ABSTRACT
Metastatic spread in the bone marrow (BM) at diagnosis is the worst prognostic factor for neuroblastoma (NB) patients. Here, we analyzed the presence of two immunosuppressive cell subsets, CD4+CD25hiCD127− regulatory T (Treg) cells and CD4+CD45R0+CD49b+LAG3+ type 1 regulatory (Tr1) cells, in BM and peripheral blood (PB) samples from NB patients and controls. Frequency of both regulatory cell subsets was lower in BM and PB samples from NB patients than in respective healthy controls. No correlation was found between the frequency of Treg and Tr1 cells and prognostic factors at diagnosis, such as age and stage. Only MYCN amplification correlated to a higher number of Treg in BM and of Tr1 in PB. These findings suggested an altered trafficking of regulatory T cells in NB, but delineated a limited role of these subsets in BM microenvironment and/or periphery in NB. These observations should be considered designing immunotherapeutic approaches for metastatic NB.
KEYWORDS:
Introduction
Neuroblastoma (NB) is a pediatric tumor arising from the sympathetic nervous system, representing the most frequent extracranial solid tumor of pediatric age. NB has an incidence of 1/100,000 case children/year, and accounts for 15% of cancer deaths in children. Although the causes are unknown, 1–2% of NB may have a family history of the disease. In addition, several genetic alterations have been characterized in NB, i.e., gain-of-function of ALK gene,Citation1 germline mutation of PHOX2B gene,Citation2 amplification of the MYCN gene, and multiple chromosomal abnormalities.Citation3 The clinical course of patients with NB is heterogeneous, ranging from spontaneous remission to progressive metastatic disease, with dissemination to multiple organs, including bone marrow (BM).Citation4 BM infiltration is indeed a predictor of poor clinical outcome.Citation5 NB patients are now classified in different risk classes following the International Neuroblastoma Risk Group staging system,Citation6 that consider genetic alterations, DNA ploidy, histological features, age, and stage as classification criteria. Low/intermediate risk NB patients have a good prognosis with surgery alone or with standard chemotherapy. In contrast the prognosis of high-risk NB patients is poor, in spite of aggressive multimodal treatments, with a survival rate of 35% at 5 y.Citation7
CD4+CD25+FOXP3+regulatory T cells (Treg) are important immunosuppressive cells that dampen antitumor immune response.Citation8 Treg cells have been previously characterized in peripheral blood (PB) from very small cohorts of NB patients.Citation9-11 A higher percentage of Treg in NB patients than healthy children was reported by us and Tilak et al., whereas Gowda et al. showed that their number was similar in low- and high-risk patients.
Type 1 regulatory (Tr1) cells were firstly identified in mice as CD4+ T cells secreting high amounts of IL-10.Citation12 Subsequently, Tr1 cells have been characterized in humans,Citation13-15 and are now identified as CD4+CD45R0+CD49b+LAG-3+ T cells.Citation16 Only recently the presence of Tr1 cells was documented in tumor patients. Precisely, an increased numbers of Tr1 cells was shown to associate to tumor progression in colorectal cancer,Citation17 whereas we showed in a small cohort of NB patients that PB Tr1 cells were present in lower percentage than in healthy children.Citation10
In this study, we have analyzed the percentage of Treg and Tr1 cells in BM and PB samples collected from NB patients at diagnosis as compared to healthy subjects. Moreover, we analyzed potential associations of this frequency to the most important NB prognostic factors, such as age, stage of disease, and MYCN gene amplification.
Materials and methods
Patients and controls
The study was approved by the Ethics Committee of the Giannina Gaslini Institute, Genoa, Italy.
Fresh BM samples collected at diagnosis from 27 children diagnosed with NB from January to June 2016 at the Italian Centers participating to the Associazione Italiana di Emato-Oncologia Pediatrica were centralized at the Istituto Giannina Gaslini in Genoa, Italy. NB patients were diagnosed at the referring center and staged according to the International Neuroblastoma Risk Group staging system.Citation6 Patients' characteristics, i.e., sex, age at diagnosis, stage, and MYCN status (single copy or amplified), are summarized in . As controls, BM aspirates were obtained from 15 healthy donors, selected according to the Transplant Unit Clinical Protocol of Ematologia 2 at the IRCCS San Martino – IST in Genoa, following a written informed consent at the time of donation. Samples were processed as described.Citation18 At the end of processing, an aliquot was taken to perform quality control tests, such as CD34+ cell count, in vitro progenitors' cell growth and sterility. The remaining from this aliquot was used in this study. BM donor's characteristics are summarized in .
Table 1. Characteristics of NB patients and controls.
Fresh PB samples were collected at diagnosis from 21 out of 27 NB patients. As controls, PB samples were obtained from 13 age- and sex-matched healthy children admitted to the Gaslini Institute for accidental injuries. Healthy children's characteristics are summarized in .
Flow cytometry
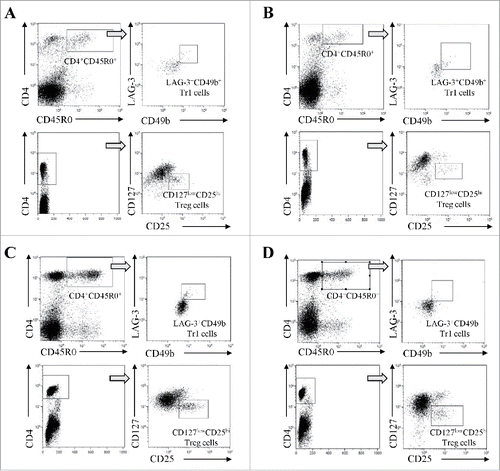
Flow cytometric analysis has been performed on whole PB or BM blood samples, using 50 µL of whole blood/tube. Treg were identified using Treg detection kit (Miltenyi Biotec), containing anti-CD4 FITC, anti-CD25 APC, and anti-CD127 PE antibodies, following manufacturer's protocol. Tr1 were evaluated using anti-CD4 PE-Cy7 (eBioscience), anti-CD45R0 APC (eBioscience), anti-CD49b PE (R&D Systems), and anti-LAG-3 FITC (R&D Systems) antibodies. Samples were incubated with specific antibodies (20′ at 4°C in the dark) and then subjected to erythrocytes lysis using BD FACS lysis (BD Biosciences) by incubating 15′ at RT in the dark. Cells were then washed with PBS 0.5% BSA, resuspended in PBS 0.5% BSA, and then run on a Gallios cytometer (Beckman Coulter), acquiring at least 5×104 events. Data were analyzed using Kaluza software (Beckman Coulter). Analysis was performed gating on lymphocytes for both cell populations. As shown in , Treg were identified as CD4+CD25highCD127−/low cells (upper panels), whereas Tr1 cells were identified as CD4+CD45R0+CD49b+LAG-3+ cells (lower panels), considering the threshold obtained in cells stained with CD4 and CD45R0 antibodies only.
Figure 1. Representative dot plots and gating strategies for the characterization of CD4+CD127lowCD25hi Treg cells (upper panels) and CD4+CD45R0+LAG-3+CD49b+ Tr1 cells (lower panels) in BM samples from healthy donors (panel A) and NB patients (panel B) and in PB samples from healthy children (panel C) and NB patients (panel D).
Statistics
Normal distribution of data was tested using Kolgomorov–Smirnov test, using Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA). Since data distribution was not normal, differences in the percentage of Treg and Tr1 cells between (1) patients and controls or (2) different groups of patients were compared by Mann–Whitney test, using Prism 5.0 software. A p value <0.05 was considered as statistically significant. Significance ranges are the following: *p < 0.05; **p < 0.005; ***p < 0.0005.
Results and discussion
Since the BM is the most important site of metastasis in NB patients,Citation5 we first analyzed the percentage of total CD4+ T cells, memory CD4+CD45R0+ T cells, Treg, and Tr1 cells in BM samples from NB patients (n = 27) and healthy subjects (n = 15). shows gating strategies for the characterization of Treg and Tr1 cells, and representative dot plots for BM samples derived from healthy donors and NB patients (panels A and B, respectively) and for PB samples derived from healthy children and NB patients (panels C and D respectively). As shown in , panel A, the percentage of total CD4+ T cells was similar between NB patients (percentage of total cells, median ± SE : 3.67 ± 0.26) and healthy donors (2.86 ± 0.62), whereas the percentage of memory CD4+CD45R0+ T cells was lower in NB patients (0.38 ± 0.16) than in controls (1.44 ± 0.13, p <0.0001). The percentage of Treg cells was significantly lower in NB patients (0.19 ± 0.03) than in healthy controls (0.39 ± 0.03, p = 0.0003). This observation is in contrast with previous studies performed in patients with other solid tumors that metastasize to the BM, such as prostate cancerCitation19 and Ewing's sarcoma,Citation20 reporting higher percentage of BM Treg cells in patients than controls. The BM has been described as a preferential site for migration and/or detainment of Treg cells, and may function as an immune regulatory organ.Citation21 Since the migration of Treg cells to the BM is mediated by the CXCL12/CXCR4 axis, the observation that BM from NB patients had less Treg cells than healthy subject can be ascribed to the low and frequently absent expression of BM CXCL12 in NB patients.Citation22 As for Tr1 cells, their percentage was very low in both NB children and healthy subjects (0.03 ± 0.006 and 0.05 ± 0.005, respectively) and the difference was not statistically significant. In addition, this limited difference may be due to a general depletion of total CD4+CD45R0+ memory T cells observed in BM samples derived from NB patients. To our knowledge, however, this is the first report on BM Tr1 cells in tumor patients and healthy subjects. Nevertheless, the very low number of Tr1 cells found in NB patients' BM does not support a role of these cells in the BM disease of NB patients.
Figure 2. Frequency of total CD4+ T cells, CD4+CD45R0+ memory T cells, CD4+CD25hiCD127− Treg, and CD4+CD45R0+CD49b+LAG3+ Tr1 cells has been analyzed in BM (A, B) and PB (C, D) samples from NB patients (gray circles) and healthy controls (white circles). Results are expressed as percentage of total cells. Horizontal bars indicated medians. p values are indicated where the difference is statistically significant.
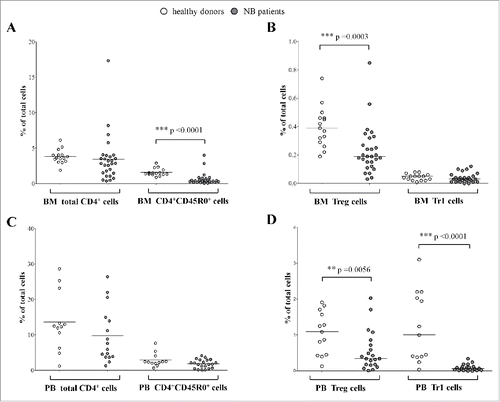
Next, we analyzed the percentage of the same cell populations in PB samples collected from NB patients (n = 21) as compared to age-matched healthy children (n = 13). As shown in , panel C, the percentage of both total CD4+ T cells and memory CD4+CD45R0+ T cells was similar between NB patients (9.72 ± 7.7 and 1.8 ± 1.2, respectively) and controls (13.58 ± 8.2 and 2.89 ± 1.9, respectively). In contrast, the percentage of both Treg and Tr1 cells was significantly lower in NB patients (0.34 ± 0.11 and 0.07 ± 0.019, respectively) than in healthy children (1.09 ± 0.16 and 1 ± 0.27, respectively) (p = 0.0056 and p < 0.0001), reflecting the observations in the BM samples. These data, obtained in a larger number of PB samples from NB patients and controls than our previous report,Citation10 confirmed the lower percentage of PB Tr1 cells and suggested that the higher percentage of PB Treg previously reported was inaccurate due to the small sample size.
Collectively, the data presented here suggested that both regulatory T cell populations are partially depleted from PB of NB patients, whereas only Treg are depleted in BM samples from NB patients. This effect was not related to the depletion of total CD4+ T cells since the percentage of these cells were similar between patients and controls. The lower percentage of Tr1 and Treg cells observed in PB samples from NB patients might be related to a specific recruitment of these regulatory cell populations in the tumor microenvironment, similarly to what observed in other solid tumors.Citation17,23,24 However, no information on the presence of Treg and/or Tr1 cells in the primary NB tumors is so far available.
Next, we asked whether the percentage of Treg and Tr1 cells in NB BM and PB samples correlated with the established prognostic factors, i.e., age, tumor stage, and amplification of MYCN gene.Citation6
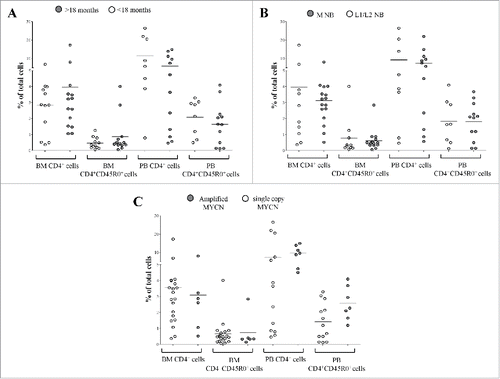
As shown in , the percentage of total CD4+ T cells and memory CD4+CD45R0+ T cells was similar between NB patients (1) with an age at diagnosis above (BM: n = 12, CD4+ 3.96 ± 4.2, CD4+CD45R0+ 0.86 ± 1.1, PB: n = 10, CD4+ 5.94 ± 5.35, CD4+CD45R0+ 1.6 ± 1.3) or below (BM: n = 15, CD4+ 2.83 ± 1.8, CD4+CD45R0+ 0.46 ± 0.35, PB: n = 11, CD4+ 11.6 ± 9.8, CD4+CD45R0+ 2 ± 1.1) 18 mo (panel A), (2) with stage M (BM: n = 13, CD4+ 3.12 ± 1.7, CD4+CD45R0+ 0.62 ± 0.63, PB: n = 9, CD4+ 7.48 ± 6.42, CD4+CD45R0+ 1.8 ± 1.1) or L1/L2 (BM: n = 11, CD4+ 3.96 ± 5, CD4+CD45R0+ 0.76 ± 1.1, PB: n = 9, CD4+ 9.3 ± 9.8, CD4+CD45R0+ 1.8 ± 1.4) disease (panel B), and (3) with (BM: n = 7, CD4+ 3 ± 2.7, CD4+CD45R0+ 0.73 ± 1, PB: n = 7, CD4+ 9.6 ± 4, CD4+CD45R0+ 2.5 ± 1) or without (BM: n = 20, CD4+ 3.5 ± 3.5, CD4+CD45R0+ 0.66 ± 0.84, PB: n = 14, CD4+ 7.4 ± 9.2, CD4+CD45R0+ 1.4 ± 1.1) MYCN amplification (panel C).
Figure 3. Frequency of total CD4+ T cells and CD4+CD45R0+ memory T cells has been analyzed in BM and PB samples from NB patients with an age at diagnosis above (gray circles) or below (white circles) 18 months (A), with metastatic (gray circles) or localized (white circles) tumors (B) and with amplified (gray circles) or single copy (white circles) MYCN gene (C). Results are expressed as percentage of total cells. Horizontal bars indicated medians. p values are indicated where the difference is statistically significant.
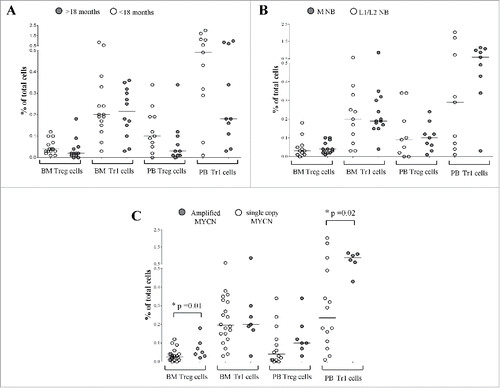
As shown in , panel A, no difference in the percentage of Treg and Tr1 cells was found between NB patients with an age at diagnosis above (BM: n = 12, Treg 0.02 ± 0.01, Tr1 0.21 ± 0.03, PB: n = 10, Treg 0.03 ± 0.03, Tr1 0.18 ± 0.11) or below 18 months (BM: n = 15, Treg 0.04 ± 0.008, Tr1 0.2 ± 0.05; PB: n = 11, Treg 0.1 ± 0.03; Tr1 0.49 ± 0.2). Similarly, no significant difference in Treg and Tr1cells' frequency was found by comparing NB patients with stage M (BM: n = 13, Treg 0.04 ± 0 .008, Tr1 0.19 ± 0 .05; PB: n = 9, Treg 0.1 ± 0.02, Tr1 0.59 ± 0.12) or L1/L2(BM: n = 11, Treg 0.03 ± 0.01, Tr1 0.2 ± 0.04; PB: n = 9, Treg 0.09 ± 0.04, Tr1 0.29 ± 0.25) (, panel B). The observed higher percentage of PB Tr1 cells in patients with M than in those with L1 disease was not statistically significant. As for MYCN status (, panel C), Treg percentage in BM samples was significantly higher in patients with MYCN amplification (n = 7, 0.05 ± 0.02) than in those with single copy gene (n = 20, 0.02 ± 0 .05, p = 0.02), whereas Tr1 cells showed similar percentages (amplified MYCN: n = 7, 0.2 ± 0.09; MYCN single copy: n = 20, 0.19 ± 0.02). In PB samples, Treg frequency was similar in patients with (n = 7, 0.1 ± 0.03) or without (n = 14, 0.04 ± 0.02) MYCN amplification, whereas Tr1 cells frequency was significantly higher in patients with MYCN amplification (n = 6, 0.85 ± 0.11) than in those with single copy gene (n = 14, 0.23 ± 0.16, p = 0.01).
Figure 4. Frequency of CD4+CD25hiCD127− Treg and CD4+CD45R0+CD49b+LAG3+ Tr1 cells has been analyzed in BM and PB samples from NB patients with an age at diagnosis above (gray circles) or below (white circles) 18 months (A), with metastatic (gray circles) or localized (white circles) tumors (B) and with amplified (gray circles) or single copy (white circles) MYCN gene (C). Results are expressed as percentage of total cells. Horizontal bars indicated medians. p values are indicated where the difference is statistically significant.
Collectively, these findings confirmed that Treg and Tr1 recruitment in the BM was not affected by the presence of metastatic disease, as already seen by stratifying by stage, but showed that BM infiltration may increase Tr1 cells in the periphery, as observed also in the presence of MYCN amplification. These differences in regulatory T cells frequencies were not related to an increase or depletion of total and/or memory CD4+ T cells. It is worth noting that the higher frequencies observed in the presence of MYCN amplification or BM infiltration were similar to those found in healthy subjects, thus, reducing the potential role of immunosuppressive Treg and Tr1 cells in the natural history of metastatic NB.
Conclusions
In conclusion, we delineated novel aspects of regulatory T-cell trafficking in NB, demonstrating that in NB patients BM and PB Treg and Tr1 cells were present at a lower frequency than in healthy subjects. In fact, the lower frequency observed might be related to a different recruitment of these cells in BM and (hypothetically) in the microenvironment of primary tumor. The frequency of Treg and Tr1 cells in BM and PB samples did not associate to established NB prognostic factors. Therefore, these findings do not support a role of these immunosuppressive cells in influencing NB disease in the periphery and/or in the BM. Future immunotherapeutic approaches in high risk NB patients should take these findings into account.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mrs. Camilla Valentino for excellent secretarial assistance.
Funding
This study was supported by Ministero del Lavoro, della Salute e delle Politiche Sociali (Progetti di Ricerca Corrente – 5 per mille) and Fondazione Italiana per la Lotta al Neuroblastoma.
References
- Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008; 455:930-5; PMID:18724359; http://dx.doi.org/10.1038/nature07261
- Mosse YP, Laudenslager M, Khazi D, Carlisle AJ, Winter CL, Rappaport E, Maris JM. Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet 2004; 75:727-30; PMID:15338462; http://dx.doi.org/10.1086/424530
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol 1999; 17:2264-79; PMID:10561284; http://dx.doi.org/10.1200/jco.1999.17.7.2264
- Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 2015; 33:3008-17; PMID:26304901; http://dx.doi.org/10.1200/JCO.2014.59.4648
- Morandi F, Corrias MV, Pistoia V. Evaluation of bone marrow as a metastatic site of human neuroblastoma. Ann NY Acad Sci 2015; 1335:23-31; PMID:25315505; http://dx.doi.org/10.1111/nyas.12554
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009; 27:289-97; PMID:19047291; http://dx.doi.org/10.1200/JCO.2008.16.6785
- Vo KT, Matthay KK, Neuhaus J, London WB, Hero B, Ambros PF, Nakagawara A, Miniati D, Wheeler K, Pearson AD et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol 2014; 32:3169-76; PMID:25154816; http://dx.doi.org/10.1200/JCO.2014.56.1621
- Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol 2015; 33:101-11; PMID:25728990; http://dx.doi.org/10.1016/j.coi.2015.02.003
- Gowda M, Godder K, Kmieciak M, Worschech A, Ascierto ML, Wang E, Marincola FM, Manjili MH. Distinct signatures of the immune responses in low risk versus high risk neuroblastoma. J Transl Med 2011; 9:170; PMID:21978632; http://dx.doi.org/10.1186/1479-5876-9-170
- Morandi F, Croce M, Cangemi G, Barco S, Rigo V, Carlini B, Amoroso L, Pistoia V, Ferrini S, Corrias MV. IL-10 and ARG-1 concentrations in bone marrow and peripheral blood of metastatic neuroblastoma patients do not associate with clinical outcome. J Immunol Res 2015; 2015:718975; PMID:25961062; http://dx.doi.org/10.1155/2015/718975
- Tilak T, Sherawat S, Agarwala S, Gupta R, Vishnubhatla S, Bakhshi S. Circulating T-regulatory cells in neuroblastoma: a pilot prospective study. Pediatr Hematol Oncol 2014; 31:717-22; PMID:24684178; http://dx.doi.org/10.3109/08880018.2014.886002
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997; 389:737-42; PMID:9338786; http://dx.doi.org/10.1038/39614
- Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol 2006; 18:120-7; PMID:16464609; http://dx.doi.org/10.1016/j.smim.2006.01.007
- Gregori S, Goudy KS, Roncarolo MG. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol 2012; 3:30; PMID:22566914; http://dx.doi.org/10.3389/fimmu.2012.00030
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006; 212:28-50; PMID:16903904; http://dx.doi.org/10.1111/j.0105-2896.2006.00420.x
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013; 19:739-46; PMID:23624599; http://dx.doi.org/10.1038/nm.3179
- Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol 2014; 31:82; PMID:25034363; http://dx.doi.org/10.1007/s12032-014-0082-9
- Vicente D, Podesta M, Pitto A, Pozzi S, Lucchetti S, Lamparelli T, Tedone E, Ibatici A, Figari O, Frassoni F et al. Progenitor cells trapped in marrow filters can reduce GvHD and transplant mortality. Bone Marrow Transplant 2006; 38:111-7; PMID:16751783; http://dx.doi.org/10.1038/sj.bmt.1705413
- Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 2012; 1:152-61; PMID:22720236; http://dx.doi.org/10.4161/onci.1.2.18480
- Brinkrolf P, Landmeier S, Altvater B, Chen C, Pscherer S, Rosemann A, Ranft A, Dirksen U, Juergens H, Rossig C. A high proportion of bone marrow T cells with regulatory phenotype (CD4+CD25hiFoxP3+) in Ewing sarcoma patients is associated with metastatic disease. Int J Cancer 2009; 125:879-86; PMID:19480009; http://dx.doi.org/10.1002/ijc.24461
- Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res 2004; 64:8451-5; http://dx.doi.org/10.1158/0008-5472.CAN-04-1987
- Scaruffi P, Morandi F, Gallo F, Stigliani S, Parodi S, Moretti S, Bonassi S, Fardin P, Garaventa A, Zanazzo G et al. Bone marrow of neuroblastoma patients shows downregulation of CXCL12 expression and presence of IFN signature. Pediatr Blood Cancer 2012; 59:44-51; PMID:21994039; http://dx.doi.org/10.1002/pbc.23339
- Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol 2006; 16:98-105; PMID:16378733; http://dx.doi.org/10.1016/j.semcancer.2005.11.003
- Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res 2009; 69:7895-8; PMID:19808962; http://dx.doi.org/10.1158/0008-5472.CAN-09-1642
