ABSTRACT
Prophylactic vaccination is typically utilized for the prevention of communicable diseases such as measles and influenza but, with the exception of vaccines to prevent cervical cancer, is not widely used as a means of preventing or reducing the incidence of cancer. Here, we utilize a peptide-based immunotherapeutic approach targeting ERBB3, a pseudo-kinase member of the EGFR/ERBB family of receptor tyrosine kinases, as a means of preventing occurrence of colon polyps. Administration of the peptide resulted in a significant decrease in the development of intestinal polyps in C57BL/6J-ApcMin mice, a model of familial adenomatous polyposis (FAP). In addition, even though they were not vaccinated, ApcMin offspring born to vaccinated females developed significantly fewer polyps than offspring born to control females. Lastly, to validate ERBB as a valid target for vaccination, we found no overt toxicity, increases in apoptosis, or morphological changes in tissues where Erbb3 was ablated in adult mice. These results indicate that prophylactic vaccination targeting ERBB3 could prevent the development of colon polyps in an at-risk patient population.
Introduction
The ability to prevent the occurrence of cancer in an at-risk population represents a novel approach to treating patients known to have a greatly increased risk of developing cancer in their lifetimes. Both familial adenomatous polyposis (FAP) and Lynch Syndrome are inherited in an autosomal dominant fashion resulting from a germline mutation in the adenomatous polyposis coli (APC) gene for FAP or in a DNA mismatch repair gene for Lynch Syndrome.Citation1,2 These diseases are characterized by a greatly increased risk of developing colon cancer, with close to 100% of FAP and 60% of Lynch Syndrome patients developing the disease during their lifetime.Citation3 These patients represent a high-risk patient population potentially suitable for prophylactic vaccination as a means of preventing or reducing cancer occurrence.
ApcMin mice are used as a model for FAP as they have a truncation mutation in the Apc gene and develop tens to hundreds of polyps in their intestinal tract.Citation4 Our strategy was to design a peptide immunotherapy targeting an appropriate cell-surface receptor that is implicated in the development of colon cancer and test whether vaccination with this peptide could prevent or reduce the incidence of polyps. We chose ERBB3 as the target antigen based upon previous work our group published showing the requirement of proper ERBB3 signaling for polyp formation in ApcMin mice.Citation5 Although small-molecule inhibition of ERBB3 is not possible due to the lack of a functional kinase domain,Citation6 a number of monoclonal antibodies targeting ERBB3 have recently entered clinical trials. These antibodies have been designed to target either domain I or III of the proteinCitation7 and inhibit the binding of ligand or interfere with heterodimer formation. Our strategy was to induce an autoimmune reaction to a specific region of ERBB3 by targeting this reaction to a recently identified critical aspartic acid residue in Domain IICitation8 through creation of two separate antigens. Here, we show that immunization against ERBB3 results in a significant decrease in polyp formation in vaccinated ApcMin mice as well as offspring of vaccinated females. In addition, we show that ablation of Erbb3 in adult mice does not result in apparent macroscopic or microscopic toxicity, validating the use of ERBB3 as a therapeutic target.
Materials and methods
EBX and EB3IV preparation
EBX peptide was chemically synthesized with the purity (> 95%) verified by LC–MS/MS (Genscript). The peptide was attached to KLH through maleimide-assisted conjugation. Erbb3 cDNA was generated from mouse liver and inserted into the pCR2.1-TOPO vector (Life Technologies). EB3IV was amplified using the cloned Erbb3 cDNA as template with the primers EB3IV_F (5′- CTGGGATCCACGTTCCAGCTTGAG-3′) and EB3IV_R (5′-CAAAAGCTTAATCTCCCGGACTGT-3′) and cloned into the BamHI and HindIII sites in the pQE-80L vector (Life Technologies). The plasmid was transfected into BL21 Escherichia coli (Life Technologies) with protein expression induced by 1mM IPTG for 4 h. Protein was purified using a Nickel-NTA column (Qiagen) with 20 column volumes of wash buffer containing 0.1% Triton X-114 utilized to eliminate endotoxin.Citation9 Endotoxin levels in protein preparations were determined by a chromogenic LAL Endotoxin Assay (Genscript) and found to be <0.1 EU.
Mice
Male C57BL/6J mice carrying the ApcMin allele were purchased from Jackson Laboratories. Mice were genotyped for the ApcMin allele as previously described.Citation10 Erbb3f/f mice were generated as previously described.Citation5 Male and female Cre-ER mice were purchased from the Jackson Laboratories and crossed to Erbb3f/f mice to generate Erbb3f/+Cre-ER mice. These mice were further backcrossed to Erbb3f/f mice to get the conditionally targeted mice (B3f/fCre-ER). Cre-ER mice were identified using PCR with CreER1 (5′- AAAGTCGCTCTGAGTTGTTAT-3′) and CreER3 (5′-CCTGATCCTGGCAATTTCG-3′), which produces a ∼800 bp PCR product.
EBX and EB3IV immunizations
Three weeks old male and female ApcMin mice were immunized i.p. with 100 μg of EBX peptide, EB3IV or KLH in 100 μl of a 50/50 mixture of antigen and CFA (Sigma). Follow up immunizations were performed at 5 and 9 weeks of age with 100 μg of antigen in 100 μL of a 50/50 mixture of antigen and IFA (Sigma). I.P. immunization was chosen for its ease and reproducibility compare with i.v. injection. The Institutional Animal Care and Use Committee at North Carolina State University approved all experiments involving mice.
Macroadenoma counts
At 100 d of age the mice were euthanized through CO2 asphyxiation, and the entire intestinal tract including the colon was removed. The intestines were splayed open onto bibulous paper, fecal material was gently flushed away with PBS and the intestines were sprayed with 70% ethanol before putting in 10% neutral-buffered formalin overnight. Polyps were counted and their location was recorded using a dissecting scope (Leica).
ELISA and ELISPOT analysis
Sera were collected from mice prior to immunization, every 2 weeks after immunization and at sacrifice. Standard ELISA conditions were followed for the detection of anti-EBX, anti-EB3IV, and anti-KLH antibodies. Titer was determined as the lowest dilution of antibody that resulted in an absorbance reading at least three times above binding to BSA. Interferon gamma (IFNγ) producing T cells were quantified using the Ready-Set-Go ELISPOT kit according to the manufacturer's instructions (eBioscience). Briefly, single cell suspensions of splenocytes were prepared by passing spleens from 100-d-old mice through a 100 μm strainer and seeded at a density of 1×106/well in a 96-well plate with 10 μg/mL antigen. For the depletion experiments, cells were treated with Mouse CD4+ or CD8+ Dynabeads (Life Technologies) prior to seeding.
ERBB3 activation assay
Human SW620 colorectal cancer cells were maintained in RPMI1640 supplemented with 10% FBS at 37 °C in a humidified atmosphere of 5% CO2. Two to 3 d prior to the assay, 1 × 104 cells were passed to 48-well plates and grown to ∼70% confluency. The cells were serum starved for 24 h prior to initiation of the experiment. Sera were diluted 1:10 in culture media and added to the cells for 30 min at 37 °C. The solution was aspirated off and culture media containing 12.5 ng/mL neuregulin was added to the cells for 10 min at 37 °C. The cells were lysed in RIPA buffer (50 mM Tris–HCl [pH 7.0], 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% TritonX-100, 500 μM AEBSF, 150 nM Aprotinin, 1 μM E-64, 1 μM leupeptin). The concentration of cleared lysate was determined by the BCA Assay (Bio-Rad) and 1 μg of protein lysate was separated on a 4% stacking/10% separating acrylamide gel before transferring to nitrocellulose (Bio-Rad). The membrane was blocked with 5% non-fat dry milk for 1 h before incubation with rabbit anti-phospho ERBB3 antibody (Abcam) for 2 h. After incubation with primary antibody the membrane was washed thrice with TBS-T before incubation with goat anti-rabbit and anti-β-actin antibodies conjugated to HRP for 1 h at room temperature. The membrane was washed thrice with TBS-T, and reactive bands were visualized with the HRP substrate kit (Bio-Rad).
ERBB3 inactivation with tamoxifen
To aid in dissolution of tamoxifen, corn oil was heated to 42 °C for 30 min before addition of 50 mg tamoxifen. The conical tube was kept wrapped in aluminum foil to minimize exposure to light. The solution was vigorously vortexed and rocked at 37 °C for 3 h. Tamoxifen solutions were prepared fresh for each round of injections and stored at 4 °C between injections. Two-months-old B3f/fCre-ER mice were injected i.p. with 1 mg tamoxifen daily for five consecutive days. The mice were then aged for 6 weeks before beginning the next round of injections. A total of three rounds of injections were performed with the mice being aged an additional 6 weeks after the final round of injections. Mice injected with only corn oil served as the negative controls. Mice were euthanized by CO2 asphyxiation and blood and tissues were collected. DNA was extracted from all tissue using the Maxwell 16 System (Promega). PCR analysis was utilized to check for Cre-mediated excision with mErbb3-S1 (5′-TCCAGCGTGGAAAAGTTCAC-3′) and mErbb3-AS1 (5′-AAGCCTTCTCTATGGAAAG- TG-3′) primers. The primers give a 488-bp product for the Erbb3f allele and a 193-bp product for the Erbb3fd Cre-deleted allele.
Histology, immunohistology, and TUNEL assay
Tissue pieces were fixed in 10% neutral buffered formalin before being embedded in paraffin and sectioned (7 μm). Every 50 μm sections were stained with H&E. For immunohistology, each section was boiled for 20 min in citrate buffer, pH 6.0 to perform antigen-retrieval. Sections were treated with 0.3% hydrogen peroxide in PBS for 30 min before being washed in PBS and blocked in PBS containing 3% BSA and 0.1% Triton X-100, and then incubated with anti-ERBB3 primary antibody (Abcam) and HRP-conjugated goat anti-rabbit secondary antibody. DAB peroxidase substrate kit (Millipore) was used following the manufacturer's protocol to detect antigen-antibody complexes. Apoptotic cells were quantified using the ApopTag fluorescein in situ apoptosis detection kit (Chemicon) according to the manufacturer's protocol. The paraffin embedded tissues were deparaffinized and then incubated in proteinase K for 15 min at room temperature. The sections were then incubated with terminal deoxynucleotidyl transferase enzyme for one hour at 37 °C, washed thrice in PBS and incubated in a humidified chamber with anti-digoxigenin conjugate (Peroxidase) for 30 min at room temperature. The tissues were then incubated with DAB for 1 to 2 min and counterstained with DAPI. The scoring of apoptotic cells was done at 200 × magnification.
Statistical analyses
The nonparametric Wilcoxon rank-sum test was used to analyze polyp counts. Differences in ELISA reactivity's were analyzed by Student's t-test. Statistical analysis was performed using StatMac Plus. One-sided p values are given with values less than 0.05 considered statistically significant.
Results and discussion
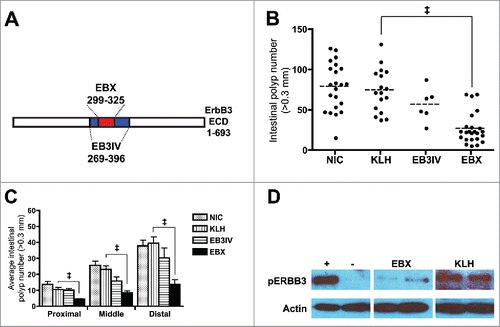
We designed a synthetic peptide (EBX) corresponding to extracellular amino acid residues 299–323 of ERBB3 and attached it to keyhole limpet hemocyanin (KLH) for immunization. In addition, we produced a larger, recombinant portion of ERBB3 that encompassed EBX (EB3IV; residues 269–396; ). Following immunization at weaning, 2 weeks, and 6 weeks of age we found a significant decrease in the number of polyps in EBX-immunized mice compare with KLH-immunized mice or non-immunized controls at 100 d of age () with the decrease in polyps evident throughout the entire intestinal tract (). Surprisingly, this effect was not seen with EB3IV immunization even though EB3IV contains the EBX sequence, is highly immunogenic, and initiates a robust antibody response. We then tested whether serum from EBX-immunized mice inhibited ERBB3 activation in a cell-based ERBB3 activation assay. We utilized a human colon cancer cell line (SW620) as the sequence of EBX is almost identical between the human and mouse forms of ERBB3 (23/25 residues). Results showed that sera from EBX-immunized mice, but not from KLH-immunized mice, inhibited the activity of ERBB3, signifying that an immunological response to EBX could be causing the decrease in polyp formation ().
Figure 1. EBX peptide inhibits polyp formation. (A) Schematic showing relation of EBX peptide (ERBB3 residues 299–325) and EB3IV recombinant protein (ERBB3 residues 269–396) to ERBB3 extracellular domain (ECD). (B) Polyp burden in 100 day old ApcMin mice after no treatment (NIC) or treatment with carrier protein (KLH), recombinant protein (EB3IV) or peptide (EBX). Mice were treated at 3, 5, and 9 weeks following birth. The dashed line represents the mean for each group. (C) Mean polyp burden for different portions of the intestinal tract from ApcMin mice treated with KLH, EB3IV, EBX, or control. (D) Erbb3 activation assay in SW620 cells with (+) or without (−) 12.5 nM neuregulin in the presence of sera from two separate EBX-or KLH-immunized mice. All error bars show means ± SEM Statistics: ‡p < 0.001; **p < 0.01.
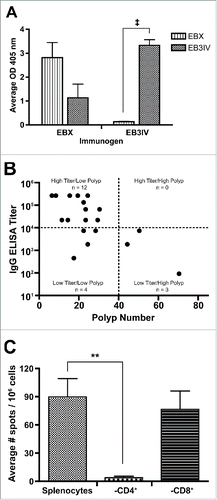
When we interrogated the immunological response further, we found that although mice immunized with EBX develop both EBX- and EB3IV-specific antibodies, mice immunized with EB3IV did not develop antibodies that react with EBX (). These results show the importance of specifically targeting the region surrounding the previously identified critical D313 residue as only those mice with antibodies targeting the EBX portion of ERBB3 had a reduced polyp burden. While no direct correlation was found between anti-EBX titer and polyp number, we found a majority of the EBX-immunized mice could be grouped into a “high titer/low polyp” group (12/19, 63%) with a much smaller number in the “low titer/high polyp” (3/19, 16%) or “low titer/low polyp” (4/19, 21%) groups (). In addition to examining antibody titers, we also tested activation of T cells to peptide through an IFNγ ELISPOT and found a robust IFNγ response to peptide from cultured splenocytes. We determined the specificity of this response by performing the ELISPOT with CD4+ and CD8+ T-cell depletion and showed the response to peptide was composed almost exclusively of CD4+ T cells ().
Figure 2. Evaluation of anti-EBX and anti-EB3IV response. (A) Average ELISA readings for sera tested for reactivity to EBX and EB3IV from mice immunized with either antigen. (B) Comparison of EBX-specific IgG titer and total polyp burden in EBX-immunized mice. (C) ELISPOT analysis of splenocytes performed with total splenocytes as well as after depletion of CD4+ or CD8+ T cells. All error bars show means ± SEM. Statistics: ‡p < 0.001; **p < 0.01.
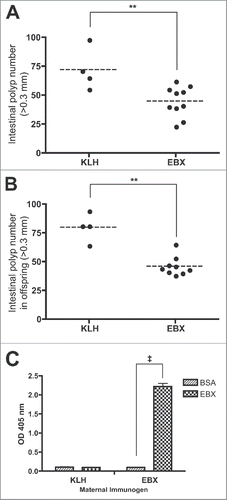
We next investigated whether the timing of immunization might affect polyp burden in ApcMin mice. First, we aged ApcMin mice for 4 weeks after weaning, when the majority of polyps have formed, before immunization. Upon examination of the intestines at 100 d of age, we found that even with a delay in treatment there was still a significant decrease in the total polyp burden in EBX treated mice compare with KLH treated mice, albeit with a greater average number of polyps than those mice who began treatment at weaning (). Next, we examined whether it would be possible to treat mice prior to weaning through immunization of females and the transfer of anti-EBX antibodies to offspring, as mice are capable of passing IgG antibodies through lactation.Citation11 We immunized female C57BL/6J mice with EBX peptide according to the standard immunization protocol and, on the day of the final immunization, mated them to ApcMin males. No difference in litter size was noted when comparing females immunized with EBX-KLH or KLH alone. The untreated ApcMin offspring were then aged to 100 d before examining polyp formation in the intestinal tract. We found that offspring from EBX-immunized females developed significantly fewer polyps than mice from KLH-immunized females (), even though the mice were never treated directly with antigen. To help determine the cause of the effect, we examined milk from immunized females and discovered a high anti-EBX IgG titer present 10 d after the litters were born (). Whether the antibodies had an effect in utero was not examined.
Figure 3. Timing of EBX treatment. (A) Polyp burden in 100-d-old ApcMin mice following treatment with carrier protein (KLH) or peptide (EBX) at 7, 9, and 13 weeks following birth. (B) Polyp burden in 100-d-old ApcMin mice born to females (n = 3) immunized with either carrier protein (KLH) or peptide (EBX). (C) ELISA analysis of IgG in milk isolated 10 d following birth from females immunized with either carrier protein (KLH) or peptide (EBX). Statistics: ‡p < 0.001; **p < 0.01.
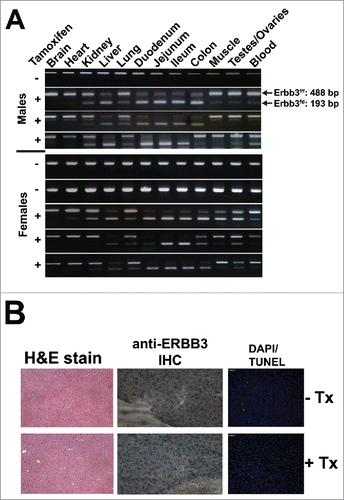
Vaccination as a means to inhibit cancer formation will necessitate a theoretical life-long immune response against the target antigen. We chose to target ERBB3 due to its importance in colon cancer development, and although ERBB3 is known to be vital for development,Citation12 its role in fully differentiated tissues is less clear. We tested the effects of systemic ERBB3 ablation using an inducible Cre-loxP strategy by crossing mice with loxP sites flanking exon 2 of Erbb3 (Erbb3f/f) with transgenic mice harboring Cre-ERT2 under control of the ubiquitous ROSA26 locus to generate Erbb3f/fCre-ERT2 mice. The Cre-ERT2 system is activated upon administration of tamoxifen, thus, enabling the ablation of ERBB3 in adult mice.Citation13 After three cycles of tamoxifen treatment, we examined the level of Erbb3 inactivation through PCR analysis and found a high level of inactivation in tissues where ERBB3 is highly expressed (). We performed further microscopic evaluation using liver as a model tissue and found no detectable ERBB3, no morphological changes, and no increase in apoptosis (). This is in agreement with our previous research on ablation of ERBB3 in intestinal tissue that found a similar lack of deleterious side effects due to the absence of ERBB3.Citation5
Figure 4. ERBB3 deletion in adult B3f/fCre-ER mice following treatment with tamoxifen at 2 mo of age followed by two additional cycles 6 weeks apart. (A) PCR analysis showing ablation of ERBB3 in different tissues from B3f/fCre-ER mice treated with corn oil (−) or tamoxifen diluted in corn oil (+). (B) Staining of liver tissue from B3f/fCre-ER mice treated with corn oil (− Tx) or tamoxifen diluted in corn oil (+ Tx).
The results of these experiments show that an active immunotherapy targeting ERBB3 causes a decrease in total polyp burden in a mouse model of hereditary colon cancer. Although previous studies have examined the use of conditionally expressed proteins as antigens for a prophylactic cancer vaccine,Citation14 this study utilized a broadly expressed protein that is vital for proper fetal development, but which appears to have few if any required activities in mature tissues. There are few reports of ERBB3 being overexpressed in tumors, but we previously demonstrated the requirement for ERBB3 activity during polyp growth.Citation5 Thus, it is not necessary for a target antigen to be overexpressed, only that the cancer is dependent upon its expression and signaling for development. ERBB3 is not conditionally expressed in any tissue; however, the necessary activity of ERBB3 is conditionally expressed in that it is a requirement in developing tissues, as ERBB3 ablation is embryonic lethal in mice,Citation12 but may not be required in adult tissue. To test for conditional activity, we ablated ERBB3 in adult mice and noticed no overt toxicity, suggesting that although the expression of ERBB3 is not conditional, ERBB3 activity may be unnecessary in fully developed tissues.
We targeted an aspartic acid residue in domain II of ERBB37 that has previously been shown to be vital for proper ERBB3 signaling.Citation8 Interestingly, although our 25-mer peptide was able to inhibit the development of polyps in ApcMin mice, a larger recombinant portion of ERBB3 overlapping the same domain did not have any significant effect on polyp number even though it resulted in a robust antibody response. Results such as these have been seen before, such as the case of a peptide of ERBB2/Neu that resulted in an anticancer response but a larger recombinant protein encompassing that region that did not offer the same results.Citation15 Specificity of immune response is a known requirement for attaining the desired effect, as antibodies raised to different portions of the same protein can either inactivate or enhance activity of the target.Citation16 The anti-polyp effect seen with administration of EBX validates our approach to targeting the previously identified essential aspartic acid residue and reinforces the notion that it is not just the target antigen that is important, but identifying the specific region of the target that can determine the effectiveness of a therapy.
These data suggest that a monoclonal antibody targeting the EBX region of ERBB3 may be clinically beneficial. There are a number of anti-ERBB3 antibodies currently in clinical development,Citation17 with one designed to target domain II that is currently in Phase 1 clinical testing.Citation18 Based upon our data, anti-ERBB3 antibodies directed against domain II may exhibit unique properties compared with other anti-ERBB3 antibodies currently under development. In any event, the data presented here suggest that prophylactic vaccination represents a novel approach to preventing or reducing the occurrence of colorectal cancer in at-risk patient populations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
D.J.B. and D.W.T. developed the methodology, designed, and performed experiments, analyzed the data, and wrote the manuscript. A.S. performed ERBB3 Cre-ER experiments.
Acknowledgments
We thank Dr. Ryan Gordon for his help with the ERBB3 activation assay.
Funding
This work was supported by National Institutes of Health grant CA079869.
References
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D et al. Identification of FAP locus genes from chromosome 5q21. Science 1991; 253:661-5; PMID:1651562; http://dx.doi.org/10.1126/science.1651562
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spiro L, Robertson M et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991; 66:589-600; PMID:1651174; http://dx.doi.org/10.1016/0092-8674(81)90021-0
- Gryfe R. Inherited colorectal cancer syndromes. Clin Colon Rectal Surg 2009; 22:198-208; PMID:21037810; http://dx.doi.org/10.1055/s-0029-1242459
- Moser AR, Luongo C, Gould KA, McNeley MK, Shoemaker AR, Dove WF. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur J Cancer 1995; 31:1061-4; http://dx.doi.org/10.1016/0959-8049(95)00181-H; PMID:7576992
- Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K, Pannicia C, Kurie JM, Threadgill DW. Tumor-specific apoptosis caused by deletion of the ERBB3 psuedo-kinase in mouse intestinal epithelium. J Clin Invest 2009; 119:2702-13; PMID:19690388; http://dx.doi.org/10.1172/JCI36435
- Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther 2008; 15:413-48; PMID:18404164; http://dx.doi.org/10.1038/cgt.2008.15
- Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 2002; 297:1330-3; PMID:12154198; http://dx.doi.org/10.1126/science.1074611
- Buac K, Watkins-Chow DE, Loftus SK, Larson DM, Incao A, Gibney G, Pavan WJ. A Sox10 expression screen identified an amino acid essential for Erbb3 function. PLoS Genet 2008; 4:e1000177; PMID:18773073; http://dx.doi.org/10.1371/journal.pgen.1000177
- Liu S, Toblas R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clin Biochem 1997; 30:455-63; PMID:9316739; http://dx.doi.org/10.1016/S0009-9120(97)00049-0
- Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA 2002; 99:1521-6; PMID:11818567; http://dx.doi.org/10.1073/pnas.032678499
- Van de Perre P. Transfer of antibody via mother's milk. Vaccine 2003; 21:3374-6; PMID:12850343; http://dx.doi.org/10.1016/S0264-410X(03)00336-0
- Erickson SL, O'Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development 1997; 124:4999-5011; PMID:9362461.
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem-cell loss. Cell Stem Cell 2007; 1:113-26; PMID:18371340; http://dx.doi.org/10.1016/j.stem.2007.03.002
- Jaini R, Kesaraju P, Johnson JM, Altunas CZ, Jane-wit D, Tuohy VK. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med 2010; 16:799-803; PMID:20512124; http://dx.doi.org/10.1038/nm.2161
- Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA. Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol 1996; 156:3151-8; PMID:8617935
- Stancovski I, Hurwitz E, Leitner O, Ullrich A, Yarden Y, Sela M. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci USA 1991; 88:8691-5; PMID:1717984; http://dx.doi.org/10.1073/pnas.88.19.8691
- Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget 2012; 3:744-58; PMID:22889873; http://dx.doi.org/10.18632/oncotarget.550
- Xiao Z, Carrasco RA, Schifferli K, Kinneer K, Tammali R, Chen H, Rothstein R, Wetzel L, Yang C, Chowdhury P, Tsui P, Steiner P, Jallal B, Herbst R, Hollingsworth RE, Tice DA. A Potent HER3 Monoclonal Antibody That Blocks Both Ligand-Dependent and -Independent Activities: Differential Impacts of PTEN Status on Tumor Response. Mol Cancer Ther 2016; 15:689-701; PMID:26880266; http://dx.doi.org/10.1158/1535-7163.MCT-15-0555
