ABSTRACT
IL-21 has reported activity in promoting both Th1 and Th17 immune responses. Its role in sporadic human colorectal cancer is unknown. We aimed to delineate the role of IL-21 in a model of sporadic intestinal carcinogenesis. We found that in APCMIN/+ mice, ablation of IL-21 increased intestinal tumorigenesis. Expression of pro-inflammatory Th17-associated genes, including RORγt and IL-17A, was increased in the intestine in the absence of IL-21, while expression of antitumor Th1-associated genes Tbet, IFNγ, granzyme B, and perforin was decreased. Similarly, the IL-21-deficient APCMIN/+ mouse intestines had fewer infiltrating T cells as well as decreased effector memory T cells, NK cells, and granzyme B-expressing cells. Finally, our data suggest that IL-21 impairs Th17 immune responses as mesenteric lymph nodes from IL-21-deficient mice had increased IL-17A expression, and naive helper T cells from IL-21-deficient mice were more prone to differentiate into IL-17A-secreting cells.
Introduction
Human cancers consist not only of neoplastic cells but also a microenvironment of immune cells and mediators. In colorectal cancer, the concentration of tumor-infiltrating T cells and genes associated with T cells is associated with metastasis and survival. While high expression of molecules associated with the Th1 cytotoxic pathway is associated with improved outcomes, expression of molecules associated with the Th17 pathway is associated with aggressive tumor biology.Citation1
IL-21 is involved in the proliferation of T and B cells as well as the cytotoxic activity of T and NK cells,Citation2,3 IL-21 was initially viewed as a potential anticancer treatment for its enhancement of the Th1 pathwayCitation4,5 without a compensatory increase in regulatory T cells (Tregs).Citation6,7 More recently, however, IL-21 has been reported to be associated with the promotion of Th17 pathway inflammation,Citation8,9 and its inhibition has been associated with improvement in colitis-associated colorectal cancer,Citation10,11 The immune mechanisms in the development of colitis-associated colorectal cancer and sporadic colorectal cancer likely differ. In sporadic colorectal cancer, there is a balance between tumor-promoting inflammation and antitumor immunity, whereas in colitis-associated colorectal cancer, the immune system appears to have a mostly inflammatory and pro-tumorigenic role.Citation12 The goal of this study was to delineate the role of IL-21 in sporadic intestinal carcinogenesis.
Materials and methods
Mice
C57BL/6J (WT), C57BL/6J-ApcMin/J (APCMIN/+) mice (Jackson Laboratories), and B6.129S-Il21tm1Lex/Mmucd (IL-21−/−, IL-21KO) mice (Mutant Mouse Resource and Research Center) were used. IL-21−/−.APCMIN/+ (IL-21KO-APCMIN/+) mice were generated through breeding. Protocols were approved by the VA Boston Healthcare System Institutional Animal Care and Use Committee.
Quantification of polyps
Murine small intestine was flushed and divided into three segments. Polyps were counted and measured at their greatest diameter. Tumor load was calculated by summing the maximum diameter of all polyps.Citation13,14
Preparation of intestinal leukocytes and flow cytometry
Ileum was processed per instructions in the mouse Lamina Propria Dissociation Kit (Miltenyi). Cells were then separated with a 40/80% Percoll gradient (GE Healthcare), blocked with anti-mouse CD16/32 (BD Biosciences), and stained with anti-mouse CD45 (clone 30-F11), CD8+ (53–6.7), CD44 (IM7), CD3 (145–2C11), and CD4+ (GK1.5) (all BD Biosciences) antibodies as well as anti-mouse NK1.1 (PK136; eBioscience) for 20 min. Cells were washed and run on a BD LSRFortessa (BD Biosciences). Data were analyzed with FlowJo v10.0.7.
Immunohistochemistry
Ileum was fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. Tissues were deparaffinized in Histo-Clear (National Diagnostics) and rehydrated in ethanol. Antigen retrieval was performed in pH 6.0 sodium citrate for FoxP3, B220, and granzyme B staining or in pH 8.0 EDTA for CD3 staining using a pressure cooker. Staining with anti-mouse FoxP3 (clone FJK-16, eBioscience), CD3 (CD3–12, Abcam), CD8α (53–6.7, R&D Systems), NKp46 (29A1.4, BioLegend), B220 (RA3–6B2, BD Biosciences), and granzyme B (R&D Systems) was performed as previously described.Citation15
Immunofluorescence
Mouse ileum was fixed in 10% formalin, washed and transferred to 15% sucrose for 1 h and then to 30% sucrose. Tissue was frozen in Optimal Cutting Temperature compound (Sakura Finetek) and cut into 10-μm sections. Slides were incubated at 37°C for 20 min followed by a bath in cold acetone and PBS with Triton-X for 30 min. Slides were blocked with 10% donkey serum (S30, EMD Millipore) for 30 min and briefly placed into cold sodium glycine. Tissue sections were stained with primary antibodies diluted in 10% donkey serum and incubated overnight. Slides were rinsed with PBS with 0.1% Tween-20. Donkey anti-goat IgG conjugated with Alexa Fluor 488 or Alexa Fluor 647 (Abcam) was applied for 2 h at room temperature. Slides were then rinsed with PBS with 0.1% Tween-20. DAPI (Invitrogen) was applied for 5 min.
RNA extraction and qRT-PCR
Tissue was frozen at −80°C. Cell lysates were prepared with QIAzol Lysis Reagent (Qiagen) followed by homogenization (Tissue Lyser II, Qiagen). The RNeasy Plus Universal Kit (Qiagen) was used for RNA extraction. cDNA was synthesized using the SuperScript III First Strand Synthesis System (Life Technologies) with oligo-dT as the first strand primer. Reactions were run on the ABI PRISM 7900HT Sequence Detection System (Life Technologies). Relative expression was normalized to GAPDH using the 2−ΔΔCt method. See Supplementary Table for primers.
Mouse Th17 polarization
Th17 polarization was performed as previously described.Citation16 In short, naïve splenic CD4+ cells extracted from 6–8-week-old mice with the CD4+CD62L+ T Cell Isolation Kit II (Miltenyi) were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies along with soluble anti-IL-4 and anti-IFNγ antibodies as well as IL-6, TGF-β, IL-23, and IL-1β for 6 d. Cells were then re-stimulated with PMA/ionomycin for 5 h. Brefeldin A was added after 1 h. Fc receptors were blocked and cells labeled with antibodies to surface markers. Cells were then fixed and permeabilized and stained with anti-mouse IL-17A and IFNγ. Flow cytometry was performed as above.
Statistical methods
All comparisons were made using the Student's t-test with p < 0.05 considered significant.
Results and discussion
Intestinal polyposis is increased in IL-21KO-APCMIN/+ mice
The APCMIN/+ mouse model is commonly employed to study intestinal carcinogenesis. APCMIN/+ mice develop small intestine tumors due to a germline mutation in Apc. Mutated APC is an important driver of human sporadic colon cancer. While previous studies have examined the role of IL-21 in inflammatory models of intestinal carcinogenesis, to our knowledge, the relevance of IL-21 in spontaneous intestinal tumorigenesis is not known.
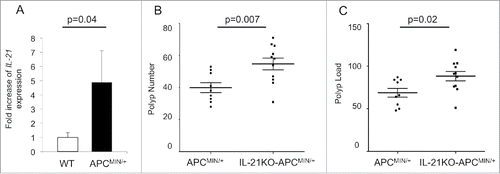
We began by comparing ileal IL-21 levels between APCMIN/+ mice and wild-type (WT) mice. We found that IL-21 expression was increased over 4-fold in polyp-bearing APCMIN/+ ileum of 15-week-old mice (). We also noted an approximately 16-fold increase in IL-21 protein in the polyp-bearing jejunum of APCMIN/+ mice at 15 weeks (Fig. S1A). As IL-21 has been implicated in both tumor-promoting and antitumor responses, we crossed APCMIN/+ mice with IL-21KO mice to further delineate the role of IL-21 in intestinal tumorigenesis. IL-21KO-APCMIN/+ mice had an increased number of polyps as well as an increased tumor load at 15 weeks ( and ), indicating that IL-21 may function in suppressing tumor growth. An increase in adenoma burden could also be seen in IL-21KO-APCMIN/+ mice at 8 weeks of age, although the overall polyp burden was much lower (Fig. S2). Similarly, 8-week old APCMIN/+ mice appeared to have increased expression of ileal IL-21 compared with WT animals, although the magnitude of the difference was much lower than that seen for the 15-week-old animals and was not statistically significant, possibly due to the lower adenoma load (Fig. S1B). The decreased adenoma burden seen in IL-21-deficient animals is in line with previous work showing that colon cancers engineered to overexpress IL-21 exhibit decreased growth and were even found to regress in subcutaneous tumor challenge models.Citation4,17 Our results, however, contradict previous studies examining the relationship between IL-21 and intestinal tumorigenesis induced by dextran sulfate sodium (DSS) and azoxymethane (AOM).Citation10,11,18 DSS and AOM are commonly used to mimic colitis-associated colon cancer. Notably, one study used AOM alone in APCMIN/+ mice.Citation18 The differences between the APCMIN/+ and the AOM-APCMIN/+ model could conceivably be related to the fact that in the AOM-APCMIN/+ model, colon tumors were examined, whereas in the APCMIN/+ model, the preponderance of the adenomas develop in the small intestine. More likely, however, the differences are related to administration of AOM, which has been shown to induce colitis-associated crypt changes in the colon.Citation19 It should also be noted that our experiments as well as the AOM-APCMIN/+ and AOM-DSS studies primarily relied on mice with a germline IL-21 deficiency. Given the well-characterized influences of IL-21 on several immune cell populations, the effect of the absence of IL-21 during development of the immune system cannot be assessed despite the fact that IL-21KO mice do not have an overt immunologic phenotype under homeostatic conditions. One study utilizing the AOM-DSS model obtained similar results regardless of whether the authors used IL-21KO mice or WT mice treated with an IL-21-blocking antibody.Citation11
Figure 1. IL-21 is overexpressed in polyp-bearing APCMIN/+ mouse intestine and deficiency of IL-21 leads to increased polyposis. (A) qRT-PCR of IL-21A expression in 15-week-old polyp-bearing APCMIN/+ mouse ileum compared with that of wild-type (WT) mice (n = 4 per group). Polyp number (B) and polyp load (C) in the small intestine of 15-week-old mice (n = 9–11 per group). The middle line represents the mean and the error bars represent SEM.
Th17-associated genes are upregulated and Th1-associated genes downregulated in IL-21KO-APCMIN/+ ileum
As IL-21 has been implicated in multiple T helper pathways, most importantly the Th1 and Th17 pathways, we next sought to assess differences in T helper differentiation associated with IL-21 deficiency as a possible cause of the increased polyposis. We examined T helper cell gene expression profiles in the intestine of IL-21KO-APCMIN/+ and APCMIN/+ mice using qRT-PCR ( and Fig. S3). IL-21KO-APCMIN/+ ileum was found to have decreased expression of Th1-related genes such as the transcription factor Tbet as well as the cytokine IFNγ and the effector cytotoxic molecules granzyme B and perforin. Previous studies have demonstrated a role for IL-21 in the release of granzyme B and perforin by T and NK cells.Citation20 Similarly, our data are in line with another study where in the AOM-APCMIN/+ model of intestinal polyposis, mice lacking IL-21 were found to have a reduction in the fraction of both perforin and granzyme B producing NK and CD8+ cells among tumor-infiltrating lymphocytes.Citation18
Figure 2. Deficiency of IL-21 in APCMIN/+ mice intestine favors increased expression of Th17-associated transcripts and a decrease in Th1-associated transcripts. Gene expression was determined by qRT-PCR on 15-week-old mouse ileum (n = 4 per group).
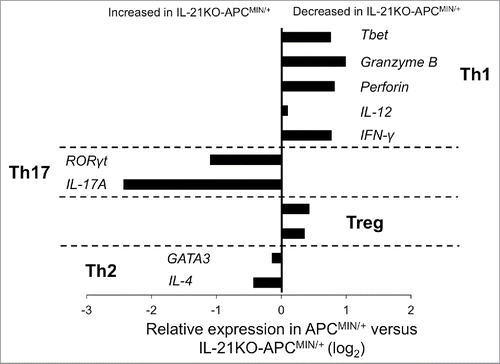
In contrast to the downregulation of the Th1-associated gene profile in IL-21 deficient mice, we found increased expression of pro-inflammatory Th17 genes with a greater than 2-fold increase in expression of the prototypical Th17 transcription factor RORγt and a greater than 5-fold increase in expression of the eponymous cytokine IL-17A at 15 weeks. The role of IL-17A in promoting carcinogenesis in the APCMIN/+ model has previously been demonstrated,Citation16,21 and genes associated with Th17 polarization have been correlated with decreased survival in human colorectal cancer.Citation1 Nevertheless, IL-21 has been shown to enhance Th17 polarization in vitro when combined with other cytokines. Thus, it was surprising that deficiency of IL-21 was associated with decreased Th17 polarization in this model. Our results are also surprising given that reduced expression of IL-17A was seen in tumors from IL-21-deficient AOM-APCMIN/+ mice.Citation18 As discussed further above, we believe the differences in the APCMIN/+ and the AOM-APCMIN/+ models are likely related to the use of AOM, which has been shown to induce inflammatory changes.
Expression of genes associated with the Th2 and Treg pathways was essentially unchanged between the groups.
IL-21KO-APCMIN/+ mouse intestine has fewer infiltrating T cells, effector memory T cells, B cells, NK cells, and granzyme B-expressing cells with an increased CD4+/CD8+ ratio
Given the decreased expression of Th1-associated genes in the polyp-bearing intestine of IL-21-deficient APCMIN/+mice, we next explored for alterations in lymphocyte number and phenotype relevant to a Th1 bias. We first evaluated the number of T cells and B cells in polyp-bearing ileum using immunohistochemistry. T cells were decreased over a third in IL-21KO-APCMIN/+ compared with APCMIN/+ mice. The number of B cells was also decreased in IL-21KO-APCMIN/+ mice, although the total number of infiltrating B cells was low in both cases. There was no alteration in Treg number ().
Figure 3. Deficiency of IL-21 in APCMIN/+ mice results in decreased infiltrating T cells, effector memory T cells, B cells, NK cells, and granzyme B-expressing cells with an increased CD4+/CD8+ ratio. (A) Density of CD3+ (T cells), FoxP3+ (Tregs), and B220+ cells (B cells) in sections of paraffin-embedded ileum as determined by immunohistochemistry (n = 6 per group).*p < 0.05. Flow cytometry was performed on leukocytes isolated from ileum and gated on live, single, CD45+ cells. Representative density plots of CD4+ and CD8+ cells among CD3+ cells (B) and a histogram of the of CD4+/CD8+ cell ratio (C) are shown (n = 4 per group). The percentage of CD8+CD44+ (D) or NK1.1+ (E) leukocytes is compared (n = 4 per group). (F) Granzyme b-expressing cells were determined by immunohistochemical staining of ileum. (G) The density of granzyme b-expressing cells is shown (n = 6 per group). (H) The percentage of granzyme b-expressing cells that also express CD3 (T cells), CD8 (CD8+ T cells), B220 (B cells), and NKp46 (NK cells) was determined in APCMIN/+ mouse intestine by immunofluorescent staining of ileum (n = 3). All mice were 15-week old.
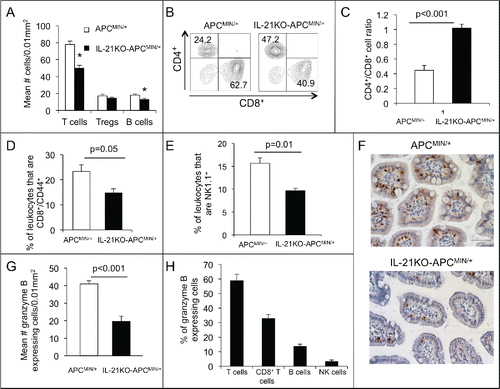
We next analyzed single-cell preparations of polyp-bearing ileum by flow cytometry to further characterize T cell populations in relation to IL-21 deficiency. We first explored the CD4+/CD8+ T cell ratio. A lower ratio represents a higher number of cytotoxic CD8+ T cells in relation to helper CD4+ T cells and has been correlated with a good prognosis in colorectal cancer.Citation22 Consistent with the hypothesis that IL-21 promotes a Th1 bias and favors antitumor immunity, in this model, the CD4+/CD8+ T cell ratio was more than two times higher in the IL-21KO-APCMIN/+ mice intestines as compared with that of APCMIN/+ mice ( and ). We also looked at the effector memory CD8+ T cell population in the adenoma-bearing ileum as a marker of a robust antitumor immune response. The percentage of effector memory (i.e., CD44+) CD8+ T cells was almost twice as high in APCMIN/+ as compared with IL-21KO-APCMIN/+ intestine (). As NK cells, like cytotoxic CD8+ T cells, can function as an important effector of Th1 responses and antitumor immunity, we similarly examined the effect of IL-21 deficiency on the number of infiltrating NK cells. NK cells were decreased in the polyp-bearing ileum of IL-21KO-APCMIN/+ compared with APCMIN/+ mice in a similar proportion to total T cells ().
The cytotoxic function of both CD8+ T cells and NK cells is mediated by the enzyme granzyme B. IL-21 has been shown to upregulate granzyme B in these cells. Thus, we investigated whether the number of granzyme B-expressing cells in the polyp-bearing ileum of APCMIN/+ mice was altered by IL-21 deficiency. Compared to the APCMIN/+ mouse intestine, that of the IL-21KO-APCMIN/+ mouse had approximately half as many cells staining for granzyme B on immunohistochemistry ( and ). To investigate which cells are responsible for any granzyme B-mediated cytotoxicity in APCMIN/+ mice, we doubly stained tissue sections for granzyme B and cell type markers by immunofluorescence. We found that over half of the granzyme B-staining cells also co-stained for CD3, indicating that T cells were the major producers of granzyme B with a smaller contribution from B cells and NK cells ().
Our finding of alterations in the profile of infiltrating leukocytes indicating that IL-21 promotes a cytotoxic antitumor response in colorectal cancer is in line with prior studies and further explains our gene expression results. The role of IL-21 in the expansion of T cells and NK cells has been previously demonstrated in vitro.Citation2,6 Mice overexpressing IL-21 have increased populations of effector memory T cells,Citation5,6,20 and IL-21 can enhance the cytotoxic activity of T and NK cells via release of granzyme B and perforin.Citation20 In experiments where IL-21 was overexpressed in subcutaneous tumor challenge models, IL-21 was found to increase infiltration of T and NK cellsCitation4 and to enhance lysis of tumor cells by splenocytes.Citation17 Furthermore, increased expression of IL-21 has been correlated with dramatically improved disease-free survival in human colorectal cancer.Citation23
Deficiency of IL-21 increases the tendency to polarize to the Th17 phenotype
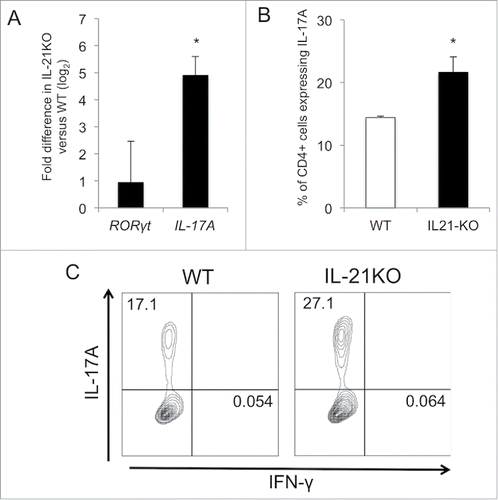
While IL-21 is well known to enhance cytotoxic lymphocyte responses and antitumor immunity, multiple studies, using predominantly in vitro experiments, have indicated that IL-21 not only promotes, but also sustains the Th17 pathway.Citation24-26 As our data appeared to conflict with these earlier reports, we went on to further investigate the potential relationship between loss of IL-21 and Th17 differentiation in vivo. We first aimed to assess for differences in baseline RORγt and IL-17A expression related to IL-21 deficiency in mesenteric lymph nodes draining the intestine using qRT-PCR. We found that IL-17A expression was increased by 30-fold in IL-21KO mice. RORγt expression also appeared to be increased 2-fold, although the difference was not statistically significant (). We next sought to determine whether the absence of IL-21 influences the ability of naïve CD4+ T cells to polarize to the Th17 phenotype. Naïve T cells from spleens of WT and IL-21KO mice were subjected to Th17 polarizing conditions and expression of IL-17A was measured. Naïve CD4+ T cells from IL-21KO mice proved to be more easily polarized to the Th17 pathway with approximately 1.5 times the number of IL-17A-producing cells after polarization compared with those from WT mice ( and ).
Figure 4. Deficiency of IL-21 results in an increase in theTh17 phenotype. (A) Comparative expression of RORγt and IL-17A in mesenteric lymph nodes from IL-21KO as compared with that from wild-type (WT) mice as determined by qRT-PCR (n = 5 per group) *p < 0.05. (B) Percent of naïve splenic CD4+ cells expressing IL-17A after polarization toward the Th17 phenotype as determined by flow cytometry with intracellular staining. (C) Representative density plots of IL-17A- and IFNγ-expressing cells after polarization are shown. Gating was performed on live, single cells.
These results may help to better understand the role of IL-21 in helper T cell differentiation, particularly with regard to Th17 responses. The exact role of IL-21 in the Th17 pathway has been controversial. Several in vitro studies using human and mouse cells have proposed that IL-21 is involved in amplification of the Th17 differentiation loop.Citation24,27,28 It has even been argued that IL-21 is necessary for the development of Th17 cells.Citation29,30 Subsequent in vitro studies, however, demonstrated that IL-6 with or without TGF-β is sufficient to drive Th17 polarization of naïve CD4+ mouse T cells deficient in IL-21 or its receptor IL-21R. These studies also showed that Th17-mediated mouse experimental autoimmune encephalomyelitis (EAE), is preserved in the absence of IL-21 and IL-21R using in vivo models based on immunization.Citation31,32 In contrast, IL-21R was subsequently found to be required for Th17-mediated EAE in a T cell receptor (TcR) transgenic mouse model.Citation33 Further studies are needed to explore why IL-21 is required to induce a Th17 response in vivo in some models, such as colitis and TcR transgenic EAE, whereas in other models such as immunization-induced EAE, APCMIN/+ intestinal carcinogenesis, or untreated C57/Bl6 mice, Th17 responses are not impaired and may even be enhanced in the absence of IL-21. Emerging evidence indicates that the local environmental context composed of cytokine and cellular elements is critically important in directing the effect of IL-21 on T cell proliferation and differentiation.Citation34
In summary, we are the first to demonstrate that in a mouse model of spontaneous intestinal tumorigenesis, IL-21 impairs tumor growth and is associated with enhancement of T and NK cell proliferation and an infiltrating leukocyte profile suggesting cytolytic activity. In addition, we discovered that deficiency of IL-21 is associated with enhancement of the Th17 axis both in sporadic intestinal tumorigenesis and in healthy mice. Together, these results offer new information on the role of IL-21 in sporadic intestinal carcinogenesis as well as on the role of IL-21 in Th1 and Th17 responses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1261776_s02.docx
Download MS Word (154.5 KB)References
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263-71; PMID:21303976; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408:57-63; PMID:11081504; http://dx.doi.org/10.1038/35040504
- Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A 2000; 97:11439-44; PMID:11016959; http://dx.doi.org/10.1073/pnas.200360997
- Hsieh CL, Hsu SC, Shen CR, Chen MY, Liu SJ, Chong P, Chen HW. Increased expression of IL-21 reduces tumor growth by modulating the status of tumor-infiltrated lymphocytes. Immunobiology 2011; 216:491-6; PMID:20884078; http://dx.doi.org/10.1016/j.imbio.2010.09.002
- Allard EL, Hardy MP, Leignadier J, Marquis M, Rooney J, Lehoux D, Labrecque N. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur J Immunol 2007; 37:3069-77; PMID:17918202; http://dx.doi.org/10.1002/eji.200637017
- Santegoets SJ, Turksma AW, Suhoski MM, Stam AG, Albelda SM, Hooijberg E, Scheper RJ, van den Eertwegh AJ, Gerritsen WR, Powell DJ, Jr. et al. IL-21 promotes the expansion of CD27+ CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells. J Transl Med 2013; 11:37; PMID:23402380; http://dx.doi.org/10.1186/1479-5876-11-37
- Attridge K, Wang CJ, Wardzinski L, Kenefeck R, Chamberlain JL, Manzotti C, Kopf M, Walker LS. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood 2012; 119:4656-64; PMID:22442347; http://dx.doi.org/10.1182/blood-2011-10-388546
- Yu J, He S, Liu P, Hu Y, Wang L, Wang X, Han Y, Zhu X. Interleukin21 promotes the development of ulcerative colitis and regulates the proliferation and secretion of follicular T helper cells in the colitides microenvironment. Mol Med Rep 2015; 11:1049-56; PMID:25371082; http://dx.doi.org/10.3892/mmr.2014.2824
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 2008; 134:1038-48; PMID:18395085; http://dx.doi.org/10.1053/j.gastro.2008.01.041
- Jauch D, Martin M, Schiechl G, Kesselring R, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut 2011; 60:1678-86; PMID:21948944; http://dx.doi.org/10.1136/gutjnl-2011-300612
- Stolfi C, Rizzo A, Franze E, Rotondi A, Fantini MC, Sarra M, Caruso R, Monteleone I, Sileri P, Franceschilli L et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med 2011; 208:2279-90; PMID:21987656; http://dx.doi.org/10.1084/jem.20111106
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010; 138:2101-14 e5; PMID:20420949; http://dx.doi.org/10.1053/j.gastro.2010.01.058
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012; 491:254-8; PMID:23034650; http://dx.doi.org/10.1038/nature11465
- Nandi B, Pai C, Huang Q, Prabhala RH, Munshi NC, Gold JS. CCR6, the sole receptor for the chemokine CCL20, promotes spontaneous intestinal tumorigenesis. PLoS One 2014; 9:e97566; PMID:24866282; http://dx.doi.org/10.1371/journal.pone.0097566
- Gonzalez G, Huang Q, Mashimo H. Characterization of oncocytes in deep esophageal glands. Dis Esophagus 2016; 29:670-80; PMID:26245938; http://dx.doi.org/10.1111/dote.12382
- Shapiro M, Nandi B, Pai C, Samur MK, Pelluru D, Fulciniti M, Prabhala RH, Munshi NC, Gold JS. Deficiency of IL-17A, but not the prototypical Th17 transcription factor RORgammat, decreases murine spontaneous intestinal tumorigenesis. Cancer Immunol Immunother 2016; 65:13-24; PMID:26559812; http://dx.doi.org/10.1007/s00262-015-1769-2
- Ugai S, Shimozato O, Kawamura K, Wang YQ, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther 2003; 10:187-92; PMID:12637939; http://dx.doi.org/10.1038/sj.cgt.7700552
- De Simone V, Ronchetti G, Franze E, Colantoni A, Ortenzi A, Fantini MC, Rizzo A, Sica GS, Sileri P, Rossi P et al. Interleukin-21 sustains inflammatory signals that contribute to sporadic colon tumorigenesis. Oncotarget 2015; 6:9908-23; PMID:25839161; http://dx.doi.org/10.18632/oncotarget.3532
- Venning FA CM, Kissow H. The Carcinogenic Agent Azoxymethane (AOM) enhances early inflammation-induced colon crypt pathology. J Cancer Sci Therapy 2013; 5:377-83; http://dx.doi.org/10.4172/1948-5956.1000229
- Sutherland AP, Joller N, Michaud M, Liu SM, Kuchroo VK, Grusby MJ. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J Immunol 2013; 190:3977-84; PMID:23479229; http://dx.doi.org/10.4049/jimmunol.1201730
- Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A 2010; 107:5540-4; PMID:20212110; http://dx.doi.org/10.1073/pnas.0912675107
- Diederichsen AC, Hjelmborg J, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother 2003; 52:423-8; PMID:12695859; http://dx.doi.org/10.1007/s00262-003-0388-5
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/10.1016/j.immuni.2013.10.003
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448:484-7; PMID:17581588; http://dx.doi.org/10.1038/nature05970
- Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol 2007; 37:3155-63; PMID:17918200; http://dx.doi.org/10.1002/eji.200737766
- Kastirr I, Maglie S, Paroni M, Alfen JS, Nizzoli G, Sugliano E, Crosti MC, Moro M, Steckel B, Steinfelder S et al. IL-21 is a central memory T cell-associated cytokine that inhibits the generation of pathogenic Th1/17 effector cells. J Immunol 2014; 193:3322-31; PMID:25172491; http://dx.doi.org/10.4049/jimmunol.1400775
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 2007; 282:34605-10; PMID:17884812; http://dx.doi.org/10.1074/jbc.M705100200
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8:967-74; PMID:17581537; http://dx.doi.org/10.1038/ni1488
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007; 448:480-3; PMID:17581589; http://dx.doi.org/10.1038/nature05969
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 2008; 454:350-2; PMID:18469800; http://dx.doi.org/10.1038/nature07021
- Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol 2008; 38:1833-8; PMID:18546146; http://dx.doi.org/10.1002/eji.200838511
- Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol 2008; 180:7097-101; PMID:18490706; http://dx.doi.org/10.4049/jimmunol.180.11.7097
- Lee Y, Mitsdoerffer M, Xiao S, Gu G, Sobel RA, Kuchroo VK. IL-21R signaling is critical for induction of spontaneous experimental autoimmune encephalomyelitis. J Clin Invest 2015; 125:4011-20; PMID:26413871; http://dx.doi.org/10.1172/JCI75933
- Tian Y, Zajac AJ. IL-21 and T Cell Differentiation: Consider the Context. Trends Immunol 2016; 37:557-68; PMID:27389961; http://dx.doi.org/10.1016/j.it.2016.06.001
