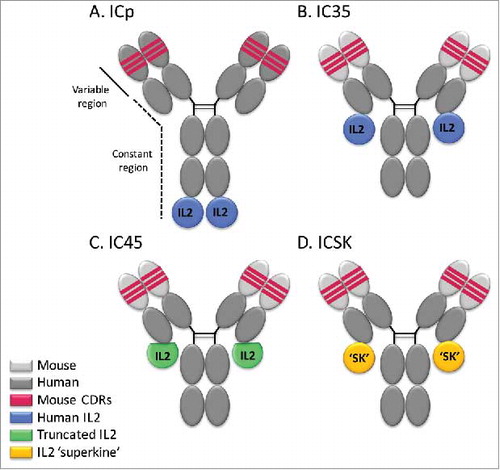
Table 1. IL2 bioactivity in the presence of anti-IL2Rα and/or anti-IL2Rβ inhibitory mAbs.
Previous work demonstrated that an anti-CD20-IL2 immunocytokine, which has IL2 located on the IgG heavy chains, activated both human αβγ-IL2Rs and βγ-IL2Rs to the same extent as soluble IL2,Citation21 and had potent antitumor activity in vivo in T-cell deficient mice bearing human CD20+ tumor xenografts. However, this same anti-CD20-IL2 showed significantly less activation of muβγ-IL2Rs when compared to soluble huIL2. A better understanding of the functional differences of the mouse and human IL2Rs may contribute to the development of IL2-based treatments in mouse models that will more accurately enable translation into the clinical setting.
Prior in vitro and in vivo studies of ICs in mice suggest that antitumor efficacy with acceptable toxicity is obtained using an IC that has full activity in activating muαβγ-IL2Rs, with attenuated (but not absent) capability of activating muβγ-IL2Rs, such as is the case with ICp. In contrast, ICp induces dose-dependent IL2-related toxicity in vivo in human patients after intravenous administration, due apparently to its more potent ability to activate huβγ-IL2Rs than muβγ-IL2Rs. Therefore, we are interested in creating and developing, for clinical use, an IC that retains signaling abilities through huαβγ-IL2Rs but has decreased ability to activate huβγ-IL2Rs. We predict that the activity in humans of an IC with these characteristics will resemble the functional behavior of anti-CD20-IL2 IC or ICp in mice (potent efficacy with little toxicity), potentially serving as a novel therapeutic strategy for treating patients and achieving antitumor benefits, while minimizing adverse side effects.
Here, we report that IC35 is a promising candidate for that purpose since it retains the activation of huαβγ-IL2Rs but exhibits significantly reduced ability to stimulate huβγ-IL2Rs. Additionally, ICSK strongly activates muαβγ-IL2Rs and retains some level of attenuated activation of muβγ-IL2Rs, showing that the use of the IL2-SK in creating this IC makes ICSK a potential surrogate molecule that will better represent in mice the level of huαβγ and huβγ-IL2R activation that we expect and desire to see in patients with an agent like IC35.
Materials and methods
Cell culture
The Kit225Citation22 human T-cell line was obtained from M. Tsudo in Kyoto University (Kyoto, Japan). The Tf-1β cell line was generated in our lab by transfecting the Tf-1 human myelomonocytic cell line (kindly provided by T. Kitamura, University of Tokyo, Tokyo, Japan) with a retroviral vector containing the IL2Rβ gene.Citation23 The CTLL-2Citation24 mouse T-cell line was obtained from the American Type Culture Collection (ATCC, Cat.TIB-214). Cell lines were grown in suspension using RPMI 1640 (Corning Cellgro, Cat.10-040-CV) complete medium supplemented with 10% FBS (GE Healthcare Hyclone, Cat.SH30910.03), 2 mM L-glutamine (Corning Cellgro, 25-005-CI), and 100 U/mL of penicillin/streptomycin (Corning Cellgro, 30-001-CI) at 37°C in a humidified 5% CO2 atmosphere. Recombinant human IL2 was added at a final concentration of 50 IU/mL for maintenance of Kit225 and CTLL-2, and at 100 IU/mL for maintenance of Tf-1β.
Recombinant IL2
Recombinant human interleukin-2 (rIL2, Hoffmann La Roche) was provided through the National Cancer Institute BRB Preclinical Repository (Rockville, MD). The NCI-BRMP standard for unit dosage was used, and the specific activity of the IL2 was 15 × 106 units/mg. This unit corresponds closely with the international standard of IL2 unitage.Citation25
Immunocytokines (ICs)
Humanized hu14.18-IL2 (ICp) (APN301, Apeiron Biologics, Vienna, Austria) was supplied by the NCI Biologics Resources Branch (Frederick, MD) via a collaborative relationship with Merck KGaA (Darmstadt, Germany) and Apeiron Biologics (Vienna, Austria). ICp is an immunocytokine consisting of intact human IL2 genetically linked to the C-termini of each human IgG1 H chain of the hu14.18 mAb.Citation2 IC35, IC45, and ICSK were constructed by fusing a synthetic sequence coding for human or variant IL2 to the coding sequence of the human kappa light chain at the C-terminal cysteine. The constructs also contained the V regions of 14.18, the mouse anti-GD2 mAb.Citation19 IC35 contains the intact human IL2 molecule, whereas IC45 has a modified human IL2 molecule with a deletion of residues 5–9. ICSK contains a mutant IL2, also called “SK,” with enhanced binding to the IL2R β-chain.Citation20 IC35, IC45, and ICSK were produced and provided by Provenance Biopharmaceuticals (Carlisle MA). To compare the IL2 activity in the soluble IL2 and different IC preparations, IL2 concentrations of IC solutions were based on weight/volume calculations of IL2 content. Since the ICs consist of 80% hu14.18 mAb and 20% IL2 by molecular weight, the concentrations were based on IL2 comprising 20% of the weight of the ICs. Thus, 50 ng/mL of IC would correspond to 10 ng/mL of IL2 within the IC.
Antibodies
MikB1, a mouse IgG2a inhibitory mAb directed against human CD122 (IL2Rβ), was kindly provided by M. Tsudo (Unitika Central Hospital, Kyoto, Japan).Citation26 GL439, a mouse IgG1 inhibitory mAb directed against human CD25 (IL2Rα), was provided by R. Robb.Citation27,28 Inhibitory antibodies against mouse IL2Rα [clone: PC61,Citation29 Rat IgG1 isotype, Cat.553864] and mouse IL2Rβ [clone:TM-β1,Citation30 Rat IgG2b isotype, Cat.553359] were purchased from BD Biosciences.
Splenocyte isolation from SCID mice
Female CB-17 SCID mice (7–8 week old) were obtained from Taconic Farms (Germantown, NY). Mice were housed in the University of Wisconsin-Madison animal facilities at the Wisconsin Institutes for Medical Research. All experimentations were performed in accordance to protocols approved by the National Institutes of Health and by the Animal Care and Use Committees of UW-Madison. For splenocyte isolation, spleens were removed from SCID mice immediately after death and placed in RPMI-1640 complete medium, supplemented with 1x MEM non-essential amino acids (Corning Cellgro, Cat.25-025-CI), 10 mM HEPES (Corning Cellgro, Cat.25-060-CI), 1 mM sodium pyruvate (Corning Cellgro, 25-000-CI), and 0.5 mM β−2-mercaptoethanol (Sigma Chemical, M-6250). The spleens were dissociated between the frosted ends of two microscope slides, and erythrocytes were lysed by brief hypotonic shock using cold water. Splenocytes were washed with media, resuspended, and counted using eosin dilution on a hemocytometer.
Proliferation assays
Cells were cultured for 72 h in round-bottom 96-well plates at a density of 5 × 103 cells/well for Kit225, CTLL-2, and Tf-1β or 5 × 104cells/well for SCID splenocytes in the presence of serial dilutions of IL2 or the different immunocytokines in a total volume of 200 µL. For the final 6 h, 3H-thymidine (1 mCi/mL; Perkin Elmer, Cat.NET027A005MCI) was added to the wells in 50 µL of media at a final concentration of 1 μCi/well. 3H-TdR-incorporation was determined using total cells harvested from the cell cultures onto glass fiber filters (Packard, Meriden, CT), using the Packard Matrix 9600 Direct β-counter (Packard, Meriden, CT). Results are presented as counts per 5 min for triplicate wells ± SD. For experiments in which mAbs were used to block the α- or β-chain of the IL2R, cells were incubated with the blocking mAbs at 10 µg/mL for 30–60 min at 4°C prior to plating with the IL2 sources. The median effective dose (EC50) value for each protein with respect to cell proliferation was obtained using GraphPad Prism by plotting the dose response curve and analyzing using nonlinear regression to identify the protein concentration that resulted in half-maximal response. For some experiments, the curves obtained for certain treatments did not exhibit a maximal response (top plateau), thereby preventing the program from fitting a unique curve through the data and leading to ambiguous EC50 values. Therefore, the EC50 values summarized in were calculated by averaging only the EC50 values obtained from properly fitted curves. The number of determinations used for this calculation is detailed under each table.
Flow cytometry detection of IC binding to IL2Rs
For the detection of IC binding to IL2Rs on the cell surface via the IL2 molecules of the IC, 2–5 × 105 Kit225, CTLL-2, SCID splenocytes, or Tf-1β cells were incubated with increasing concentrations of the ICs for 30–60 min at 4°C and the Alexa Fluor 647 goat anti-human IgG mAb (polyclonal, Invitrogen, Cat. A-21445) was used for secondary detection of IL2R-bound ICs. To reduce Fc receptor-mediated binding by the ICs, SCID splenocytes were pre-incubated (1 μg/1 × 106 cells) with anti-mouse CD16/CD32 (Mouse BD Fc block, clone: 2.4G2, BD PharMingen, Cat. 553142) for 15–30 min at 4°C. The ICs were tested in a range of concentrations similar to the concentrations used on those same cells for the proliferative assays. IC were added directly to pre-incubated cells without washing the Fc blocking mAb. Data [mean fluourescence intensity (MFI)] were acquired on the MACSQuant Analyzer 10 flow cytometer (Militenyi Biotec Inc. Auburn, CA) and analysis was performed using the FlowJo v10 software (FlowJo LLC., Ashland. OR).
Assessment of STAT5 phosphorylation by flow cytometry
IL2 responsive cells were starved by washing away IL2 used in culture and incubating at 37°C in complete media overnight. Cells were stimulated with increasing concentrations of IL2, ICp, IC35, or ICSK for 15 min at 37°C and then fixed with 1.5% paraformaldehyde (Polysciences, Inc., Cat.18814) for 10 min at room temperature. Cells were permeabilized with 100% ice-cold methanol (Fisher Scientific, Cat.A433P-4) for 30 min at 4°C and then washed and stained with FITC-labeled anti-STAT5-pY694 (Clone:SRBCZX, eBioscience, Cat.11-9010-42) for 30 min at room temperature. Intracellular staining was assessed by flow cytometry detection on the MACSQuant analyzer.
Ex vivo NK cell expansion
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood obtained from healthy donors by ficoll density gradient centrifugation. Freshly isolated PBMCs were co-incubated with (100 Gy) irradiated K562-mbIL15-41BBL cells (obtained from Dr. Dario Campana, formerly from St. Jude Children's Research Hospital, Memphis TN),Citation31 which were mixed at a 1:1 ratio (5 × 106cells/each) in 10 mL RPMI with 100 IU/mL IL2. Mixtures of PBMC and K562-mbIL15-41BBL cells were incubated at 37°C on a rocker at a constant speed. Fresh IL-2 supplemented RPMI was added every 2–3 d to the culture. All cells in the expansion cultures were harvested between expansion days 12–15 for CD3 and CD56 staining to determine NK cell percentage followed by functional analyses in the cytotoxicity assay.
Antibody-dependent cell-mediated cytotoxicity assay
M21, human melanoma cells expressing GD2, were used as target cells and labeled with 51Cr (Perkin Elmer, Cat.NEZ030010MCI) for 1.5 h. PBMCs that were stimulated overnight with 100 IU/mL IL2 or expanded NK cells were plated in round bottom 96-well plates and ICp, IC35, or ICSK were added for a final concentration of 3000 IU/mL IL2 per well. 51Cr-labeled M21 cells were added at various effectors to target (E:T) ratios and cells were co-incubated for 4 h at 37°C. Supernatants were harvested at the end of incubation using the Skatron harvesting system (Skatron, McLean, VA) and read by a gamma counter (Packard, Meriden, CT). Percent cytotoxicity was calculated as 100 × [(experimental release-spontaneous release)/(Maximum release – spontaneous release)].
Interferon-γ secretion by SCID splenocytes
Freshly isolated SCID splenocytes were plated at a density of 5 × 104 cells/well in round-bottom 96-well plates and incubated at 37°C for 72 h in the presence of serial dilutions of IL2 or the different immunocytokines in a total volume of 200 µL. On day 3, supernatants were harvested and secreted protein levels were measured using the BD™ CBA Mouse Inflammation Kit (BD Biosciences, Cat.552364). Data were acquired on the BD FACSCalibur and analysis was performed using the BD FACSArray software, both from BD Biosciences, San Jose, CA.
Statistical analyses
Cell proliferation counts and stimulation indices were evaluated for different treatment groups within various cell lines. Proliferation counts and stimulation indices were summarized using means and standard deviations (SD). Two-way ANOVA was used to compare proliferation counts between treatment groups within doses and to compare stimulation indices between treatment groups. The Bonferonni method was used to adjust for multiple treatment comparisons. p-values less than 0.05 were considered statistically significant. Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC). Statistically significant differences of biological values are indicated in graphs as described in the figure legends.
Results
IL2-dependent cell lines and SCID splenocytes serve as an in vitro system to study high and intermediate affinity IL2Rs
To investigate the ability of the IL2 molecules within the new ICs to activate high (αβγ) or intermediate affinity (βγ) IL2Rs, we established an in vitro system using mouse and human IL2-dependent cell lines expressing either αβγ- or βγ-IL2Rs. For this purpose, we used Kit225,Citation22 a human T-cell line derived from a patient with T cell chronic lymphocytic leukemia, and CTLL-2,Citation24 a mouse T-cell line cloned from a leukemic mouse spleen, which have been reported to express high affinity IL2Rs. As a model of human intermediate affinity IL2Rs, we used Tf-1β,Citation23 a human myelomonocytic cell line transfected to express the human IL2Rβ subunit in order to form βγ dimers. To our knowledge, there is no IL2-dependent mouse cell line available that only expresses mouse intermediate affinity IL2Rs. Therefore, to study muβγ-IL2Rs, we isolated resting splenocytes from SCID mice.Citation32 For each cell line (Kit225, CTLL-2, Tf-1β ) and SCID splenocytes, the surface expressions of both IL2Rα and IL2Rβ were verified via flow cytometry (Fig. S2). To confirm that the SCID splenocytes did not upregulate expression of endogenous muIL2Rα following IL2 stimulation, we verified the absent expression of IL2Rα. After a 72 h incubation with increasing concentrations of IL2, known to stimulate proliferation in these cells; even at the highest concentration of 1333 ng/mL, there was no IL2Rα detected (Fig. S3).
To validate the receptor specificity of the IL2-dependent cell lines and SCID splenocytes, we performed proliferation experiments in response to soluble human IL2 in the presence of blocking antibodies against IL2Rα and IL2Rβ (). As expected, addition of an inhibitory anti-IL2Rα mAb (10 µg/mL), which binds to the α-chain of the IL2R, did not interfere with the proliferation seen in Tf-1β or SCID splenocytes in response to IL2 (). When the anti-IL2Rα mAb was added to Kit225 and CTLL-2 cells, IL2-induced proliferation was substantially inhibited; a 3–13-fold increase in IL2 was required to induce proliferation comparable to that seen in the absence of the anti-IL2Rα mAb (, EC50 values summarized in ). To determine if the decreased proliferation was due to incomplete blocking of αβγ-IL2Rs, we increased the amount of anti-IL2Rα mAb and confirmed cell surface saturation by flow cytometry. Even with 10 times more blocking mAb (100 µg/mL), proliferation was not completely inhibited but continued to exhibit a ∼15-fold decrease (Fig. S4), indicating that Kit225 and CTLL-2 are able to retain some lower-level proliferation in response to IL2 even when IL2Rα is unavailable, suggesting the presence of functional βγ-IL2Rs on these cells. The addition of anti-IL2Rβ mAb (10 µg/mL) did not affect proliferation of Kit225 and CTLL-2 when compared to IL2 alone, demonstrating that even in the presence of the anti-IL2Rβ mAb, these cells continue to utilize high affinity IL2Rs. However, when blocking the α- and β-chains simultaneously, by adding both anti-IL2Rα mAb and anti-IL2Rβ mAb, proliferation is virtually completely inhibited (). Thus, the IL2-dependent growth observed in Kit225 and CTLL-2 cells when IL2Rα was blocked was mediated through IL2Rβ, most likely via activation of βγ-IL2Rs. This is evidenced by the increase in the effective concentrations (EC50) of IL2 needed for Kit225 and CTLL-2 when blocked with anti-IL2Rα mAb ().
Figure 2. Effect of anti-IL2Rα and anti-IL2Rβ inhibitory mAbs on IL2 induced proliferation. (A) Tf-1β, (B) Kit225, (C) SCID splenocytes, or (D) CTLL 2 cells were incubated with inhibitory mAbs. MAbs used in (A) and (B) GL439 (anti-huIL2Rα), MikB1 (anti-huIL2Rβ) and in (C) and (D) PC61 (anti-muIL2Rα) and TM-β1 (anti-muIL2Rβ), each at 10 µg/mL for 30–60 min at 4C prior to addition of IL2. In (B) and (D), a combination of species-appropriate anti-IL2Rα and anti-IL2Rβ mAbs were also tested. Cells were cultured for 72 h at 37°C and were pulse labeled with 1 µCi of 3H thymidine for 6 h. Error bars indicate SD of triplicate samples. Data are representative of 2–10 separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.001.
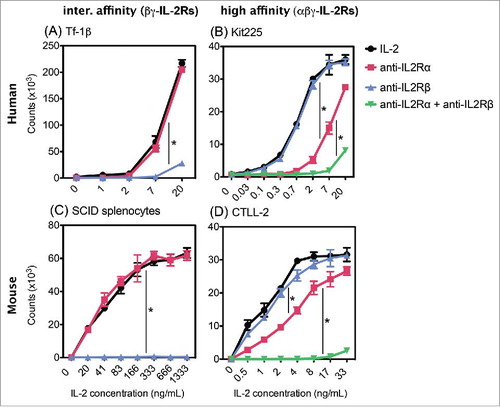
On the other hand, blocking Tf-1β and SCID splenocytes with anti-IL2Rβ mAb alone completely abrogated their proliferation in response to IL2 (). Since the absence of proliferation in the presence of this anti-IL2Rβ mAb could be due to non-specific inhibition, we confirmed the specificity of the anti-IL2Rβ blocking antibody by verifying that it did not interfere with GM-CSF-induced proliferation of these same Tf-1β cells. Even at the highest concentrations, IL2-induced proliferation was virtually abrogated when Tf-1β cells were first blocked with anti-IL2Rβ. In contrast, blocking IL2Rβ did not interfere with the ability of GM-CSF to induce proliferation of these same Tf-1β cells (Fig. S5). Taken together, these results indicate that Tf-1β and SCID splenocytes proliferate in response to IL2 solely through βγ-IL2Rs; thus, these two cell types serve as suitable in vitro models to study activation of human and mouse intermediate affinity IL2Rs, respectively.
Novel ICs with IL2 on the light chains exhibit reduced ability to stimulate βγ-IL2Rs
IC35 and IC45, with human IL2 fused to the light chains of the anti-GD2 mAb (), were designed to reduce activation of βγ-IL2Rs. We evaluated their bioactivity in comparison to the parent ICp (which has IL2 fused to the heavy chains of the mAb) and to soluble IL2 in our established in vitro systems using cells that express human and mouse αβγ- or βγ-IL2Rs (). In our human cell models, ICp-induced proliferation to the same extent as IL2 (), demonstrating that placing the IL2 molecules on the heavy chains of the mAb does not interfere with the ability of human αβγ or βγ-IL2Rs to bind and respond to the IL2 component of ICp. In contrast to the results with human cells, ICp was less potent than soluble IL2 at inducing proliferation of cells expressing muIL2Rs, illustrated by a 10–12-fold increase () in the amount of ICp needed to induce proliferation comparable to that by soluble IL2 for CTLL-2 and SCID splenocytes, respectively ().
Figure 3. Novel ICs exhibit reduced ability to induce proliferation of cells expressing βγ-IL2Rs. Comparison of IC35 and IC45 induced proliferation on: (A) Tf-1β, (B) Kit225, (C) CTLL-2, and (D) SCID splenocytes. Cells were stimulated with increasing concentrations of IL2, ICp, IC35, or IC45 and cultured for 72 h at 37°C. The counts were determined by [3H]TdR incorporation by proliferating cells. Error bars indicate SD of triplicate samples. Data are representative of two–six separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.001.
![Figure 3. Novel ICs exhibit reduced ability to induce proliferation of cells expressing βγ-IL2Rs. Comparison of IC35 and IC45 induced proliferation on: (A) Tf-1β, (B) Kit225, (C) CTLL-2, and (D) SCID splenocytes. Cells were stimulated with increasing concentrations of IL2, ICp, IC35, or IC45 and cultured for 72 h at 37°C. The counts were determined by [3H]TdR incorporation by proliferating cells. Error bars indicate SD of triplicate samples. Data are representative of two–six separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.001.](/cms/asset/fd81b6bc-dfed-4e15-a244-536f41591bde/koni_a_1238538_f0003_oc.jpg)
Table 2. IL2 bioactivity of IL2, ICp, IC35 and IC45.
When exposed to IC35, both human and mouse cells expressing αβγ-IL2Rs responded similarly to ICp (), with superimposed proliferation curves for both IC35 and ICp on Kit225 cells and on CTLL-2 cells. These results suggest that changing the location of IL2 from the heavy chains to the light chains does not interfere with the ability of IC35 to activate human or mouse αβγ-IL2Rs. However, placing the IL2 molecules on the light chains interfered with the bioactivity of IC35 on human and mouse βγ-IL2Rs. Tf-1β cells required 15–20 times more IC35 to induce proliferation comparable to that induced by ICp (). The sequestering effect achieved by having IL2 on the light chains was even more evident when evaluating muβγ-IL2Rs; IC35 was completely unable to induce proliferation of SCID splenocytes at the concentrations tested (). These data comparing proliferation induced by soluble IL2, ICp, and IC35 demonstrate the decreased capability of muIL2Rs (vs. huIL2Rs) to respond to huIL2 when fused to heavy or light chains of a mAb (EC50 values summarized in ).
Although IC35 retained the same stimulatory ability as ICp on human and mouse cells with αβγ-IL2Rs (), IC45 showed substantially less ability to stimulate these cells than IC35 or ICp. IC45 exhibited a 3-fold decrease in bioactivity when compared to ICp on Kit225 ( and ), and this effect was more pronounced on muαβγ-IL2Rs with a 30-fold decrease on CTLL-2 (). Moreover, IC45 completely lost the ability to activate βγ-IL2Rs with no proliferation seen on Tf-1β or SCID splenocytes (). These data indicate that the deletion of residues within the IL2 molecules of IC45 significantly reduced its ability to interact with both αβγ- and βγ-IL2Rs (human and mouse).
To further evaluate the selectivity of IC35 for αβγ-IL2Rs, we investigated its bioactivity in the presence of the anti-IL2Rα mAb, which blocks αβγ-IL2Rs, thus driving Kit225 and CTLL-2 cells to respond only through βγ-IL2Rs. As observed previously in , anti-IL2Rα did not alter the proliferation of Tf-1β or SCID splenocytes; both responded to IL2, ICp, and IC35 to the same extent in the presence or absence of the blocking mAb (). In contrast, shows that though Kit225 cells displayed similar proliferation curves in response to IL2, ICp, and IC35 in the absence of the blocking mAb, there was a significant decrease in proliferation when the α-chain was blocked. For both IL2 and ICp, 10 times higher concentrations were required in order to achieve proliferation of Kit225 in the presence of anti-IL2Rα that was comparable to that seen in the absence of the blocking antibody (EC50 values summarized in ). This is consistent with these Kit225 cells treated with the blocking mAb responding to proliferative signals through βγ-IL2Rs. Importantly, in the presence of the blocking antibody, IC35 showed a further significant reduction in Kit225 bioactivity, resembling the proliferation curves obtained on Tf-1β cells, with 30-fold decrease when compared to ICp (). Furthermore, blocking the α-chain on CTLL-2 cells () produced similar proliferation curves as those observed on SCID splenocytes (in the absence of blocking mAb), where more ICp is required to induce proliferation comparable to soluble IL2 and IC35-induced proliferation is completely inhibited. Together, these results demonstrate that IC35 and IC45 have a significantly reduced ability to activate βγ-IL2Rs, with this effect being more pronounced on muβγ-IL2Rs where bioactivity has been completely abrogated.
Figure 4. Blocking IL2Rα allows Kit225 and CTLL-2 to respond through βγ-IL2Rs. Inhibitory antibody against the α-chain of the IL2R effects proliferation of cells expressing high affinity IL2Rs (B) and (D) but not cells expressing intermediate affinity IL2Rs (A) and (C). (A) Tf-1β, (B) Kit225, (C) CTLL-2, and (D) SCID splenocytes were incubated with inhibitory mAbs at 10 µg/mL for 30–60 min at 4C prior to addition of IL2. Cells were cultured for 72 h at 37°C and were pulse labeled with 1 µCi of 3H thymidine for 6 h. Error bars indicate SD of triplicate samples. Data are representative of 2–10 separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.001.
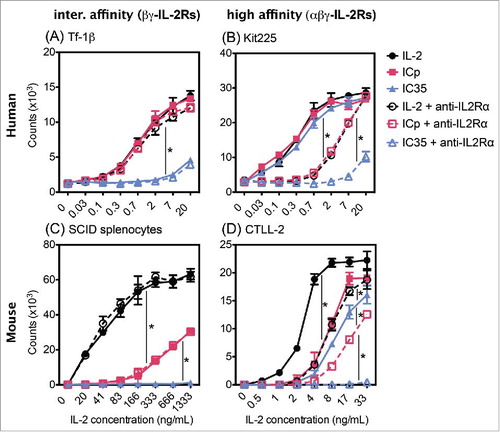
Table 3. IC bioactivity in the presence of anti-IL2Rα.
The IL2 “superkine” molecules within ICSK restore activation of βγ-IL2Rs
Proliferation curves from and show that IC35 modestly activates huβγ-IL2Rs, whereas activation of muβγ-IL2Rs is completely lost. This disparity in bioactivity suggests important differences in IL2Rs between species that must be taken into account when designing in vivo experiments. In order to more accurately simulate in mice the level of IL2R activation anticipated in patients treated with IC35, we sought to find a separate IC, capable of modest activation of muβγ-IL2Rs (comparable to the ability of IC35 to activate huβγ-IL2Rs), to be used as a surrogate in our mouse models for the activity of IC35 in the stimulation of human cells. ICSK was created using the same IC construction platform as IC35, with fusion of an intact IL2 molecule, in this case the IL2-SK, to each of the light chains of the 14.18 mAb. The soluble IL2-SK molecule harbors mutations that increase binding affinity to the β-chain of the IL2R.Citation20 shows that ICSK can activate both mouse and human αβγ-IL2Rs, with identical proliferation curves for ICp, IC35, and ICSK on both Kit225 and CTLL-2 (, ). Furthermore, ICSK induced proliferation to the same extent as soluble IL2 and ICp on Tf-1β cells (), demonstrating that incorporating the IL2-SK molecules into ICSK overcomes the sequestering effects of IL2 placement on the light chains (as seen with IC35) and renders ICSK capable of uninhibited activation of human βγ-IL2Rs. Importantly, ICSK stimulates proliferation of SCID splenocytes five times better than ICp, achieving a proliferation curve similar to that observed with IC35 on Tf-1β cells (), suggesting that ICSK might better recapitulate in mice the extent of IC35 induced activation of βγ-IL2Rs in humans (EC50 values summarized in ).
Figure 5. The “superkine” molecules within ICSK rescue the ability to elicit proliferation through βγ-IL2Rs. Comparison of IC35 and ICSK induced proliferation on: (A) Tf-1β, (B) Kit225, (C) SCID splenocytes, and (D) CTLL-2. Cells were stimulated with increasing concentrations of IL2, ICp, ICSK, or IC35 and cultured for 72 h at 37°C. The counts were determined by [3H]TdR incorporation by proliferating cells. Error bars indicate the SD of triplicate samples. Data are representative of six–eight separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.001. Bold font: These values are similar when compared to one another.
![Figure 5. The “superkine” molecules within ICSK rescue the ability to elicit proliferation through βγ-IL2Rs. Comparison of IC35 and ICSK induced proliferation on: (A) Tf-1β, (B) Kit225, (C) SCID splenocytes, and (D) CTLL-2. Cells were stimulated with increasing concentrations of IL2, ICp, ICSK, or IC35 and cultured for 72 h at 37°C. The counts were determined by [3H]TdR incorporation by proliferating cells. Error bars indicate the SD of triplicate samples. Data are representative of six–eight separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.001. Bold font: These values are similar when compared to one another.](/cms/asset/1d53dfe8-14cf-4299-9f8b-42f21ab99655/koni_a_1238538_f0005_oc.jpg)
Table 4. IL2 bioactivity of IL2, ICp, IC35, and ICSK.
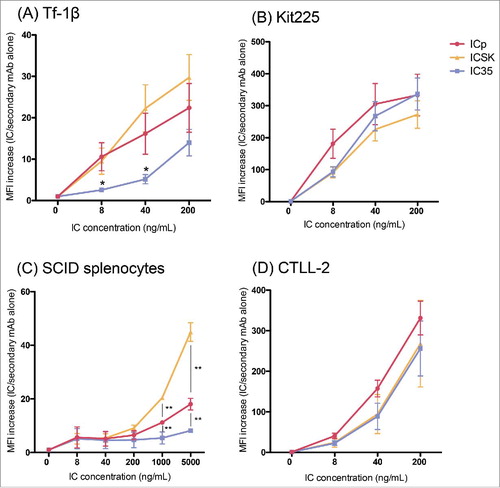
We hypothesized that these differences in IL2R activation were due to differences in the ability of these ICs to bind αβγ-IL2Rs and βγ-IL2Rs. Therefore, we performed flow cytometry-based binding titrations of the ICs on the mouse and human IL2R-expressing cells used in these proliferation experiments to assess differences in the kinetics of receptor engagement between the ICs. In , cells expressing high affinity IL2Rs show that ICp, IC35 and ICSK all exhibited similar binding capabilities on Kit225 cells, and all three of these ICs also exhibited similar binding capabilities on the CTLL-2 cells[AQ]. In addition, these ICs appear to bind slightly better to Kit225 than to CTLL-2 (plateau level binding is seen at 40 ng/mL for Kit225, whereas 200 ng/mL are required on CTLL-2), suggesting that muαβγ-IL2Rs have less affinity for the IL2 molecules within the ICs than huαβγ-IL2Rs. These binding patterns are very similar to the proliferation data presented for these ICs on these same two cell lines (). Similarly, binding on intermediate affinity IL2Rs () reflected the proliferation results obtained in , namely IC35 exhibited less binding on Tf-1β () and on SCID splenocytes () when compared to either ICp or ICSK. This difference was significant for Tf-1β cells at the lower concentrations of 8–40 ng/mL, the range in concentrations used for the proliferative studies. On SCID splenocytes, ICSK bound significantly better than ICp at the higher concentrations, which is consistent with the proliferation results that show that ICSK better activates muβγ-IL2Rs than ICp does. These binding studies provide evidence to support the hypothesis that moving the location of the IL2 molecules from the heavy to the light chains (compare data with ICp to data with IC35) does not influence binding to αβγ-IL2Rs but does interfere with the interaction with βγ-IL2Rs. Furthermore, replacing these IL2 molecules on the light chains with the SK IL2 muteins overcomes this effect and restores the interaction with βγ-IL2Rs back to (or above) the original configuration (compare data with ICSK to data with ICp).
Figure 6. IC binding to cells expressing high or intermediate affinity IL2Rs. Flow cytometry analysis of IC binding on IL2R-expressing cells. (A) Tf-1β, (B) Kit225, (C) SCID splenocytes, and (D) CTLL-2. Cells (0.2 × 106 cells/sample) were incubated with increasing amounts of the different ICs (ICp, IC35, or ICSK) in 0.2 mL of staining buffer for 30–60 min at 4°C. SCID splenocytes were pre-incubated with Mouse Fc block to minimize Fc receptor mediated binding of the ICs. For detection an Alexa fluor 647-conjugated goat anti-huIgG secondary mAb was used. MFI fold increase = (MFI of IC-stained sample/MFI of secondary mAb alone). Data represent the mean ± SD from three to five separate experiments. Statistical differences of significance are represented with an asterisk *p-value = <0.05 and **p-value = <0.01.
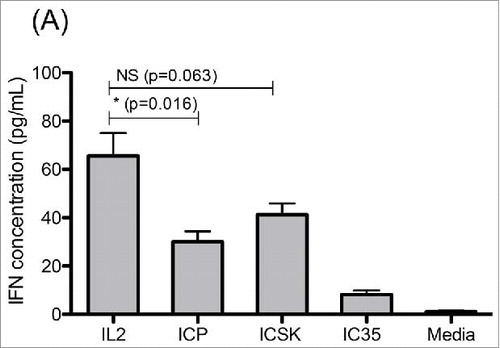
One downstream effect of IL2 stimulation on NK cells is the secretion of secondary cytokines such as IFNγ. Therefore, to further evaluate the ability of ICSK to activate mouse βγ-IL2Rs, we measured IFNγ secretion of SCID splenocytes after they had been stimulated for 72 h with IL2, ICp, and ICSK. Our results show that IL2 stimulation led to the highest accumulation of IFNγ in culture, with a median concentration of 71.8 pg/mL, whereas ICp-stimulated cells had the lowest IFNγ with 31.6 pg/mL. On the other hand, ICSK stimulated cells to produce 44.0 pg/mL IFNγ (). Blocking IL2Rβ on these cells inhibited IFNγ secretion for all treatments demonstrating that IFNγ is being produced as a result of βγ-IL2R activation (not shown). These results provide additional evidence to show that ICSK is able to activate mouse βγ-IL2Rs to a greater degree than ICp.
Figure 7. ICSK leads to increased IFNγ secretion on SCID splenocytes when compared to ICp. Comparison of IL2, ICp and ICSK induced secretion of interferon-γ on SCID splenocytes. Freshly isolated SCID splenocytes were stimulated with 7 μg/mL (equivalent to 1.3 × 103 ng/mL IL2) ICp, IC35, or ICSK for 72 h at 37°C. Supernatants were harvested and secreted IFNγ was measured via flow cytometry using a colorimetric bead array. Error bars indicate SD of five samples. Data are representative of three separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.05.
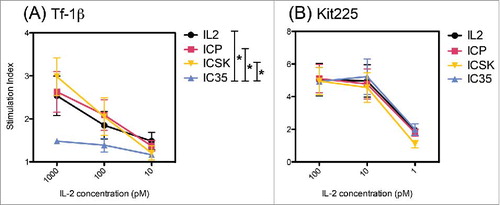
Phosphorylation of STAT5 as a direct measure of IL2R activation
One of the earliest cell signaling events that are triggered upon IL2 binding to the IL2R is the phosphorylation of STAT5 proteins. In order to confirm the selectivity of the novel ICs with a direct measure of IL2R engagement and activation, we measured phosphorylated STAT5 (pSTAT5) levels in cells expressing high or intermediate affinity IL2Rs. Using intracellular flow cytometry staining, we assessed the levels of pSTAT5 in Kit225 and Tf1b cells after 15 min incubation with IL2, ICp, IC35, and ICSK (). Similar to the proliferation results, there was no difference in pSTAT5 levels for all the treatments on Kit225 cells. This is further evidence to confirm that neither the location of the IL2 moiety nor the mutations within the SK molecules interfere with the ability of these ICs to interact and activate αβγ-IL2Rs. On Tf1b cells, IC35 was the only IC unable to induce the phosphorylation of STAT5, confirming that having the IL2 on the light chains significantly hinders that ability of IC35 to engage βγ-IL2Rs.
Figure 8. IC35 exhibits reduced phosphorylation of STAT5 via intermediate affinity IL2Rs. Comparison of IL2, ICp, IC35, and ICSK induced phosphorylation of STAT5 on: (A) Tf-1β and (B) Kit225. Cells were stimulated with increasing concentrations of IL2, ICp, ICSK, or IC35 and incubated for 15 min at 37°C. pSTAT5 levels were determined by intracellular flow cytometry staining using anti-pSTAT5(pY694). SI = (MFI stimulated cells/MFI unstimulated cells). Error bars indicate SD of triplicate samples. Data are representative of three separate experiments. Statistical differences of biological significance are represented with an asterisk *p-value = <0.05. SI, stimulation index; MFI, mean fluorescence intensity.
Changing the location of the IL2 molecules does not interfere with ADCC
The ability of the novel ICs to induce effector functions and mediate tumor cell killing via antibody-dependent cell-mediated cytotoxicity (ADCC) was assessed in a 4 h 51Cr release assay. Expanded NK cells and peripheral blood mononuclear cells (PBMCs) from healthy donors were incubated with GD2+ M21 human tumor cells in the presence of ICp, IC35, or ICSK. IL2R expression was verified by flow cytometry; both effector cell populations showed the expression of IL2Rβ and the absence of detectible IL2Rα, confirming the presence of human βγ-IL2Rs on these cells (data not shown). Our data show that all three ICs had equivalent ADCC activity at comparable effector-to-target ratios and IC concentration (). These results demonstrate that all ICs are able to bind GD2+ tumor cells and mediate ADCC to the same extent irrespectively of the position or nature of the IL2 moiety on the antibody.
Figure 9. ADCC activity is not affected by moving the IL2 molecules to the light chains of the mAb. Comparison of ICp, IC35, and ICSK-mediated ADCC on: (A) Expanded NK cells and (B) PBMCs. Effector cells were co-incubated with 51Cr-loaded M21 tumor targets in the presence of 1 μg/mL (equivalent to 195 ng/mL IL2) ICp, IC35, or ICSK for 4 h at 37°C. Supernatants were harvested and the released 51Cr from lysed target cells was measured using a gamma counter. Percentage Cytotoxicity = [(experimental release − spontaneous release)/(maximum release − spontaneous Release)]×100. Error bars indicate SD of quadruplicate samples. Data are representative of three separate experiments.
![Figure 9. ADCC activity is not affected by moving the IL2 molecules to the light chains of the mAb. Comparison of ICp, IC35, and ICSK-mediated ADCC on: (A) Expanded NK cells and (B) PBMCs. Effector cells were co-incubated with 51Cr-loaded M21 tumor targets in the presence of 1 μg/mL (equivalent to 195 ng/mL IL2) ICp, IC35, or ICSK for 4 h at 37°C. Supernatants were harvested and the released 51Cr from lysed target cells was measured using a gamma counter. Percentage Cytotoxicity = [(experimental release − spontaneous release)/(maximum release − spontaneous Release)]×100. Error bars indicate SD of quadruplicate samples. Data are representative of three separate experiments.](/cms/asset/b197186f-0a51-47a1-85a1-1f31c84d43f4/koni_a_1238538_f0009_oc.jpg)
Discussion
In this report, we sought to dissect the differences in IL2R activation between the human and murine IL2R systems using novel ICs designed to selectively activate either high or intermediate affinity IL2Rs.Citation19 Previous studies investigated the IL2 bioactivity of these novel ICs compared only between muαβγ-IL2Rs and huβγ-IL2Rs.Citation19 Here, we studied both human and murine αβγ-IL2Rs and βγ-IL2Rs in parallel. We found that unlike huIL2Rs, muIL2Rs are less able to respond to IL2 when presented on the ICp, and this reduced activation of muβγ-IL2Rs likely accounts for the relative absence of IL2-induced toxicity observed when ICp and related ICs are used to treat tumor-bearing mice.Citation4,6,Citation7,33,Citation34
Murine and human IL2 and IL2R subunits share 60–70% homology;Citation35-38 thus, it is not surprising that mouse and human IL2Rs are both responsive to human IL2 at somewhat similar levels.Citation39,40 Consequently, human IL2 has been widely used to study the biology of IL2Rs interchangeably in models that use human and murine IL2Rs.Citation41,42 However, preclinical and clinical studies with hu14.18-IL2 (ICp) have revealed somewhat distinct in vivo effects when comparing mice to humans. Although in vivo studies in mouse tumor models have demonstrated promising antitumor effects without IL2-related side effects,Citation4-7 ICp immunotherapy in patients (at doses that achieve relatively similar peak blood levels, and similar half-livesCitation8,9,Citation43 to those in mice that show antitumor benefit) is limited by dose-dependent IL2 toxicity.Citation8-11 This toxicity appears to be the result of overactivation of immune cells bearing intermediate affinity βγ-IL2Rs, leading to inflammatory cytokine release.Citation13,16,Citation17 Therefore, the relative absence of IL2-induced toxicity in mice treated with ICp suggests that the IL2 component of this IC elicits less activation of muβγ-IL2Rs than of huβγ-IL2Rs.
We evaluated IL2R function using human and mouse cells that expressed either high or intermediate affinity IL2Rs and proliferated in response to IL2. Kit225 and CTLL-2 expressed both IL2Rα and IL2Rβ and proliferated in response to low doses of IL2, consistent with the activation of human and mouse αβγ-IL2Rs, respectively. Blocking IL2Rα on these cells substantially inhibited the IL2 induced proliferation, shifting the IL2 response curve toward the concentrations needed to stimulate βγ-IL2Rs. This suggests that, in the presence of an anti-IL2Rα mAb, both Kit225 and CTLL-2 retained some lower levels of IL2 responsiveness via IL2R with intermediate affinity, thereby providing an additional tool to study the activation of βγ-IL2Rs. Surprisingly, even though IL2 interaction with the β-chain component of high affinity IL2R is required for IL2-induced signaling,Citation12 blocking IL2Rβ alone was not enough to inhibit proliferation of Kit225 or CTLL-2 cells at any of the IL2 concentrations tested. The absence of a significant shift in the dose-dependent proliferation curves demonstrates that even in the presence of the IL2Rβ blocking antibody, these cells continue to have fully functional αβγ-IL2Rs mediating IL2 signaling. This retained function of high affinity IL2Rs, in the presence of mAb against the IL2Rβ, seen by us and others,Citation26,30,Citation44 likely reflects a tight association between the IL2/IL2Rα complex and the β-chain capable of overcoming the interaction of the blocking mAb with IL2Rβ, resulting in its detachment from the trimeric IL2R αβγ complex.Citation45 When mAbs against both IL2Rα and IL2Rβ are added, the proliferative response by Kit225 cells is abrogated, due to complete blockade of both high and intermediate affinity receptors.
Flow cytometry analyses showed expression of IL2Rβ, but no detectible IL2Rα on the surfaces of Tf-1β cells and SCID splenocytes was seen, suggesting their ability to respond through βγ-IL2Rs[AQ]. Adding the blocking mAb that specifically recognizes the IL2 binding site on IL2Rβ was sufficient to abolish the proliferative response of Tf-1β and SCID splenocytes at the range of IL2 doses tested, confirming the absence of αβγ-IL2Rs on these cells and demonstrating that they respond solely through βγ-IL2Rs. The IL2Rβ-dependent proliferation observed with SCID splenocytes is particularly important to illustrate the existence of functional mouse intermediate affinity IL2Rs, since earlier work proposed that contrary to the human βγ heterodimer, muβγ-IL2Rs are unable to bind IL2 or induce intracellular signaling.Citation38,46,Citation47 In those prior studies, various cell lines were transfected with muIL2Rβ and it was concluded that expression of IL2Rα is required for the formation of functional IL2Rs in mice. However, our data are in accordance with other reports, wherein splenocytes from various mouse strains with endogenous expression of muβγ-IL2Rs exhibited IL2 induced proliferation consistent with the activation of intermediate affinity IL2Rs.Citation30,48
The evaluations of IL2R expression and specificity in these different cell lines and SCID splenocytes allowed us to establish a useful in vitro model system to analyze the behavior of the novel IC constructs on human and murine IL2Rs. We acknowledge that these cells are not perfect controls for one another, exhibiting significant biological differences, including their difference in immune cell type (T cells vs. monocytes vs. NK cells) and their IL2R expression levels. These limitations can be evidenced in the concentrations needed to achieve maximal response. For example, SCID splenocytes require ∼40 times more IL2 to achieve proliferation likely due to a combination of factors including the fact that these are primary cells that have not been sensitized to growing in culture. However, for the purpose of our study, this model allows us to draw conclusions of the behavior of these ICs by focusing on the relative patterns of response of these distinct ICs compared to each other and to soluble IL2 within each particular cell line, rather than by comparing the response of distinct cell lines quantitatively to each other.
Our results show that both ICp and soluble huIL2 have comparable IL2-weight-based IL2 activity on human cells bearing αβγ-IL2Rs and βγ-IL2Rs. This illustrates that huIL2Rs are able to recognize and be activated by huIL2, even when IL2 is presented on the IgG heavy chains as part of the fusion protein. In contrast, ICp shows decreased ability to activate murine cells expressing muIL2Rs when compared to soluble huIL2. These data are in agreement with a separate study evaluating the IL2 bioactivity of different ICs containing IL2 on the heavy chains, wherein muIL2Rs were less responsive than huIL2Rs.Citation21 Fusing IL2 to the heavy chain of ICp partially disrupts the ability of muIL2Rs to respond to the IL2 component of the IC, as shown by the decreased proliferation elicited by ICp when compared to IL2 on CTLL-2 but not on Kit225 cells. This suggests that muIL2Rs are less efficient at binding huIL2 than huIL2Rs, making them more susceptible to subtle structural changes within the huIL2 molecules that might occur when fused to the hu14.18 mAb. These conformational differences seem to be most pronounced when evaluating stimulation of muβγ-IL2Rs as evidenced by the striking decrease in proliferation of SCID splenocytes in response to ICp compared to IL2. Therefore, this difference in the IC selectivity profile for muβγ-IL2Rs may account for the successful retention of the antitumor effect and reduced IL2 toxicity observed in mice treated with ICs such as ICp.Citation4,6,Citation7,33,Citation34
In order to reduce the ability of the IC to interact and activate huβγ-IL2Rs, a new platform for constructing ICs was developed by utilizing cytokine fusion to the light chain of the mAb.Citation19 In these ICs, the fusion junction of the IL2 molecules is located adjacent to the antibody hinge region, creating a sequestering effect that restricts IL2Rβ binding. Our binding data demonstrate that IC35 is able to bind αβγ-IL2Rs with similar affinity to ICp and ICSK but has significantly reduced ability to bind βγ-IL2Rs, particularly at the lower concentrations tested. Furthermore, ICSK showed similar affinity to ICp on both αβγ-IL2Rs and βγ-IL2Rs, with higher activity on SCID splenocytes, demonstrating that the enhanced SK molecules used in this construct restore the binding ability of this IC for intermediate affinity IL2Rs. However, this new placement of IL2 does not interfere with antigen binding as confirmed with flow cytometry staining of GD2+ M21 human tumor cells (not shown). Furthermore, when compared for their ability to mediate ADCC, these novel ICs were found to have equivalent activity to ICp, demonstrating that the ability of these ICs to bind Fc receptors and mediate tumor cytotoxicity was unaltered. Therefore, fusing the IL2 molecules to the light chains of the mAb allows fine-tuning the degree of βγ-IL2R binding specificity without affecting the other functions of the IC, such as antigen and FcR binding. IC35 has one huIL2 molecule fused directly to the C-terminal cysteine residue of each L-chain. The current experiments demonstrate that IC35 retains intact bioactivity (comparable to ICp) on human and mouse αβγ-IL2Rs, whereas it shows decreased or no activation of human or mouse βγ-IL2Rs, respectively. Therefore, IC35 achieves a low-level activation on huβγ-IL2Rs comparable to that which ICp has on muβγ-IL2Rs.
To further modulate the degree of receptor specificity, the length of the N-terminus of the huIL2 was adjusted on IC45 by removing five residues.Citation19 We showed that the ability of IC45 to activate mouse and human αβγ-IL2Rs was decreased; therefore, shortening the IL2 molecules on IC45 resulted in a reduction of high affinity activation. Furthermore, IC45 was unable to stimulate proliferation of mouse and human βγ-IL2Rs in the range of concentrations tested, although it does stimulate proliferation at higher concentrations (SG unpublished results). This high level of IL2R selectivity with IC45 is likely unfavorable for effective clinical immune cell activation at the low doses normally used for IC therapy, since it has been shown that initial activation of βγ-IL2Rs is needed for the upregulation of IL2Rα.Citation49 It remains to be determined whether higher doses, typically used for antibody therapy, might benefit from this reduced activity form of IL2. In addition, clinical studies that utilized a fusion protein containing a genetically modified IL2 protein (Selectikine), which was designed to be highly selective for αβγ-IL2R, found that although it did not exhibit any IL2-related toxicity, it also did not show any objective antitumor responses as a single agent in end-stage patients with bulky disease (although several patients had long-term stable disease >4 y). These findings suggest that some minimal activation of βγ-IL2Rs is needed to mount an effective αβγ-IL2R-based therapeutic immune response.Citation18
It is important to note that in addition to toxicity mediated via the activation of immune cells expressing βγ-IL2Rs, the direct response of endothelial cells to IL2 via αβγ-IL2Rs on their surface has also been shown to contribute to IL2 toxicity.Citation50 Recent studies using IL2 muteins or IL2/anti-IL2 mAb complexes directed toward βγ-IL2Rs over αβγ-IL2Rs have demonstrated an expansion of T and NK cells accompanied with a reduction in endothelial cell activation resulting in antitumor effects with acceptable toxicity.Citation51,52 Although these results may seem contradictory, the mechanisms mediating IL2 toxicity are complex and remain to be completely understood. It is possible that in these previously reported model systems, the contribution of endothelial cells to IL2 toxicity is more prominent due to the systemic administration and exposure to αβγ-IL2Rs on blood vessels for these IL2 formulations; in this setting, it may be possible that using IL2 formulations that reduce contact with αβγ-IL2Rs on endothelial cells may reduce this component of IL2-related toxicity. In contrast, in our model, the novel immunocytokines target IL2 to malignant cells via antigen binding, increasing the concentration of IL2 within the tumor microenvironment, and resulting in the activation of tumor-infiltrating immune cells. In this scenario, over-stimulation of NK cells expressing intermediate affinity IL2Rs might contribute more significantly to IL2-induced toxicity and therefore reducing these βγ-IL2R interactions would allow safer administration of the ICs to doses that achieve clinical efficacy.
Our data suggest that IC35 administration to patients bearing GD2+ tumors might achieve the desired balance between antitumor efficacy and tolerability that we observed in mice treated with ICp. However, due to its inability to activate muβγ-IL2Rs, preclinical in vivo studies designed to study the antitumor effect and safety of this IC35 molecule have limited translatability to the clinical setting. In order to overcome this limitation and better represent in our mouse model the level of IC35-induced βγIL2R activation expected in humans using an IC with IL2 on the immunoglobulin light chains, ICSK was developed. This IC contains two IL2 “SK” molecules that have been mutated to exhibit high affinity binding to IL2Rβ, and thus overcome the sequestering effect of being placed on the light chains. Our results show that indeed, ICSK has regained the ability to stimulate huβγ-IL2Rs completely (comparable to IL2). On muβγ-IL2Rs, ICSK retains a modest level of IL2 bioactivity, exhibiting a ∼20-fold difference when compared to huIL2, similar to the ∼20-fold difference observed with IC35 on huβγ-IL2Rs (). Furthermore, when compared to ICp, ICSK stimulated muβγ-IL2Rs to secrete higher levels of IFNγ that is an integral part of the NK effector machinery.Citation53 The ability to induce increased production of this pro-inflammatory cytokine is a functional consequence of IL2R binding, showing that ICSK can better activate mouse NK cells than ICp. These results suggest that ICSK might be used in the future as a surrogate in mouse model systems to better predict the effects that we hypothesize IC35 will have in humans. In this way, we could improve our understanding of IL2-induced dose-dependent toxicity vs. antitumor efficacy in mice, and subsequently advance the design of therapeutics such as IC35 for assessment in clinical trials.
Table 5. Fold difference of the bioactivity of the ICs when compared to IL2.
In summary, our findings demonstrate that human and mouse IL2R have quantitative differences in their ability to respond to human IL2. These differences are magnified when IL2 is presented as part of a fusion protein, such as the ICs evaluated here. In order to use mouse models to simulate the results expected in clinical testing with an IL2-based clinical reagent, the IL2-based compound evaluated in mice should simulate with murine IL2Rs, the biology of the clinical reagent seen with human IL2Rs. Further work is warranted to investigate the functional capabilities of the new ICs presented here, and similar IC constructs, to elicit immune effector functions in vitro, and their stability, safety, and antitumor efficacy in vivo. These, together with a detailed in vivo analysis of the stimulatory effects of these ICs on different immune cell populations expressing αβγ- vs. βγ-IL2Rs will enhance our understanding of murine models and the mechanism of action of these molecules. As oursCitation54,55 and other preclinical dataCitation56,57 are now demonstrating the potential for augmented antitumor activity by ICs when combined with other modalities, it will be important to use ICs in the clinic that simulate the IL2R biology shown to be effective in the murine models.
Disclosure of potential conflicts of interest
Dr. Steve Gillies discloses equity ownership in Provenance Biopharmaceut-icals of Carlisle MA and ownership of intellectual property related to the light chain fusion proteins. All other authors declare no competing financial interests.
KONI_A_1238538_supplementary_data.zip
Download Zip (1 MB)Acknowledgments
We thank Dr. Jeffery Way for his help generating the PDB files used for the 3D modeling of the ICs and Karla Esquilin for her assistance using the Pymol software to model the IC constructs. We also thank Dr. Amy Gurel for comments that greatly improved this manuscript.
Funding
This research was supported by Hyundai Hope on Wheels Grant; Midwest Athletes Against Childhood; Stand Up 2 Cancer; The St. Baldrick's Foundation; American Association of Cancer Research; University of Wisconsin-Madison Carbone Cancer Center; and supported in part by Public Health Service Grants CA21115, CA23318, CA66636, CA180820, CA180794, CA21076, CA180799, CA14958, CA180816, CA166105 and CA197078; from the National Cancer Institute. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The Advanced Opportunity Fellowship through SciMed Graduate Research Scholars and the Molecular Bioscience Training Grant (MBTG) T32 GM07215 at University of Wisconsin – Madison, provided funding for Zulmarie Perez Horta.
References
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New Engl J Med 2010; 363:1324-34; PMID:20879881; https://doi.org/10.1056/NEJMoa0911123
- Gillies SD, Reilly EB, Lo K-M, Reisfeld RA. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci 1992; 89:1428-32; PMID:1741398; https://doi.org/10.1073/pnas.89.4.1428
- Niculescu-Duvaz I. Technology evaluation: EMD-273063, EMD Lexigen. Curr Opin Mol Ther 2004; 6:559-66; PMID:15537058
- Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD. Enhanced activity of hu14. 18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res 2004; 10:4839-47; PMID:15269160; https://doi.org/10.1158/1078-0432.CCR-03-0799
- Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci 1994; 91:9626-30; PMID:7937818; https://doi.org/10.1073/pnas.91.20.9626
- Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 1998; 91:1706-15; PMID:9473237
- Lode HN, Xiang R, Dolman CS, Reisfeld RA, Varki NM, Gillies SD. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer I 1997; 89:1586-94; PMID:9362156; https://doi.org/10.1093/jnci/89.21.1586
- King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol 2004; 22:4463-73; PMID:15483010; https://doi.org/10.1200/JCO.2004.11.035
- Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C. A phase I clinical trial of the hu14. 18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res 2006; 12:1750-9; PMID:16551859; https://doi.org/10.1158/1078-0432.CCR-05-2000
- Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol 2010; 28:4969-75; PMID:20921469; https://doi.org/10.1200/JCO.2009.27.8861
- Albertini MR, Hank JA, Gadbaw B, Kostlevy J, Haldeman J, Schalch H, Gan J, Kim K, Eickhoff J, Gillies SD. Phase II trial of hu14. 18-IL2 for patients with metastatic melanoma. Cancer Immunol Immun 2012; 61:2261-71; PMID:22678096; https://doi.org/10.1007/s00262-012-1286-5
- Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol 1998; 70:1-81; PMID:9755337; https://doi.org/10.1016/S0065-2776(08)60386-7
- Anderson T, Hayes T, Gately M, Bontempo J, Stern L, Truitt G. Toxicity of human recombinant interleukin-2 in the mouse is mediated by interleukin-activated lymphocytes. Separation of efficacy and toxicity by selective lymphocyte subset depletion. Lab Invest 1988; 59:598-612; PMID:3263543
- Caligiuri MA, Murray C, Robertson M, Wang E, Cochran K, Cameron C, Schow P, Ross M, Klumpp T, Soiffer R. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Investig 1993; 91:123; PMID:7678599; https://doi.org/10.1172/JCI116161
- Peace DJ, Cheever MA. Toxicity and therapeutic efficacy of high-dose interleukin 2. In vivo infusion of antibody to NK-1.1 attenuates toxicity without compromising efficacy against murine leukemia. J Exp Med 1989; 169:161-73; PMID:2783332; https://doi.org/10.1084/jem.169.1.161
- Shanafelt A, LIn Y, Shanafelt M, Forte CP, Dubois-Stringfellow N, Carter C, Gibbons JA, Cheng S, Delatia KA, Fleischer R et al. A T-cell selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat Biotechnol 2000; 18:1197-202; PMID:11062441; https://doi.org/10.1038/81199
- Gillies SD, Lan Y, Hettmann T, Brunkhorst B, Sun Y, Mueller SO, Lo KM. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res 2011; 17:3673-85; PMID:21531812; https://doi.org/10.1158/1078-0432.CCR-10-2921
- Gillessen S, Gnad-Vogt US, Gallerani E, Beck J, Sessa C, Omlin A, Mattiacci MR, Liedert B, Kramer D, Laurent J et al. A phase I dose-escalation study of the immunocytokine EMD 521873 (Selectikine) in patients with advanced solid tumours. Eur J Cancer 2013; 49:35-44; PMID:22918078; https://doi.org/10.1016/j.ejca.2012.07.015
- Gillies SD. A new platform for constructing antibody-cytokine fusion proteins (immunocytokines) with improved biological properties and adaptable cytokine activity. Protein Eng Des Sel 2013; 26:561-9; PMID:24025193; https://doi.org/10.1093/protein/gzt045
- Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P et al. Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature 2012; 484:529-33; PMID:22446627; https://doi.org/10.1038/nature10975
- Gillies SD, Lan Y, Williams S, Carr F, Forman S, Raubitschek A, Lo KM. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood 2005; 105:3972-8; PMID:15692062; https://doi.org/10.1182/blood-2004-09-3533
- Hori T, Uchiyama T, Tsudo M, Umadome H, Ohno H, Fukuhara S, Kita K, Uchino H. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood 1987; 70:1069-72; PMID:3115332
- Farner N, Voss S, Leary T, Gan J, Hakimi J, Evans G, Ju G, Sondel P. Distinction between γc detection and function in YT lymphoid cells and in the GM-CSF responsive myeloid cell line, Tf-1. Blood 1995; 86:4568-78; PMID:8541547
- Baker PE, Gillis S, Smith KA. Monoclonal cytolytic T-cell lines. J Exp Med 1979; 149:273-8; PMID:310861; https://doi.org/10.1084/jem.149.1.273
- Gearing A, Thorpe R. The international standard for human interleukin-2: calibration by international collaborative study. J Immunol Methods 1988; 114:3-9; PMID:3263444; https://doi.org/10.1016/0022-1759(88)90145-7
- Tsudo M, Kitamura F, Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci 1989; 86:1982-6; PMID:2467293; https://doi.org/10.1073/pnas.86.6.1982
- Robb RJ, Rusk CM, Neeper MP. Structure-function relationships for the interleukin 2 receptor: location of ligand and antibody binding sites on the Tac receptor chain by mutational analysis. Proc Natl Acad Sci 1988; 85:5654-8; PMID:3135551; https://doi.org/10.1073/pnas.85.15.5654
- Voss S, Sondel P, Robb R. Characterization of the interleukin 2 receptors (IL-2R) expressed on human natural killer cells activated in vivo by IL-2: association of the p64 IL-2R gamma chain with the IL-2R beta chain in functional intermediate-affinity IL-2R. J Exp Med 1992; 176:531-41; PMID:1500859; https://doi.org/10.1084/jem.176.2.531
- Lowenthal J, Corthesy P, Tougne C, Lees R, MacDonald H, Nabholz M. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol 1985; 135:3988-94; PMID:3934270
- Tanaka T, Tsudo M, Karasuyama H, Kitamura F, Kono T, Hatakeyama M, Taniguchi T, Miyasaka M. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J Immunol 1991; 147:2222-8; PMID:1918958
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69:4010-7; PMID:19383914; https://doi.org/10.1158/0008-5472.CAN-08-3712
- Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Ann Rev Immunol 1991; 9:323-50; PMID:1910681; https://doi.org/10.1146/annurev.iy.09.040191.001543
- Pancook JD, Becker JC, Gillies SD, Reisfeld RA. Eradication of established hepatic human neuroblastoma metastases in mice with severe combined immunodeficiency by antibody-targeted interleukin-2. Cancer Immunol Immunother 1996; 42:88-92; PMID:8620525; https://doi.org/10.1007/s002620050256
- Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K, Alderson KL, Gan J, Reisfeld RA, Gillies SD. Intratumoral hu14. 18–IL-2 (IC) Induces Local and Systemic Antitumor Effects That Involve Both Activated T and NK Cells As Well As Enhanced IC Retention. J Immunol 2012; 189:2656-64; PMID:22844125; https://doi.org/10.4049/jimmunol.1200934
- Zurawski SM, Mosmann TR, Benedik M, Zurawski G. Alterations in the amino-terminal third of mouse interleukin 2: effects on biological activity and immunoreactivity. J Immunol 1986; 137:3354-60; PMID:2430019
- Shimuzu A, Kondo S, Takeda S-i, Yodoi J, Ishida N, Sabe H, Osawa H, Diamantstein T, Nikaido T, Honjo T. Nucleotide sequence of mouse IL-2 receptor cDNA and its comparison with the human IL-2 receptor sequence. Nucleic Acids Res 1985; 13:1505-16; PMID:2987826; https://doi.org/10.1093/nar/13.5.1505
- Kono T, DoI T, Yamada G, Hatakeyama M, Minamoto S, Tsudo M, Miyasaka M, Miyata T, Taniguchi T. Murine interleukin 2 receptor beta chain: dysregulated gene expression in lymphoma line EL-4 caused by a promoter insertion. Proc Natl Acad Sci 1990; 87:1806-10; PMID:2155425; https://doi.org/10.1073/pnas.87.5.1806
- Kumaki S, Kondo M, Takeshita T, Asao H, Nakamura M, Sugamura K. Cloning of the mouse interleukin 2 receptor γ chain: demonstration of functional differences between the mouse and human receptors. Biochem Biophys Res Commun 1993; 193:356-63; PMID:8503926; https://doi.org/10.1006/bbrc.1993.1631
- Mosmann T, Yokota T, Kastelein R, Zurawski SM, Arai N, Takebe Y. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4). J Immunol 1987; 138:1813-6; PMID:3493289
- Rosenberg SA, Grimm EA, McGrogan M, Doyle M, Kawasaki E, Koths K, Mark DF. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science 1984; 223:1412-4; PMID:6367046; https://doi.org/10.1126/science.6367046
- Chang AE, Hyatt CL, Rosenberg SA. Systemic administration of recombinant human interleukin-2 in mice. J Immunother 1984; 3:561-72; PMID:6334141
- Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol 1978; 120:2027-32; PMID:307029
- Kendra K, Gan J, Ricci M, Surfus J, Shaker A, Super M, Frost JD, Rakhmilevich A, Hank JA, Gillies SD. Pharmacokinetics and stability of the ch14. 18–interleukin-2 fusion protein in mice. Cancer Immunol Immun 1999; 48:219-29; PMID:10478638; https://doi.org/10.1007/s002620050569
- Takeshita T, Goto Y, Tada K, Nagata K, Asao H, Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med 1989; 169:1323-32; PMID:2784485; https://doi.org/10.1084/jem.169.4.1323
- Kamio M, Uchiyama T, Arima N, Itoh K, Ishikawa T, Hori T, Uchino H. Role of α chain–IL-2 complex in the formation of the ternary complex of IL-2 and high-affinity IL-2 receptor. Int Immunol 1990; 2:521-30; PMID:2085490; https://doi.org/10.1093/intimm/2.6.521
- Nemoto T, Takeshita T, Ishii N, Kondo M, Higuchi M, Satomi S, Nakamura M, Mori S, Sugamura K. Differences in the interleukin‐2 (IL‐2) receptor system in human and mouse: α chain is required for formation of the functional mouse IL‐2 receptor. Eur J Immunol 1995; 25:3001-5; PMID:7489734; https://doi.org/10.1002/eji.1830251102
- Chastagner P, Moreau JL, Jacques Y, Tanaka T, Miyasaka M, Kondo M, Sugamura K, Thèze J. Lack of intermediate‐affinity interleukin‐2 receptor in mice leads to dependence on interleukin‐2 receptor α, β and γ chain expression for T cell growth. Eur J Immunol 1996; 26:201-6; PMID:8566067; https://doi.org/10.1002/eji.1830260131
- Sharon M, Gnarra JR, Leonard WJ. A 100-kilodalton protein is associated with the murine interleukin 2 receptor: biochemical evidence that p100 is distinct from the alpha and beta chains. Proc Natl Acad Sci 1990; 87:4869-73; PMID:2352954; https://doi.org/10.1073/pnas.87.12.4869
- Rebollo A, Silva A. Intermediate-and high-affinity interleukin-2 receptors expressed in an IL-4-dependent T-cell line induce different signals. Immunology 1993; 80:229; PMID:8262551
- Krieg C, Létourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci 2010; 107:11906-11; PMID:20547866; https://doi.org/10.1073/pnas.1002569107
- Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol 2015; 36:763-77; PMID:26572555; https://doi.org/10.1016/j.it.2015.10.003
- Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Exp Opin Biol Ther 2006; 6:1323-31; PMID:17223740; https://doi.org/10.1517/14712598.6.12.1323
- Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol 2012; 91:299-309; PMID:22045868; https://doi.org/10.1189/jlb.0611308
- Morris Z, Guy E, Francis D, Gressett M, Armstrong E, Huang S, Gilles S, Korman A, Hank J, Rakhmilevich A. Immunocytokine augments the local and abscopal response to radiation and CTLA4 checkpoint inhibition in a murine melanoma model. Int J Radiat Oncol Biol Phys 2015; 93:S93-S4; https://doi.org/10.1016/j.ijrobp.2015.07.224
- Morris ZS, Guy EI, Francis DM, Gressett MM, Armstrong EA, Huang S, Gillies SD, Korman AJ, Hank JA, Rakhmilevich AL. Immunocytokine augments local and abscopal response and animal survival when added to radiation and CTLA-4 checkpoint inhibition in a murine melanoma model. J Immunother Cancer 2015; 3:P308; https://doi.org/10.1186/2051-1426-3-S2-P308
- Johnson EE, Lum HD, Rakhmilevich AL, Schmidt BE, Furlong M, Buhtoiarov IN, Hank JA, Raubitschek A, Colcher D, Reisfeld RA. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immun 2008; 57:1891-902; PMID:18438664; https://doi.org/10.1007/s00262-008-0519-0
- Johnson EE, Yamane BH, Buhtoiarov IN, Lum HD, Rakhmilevich AL, Mahvi DM, Gillies SD, Sondel PM. Radiofrequency ablation combined with KS-IL2 immunocytokine (EMD 273066) results in an enhanced antitumor effect against murine colon adenocarcinoma. Clin Cancer Res 2009; 15:4875-84; PMID:19638464; https://doi.org/10.1158/1078-0432.CCR-09-0110
