ABSTRACT
Background and Aims: As the predominant lymphocyte subset in the liver, natural killer (NK) cells have been shown to be highly associated with the outcomes of patients with chronic hepatitis B virus infection (CHB) and hepatocellular carcinoma (HCC). Previously, we reported that NKG2A, a checkpoint candidate, mediates human and murine NK cell dysfunction in CHB. However, NK cell exhaustion and, particularly, the level of NKG2A expression within liver tumors have not been reported.
Methods: In this study, we analyzed NKG2A expression and the related dysfunction of NK cells located in intra- or peritumor regions of liver tissue samples from 207 HCC patients, in addition to analyzing disease outcomes.
Results: The expression of NKG2A in NK cells and the NKG2A ligand, HLA-E, in intratumor HCC tissues was observed to be increased. These NK cells, and particularly CD56dim NK cells, with higher NKG2A expression showed features of functional exhaustion and were associated with a poor prognosis. The increase in NKG2A expression might be induced by IL-10, which was present at a high level in the plasma of HCC patients. Blocking IL-10 could specifically inhibit NKG2A expression in NK cells.
Conclusions: These findings indicate that NKG2A expression is influenced by factors from cancer nests and contributes to NK cell exhaustion, suggesting that NKG2A blockade has the potential to restore immunity against liver tumors by reversing NK cell exhaustion.
Abbreviations
| AFP | = | α fetoprotein |
| ALT | = | alanine aminotransferase |
| CHB | = | chronic hepatitis B virus infection |
| DFS | = | disease free survival |
| HBV | = | hepatitis B virus |
| HC | = | healthy controls |
| HCC | = | hepatocellular carcinoma |
| IOD | = | integrated optical density |
| LC | = | liver cirrhosis |
| OS | = | overall survival |
Introduction
It is commonly accepted that CD8+ T cells play an important role in antitumor immune responses, and exhausted CD8+ T cells contribute to hepatocellular carcinoma (HCC) progression. However, a few studies on NK cells, which are the predominant lymphocyte subset in the liver and the first line of defense against infections and tumors, have deeply explored their specific role in tumor progression in HCC patients.
NK cells are innate lymphoid cells that exhibit natural cytotoxicity and the capacity to secrete cytokines. The proportion of NK cells in liver lymphocytes is over five times as high as that in the peripheral blood or spleen.Citation1 In addition, a significant positive correlation has been reported between the total NK cell density and a good prognosis of patients with liver cancer.Citation2 Accumulating evidence has suggested dysfunction of NK cells in intratumor tissues (IT) of both HCC patients and HCC mouse models.Citation3-5 Defects in cytotoxicity and cytokine secretion have been noted in NK cells from either the peripheral blood or tumor tissues of HCC patients, and this decreased NK cell activity appears to be highly associated with the progression of liver cancer.Citation3,6,7 Taken together, these data suggest that the decreased frequency and functional impairment of NK cells in HCC patients may contribute to a poor prognosis of patients with liver cancer.
NK cells express a series of immune receptors to identify relevant ligands on target cells, which maintain the immune balance between activation and tolerance of NK cells.Citation4,8–10 Importantly, tumor cells can escape immune surveillance by hijacking these receptor–ligand systems to impair immune cell-mediated cytolysis. Overexpression of inhibitory receptors on CD8+ T cells has been verified in various tumor types, and reversal of this inhibition by blocking the inhibitory receptor–ligand axis, such as the PD-1–PD-L1 pathway, in the clinic can successfully restore immunity against tumors, representing the beginning of the so-called immunotherapy age.Citation11 However, insufficient attention has been paid to the role of the inhibitory receptors on NK cells during tumor progression. Among the inhibitory receptors of NK cells, NKG2A has been well studied, but its clinical relevance to HCC has not been reported.
Recently, we have shown increased NKG2A expression in NK cells in both chronic hepatitis B virus (HBV) patients and a mouse model with chronic HBV infection (CHB).Citation12 Decreased cytotoxicity of NK cells, reducing their ability to clear HBV, is associated with increased NKG2A expression. Blocking the interaction between NKG2A and HLA-E (NKG2A ligand) can restore the antiviral activity of NK cells during CHB,Citation12 suggesting that NKG2A expression in patients with HCC could become a predictive factor for prognosis.Citation13 Though previous studies have described the expression of inhibitory receptors primarily on NK cells from the peripheral blood of HCC patients,Citation1,4 there is lack of evidence regarding the expression of these receptors on NK cells directly from tumor nests.
In this study, we demonstrated that NKG2A expression in in situ NK cells predicted the survival of HCC patients in a large cohort (n = 207). Additionally, we found that these NK cells with higher NKG2A expression exhibited characteristics of functional exhaustion, such as lower IFNγ production and weaker cellular cytotoxicity, as well as a shorter overall survival (OS) time. Furthermore, we observed high plasma levels of IL-10, which may strongly induce NKG2A expression in NK cells in HCC patients. Interestingly, this increased expression of NKG2A was predominantly detected in CD56dim NK cells, rather than CD56bright NK cells from intratumor tissue and not in those from peripheral blood. These findings indicate the existence of a negative regulatory mechanism in the exhausted NK cells as a result of the increased expression of the inhibitory receptor NKG2A in cancer nests and have implications for therapeutic intervention in HCC patients.
Patients and methods
Patients
Tissues from 177 HCC patients who had undergone curative resection between 2006 and 2010 and had complete follow-up data (Cohort 1; Tables S1 and S3) were obtained from the Bank of Tumor Resources at Sun Yat-Sen University. The patients were enrolled in the study to analyze OS and disease-free survival (DFS). Fresh tumor tissue samples were obtained from 30 HCC patients during surgery in the Department of Hepatobiliary Surgery of Anhui Provincial Hospital (Cohort 2; Tables S1 and S3). Among these samples, there were 23 paired samples of peritumor liver tissue (collected 2 cm distal to the tumor site) and tumor tissue from the same patients. Only single PT/IT tissues were obtained from the remaining seven HCC patients. All fresh tissues were used for the phenotypic analysis, and most of them were also used for the intracellular cytokine analysis if they had a sufficient amount of cells. Peripheral blood samples from HCC, HBV, and liver cirrhosis (LC) patients and healthy controls (HC) were obtained from the First Affiliated Hospital of Anhui Medical University (Cohort 3; Table S3). Normal liver tissues (N = 17) having distal to liver echinococcosis were obtained from the First Affiliated Hospital of Xinjiang Medical University. Pilot studies were conducted to ensuring sample sizes are large enough to detect the effects. The clinical characteristics of all tissue samples from HCC patients are summarized in Table S1. The details of all patients are provided in Table S3, according to REMARK. Written informed consent was provided in accordance with the Helsinki Declaration. The protocols for all study cohorts were approved by the Ethical Board of the Institutional Review Board of the University of Science and Technology of China.
Flow cytometry
Peripheral leukocytes were isolated via Ficoll-Isopaque (Solarbio, China) gradient centrifugation.Citation14 Liver tissue-infiltrating leukocytes (TIL) were obtained as previously described.Citation15 To digest the specimens, the tissue samples were cut into small pieces and digested in RPMI 1640 (HyClone Laboratories, USA) supplemented with collagenase IV (10 mg/mL, Sigma-Aldrich) and DNase I (33.3 mg/mL, Sigma-Aldrich, United States) at 37 °C for 1–2 h. The peripheral lymphocytes, TIL, and NK cells from the in vitro cultures were stained with fluorochrome-conjugated Abs and then analyzed through flow cytometry.Citation14 Abs against the following proteins were used for staining: CD3 (SK7), CD56 (B159), IFNγ (B27), CD107a (H4A3), Granzyme B (GB11), Perforin (δG9) (BD PharMingen, United States), NKG2A (131411), and NKG2C (134591) (R&D Systems, United States). The stained cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, United States), and the data were analyzed using FlowJo analysis software 7.6.1 (Treestar, United States).
In vitro NK cell culture system and real-time RT-PCR
Purified NK cells were enriched from whole blood via negative selection (NK Cell Isolation Kit, Miltenyi Biotec). The cells were incubated in medium alone or medium with recombinant TGF-β1 (1 ng/mL; PeproTech, United States), IL-10 (1 ng/mL; PeproTech, United States), or IL-10 (10 ng/mL; PeproTech, United States) plus IL-15 (10 ng/mL; PeproTech, United States) and IL-2 (100 U/mL). In another culture model, the NK cells were cultured in the presence of 20% HCC patient plasma or IL-10 (1 ng/mL; PeproTech, United States) with or without an anti-human IL-10 neutralizing Ab (Clone 25209, R&D Systems, United States) or control IgG (BD) for 72 h. Relative quantification of IL-10 mRNA expression using real-time RT-PCR was performed as previously reported.Citation14 Primers for IL-10 were forward 5′-GAG ATG CCT TCA GCA GAG TGA AGA-3′ and reverse 5′-AGG CTT GGC AAC CCA GGT AAC-3′.
Immunohistochemistry
Paraffin sections were dewaxed in xylene and rehydrated with distilled water. Following incubation with antibodies against human HLA-E (bs-1279R, Bioss Antibodies, Boston) or NKG2A (PA5-21949, Thermo Fisher, United States) adjacent sections were stained with the DAB Peroxidase Substrate Kit (SK-4100) (Vector Laboratories, United States). Positive and negative controls were tested before formal staining (see Supplementary data). The number of positive cells was quantified using ImagePro Plus software (Media Cybernetics, United States) as previously described.Citation16
Statistical analysis
Significant differences between groups were determined using Mann–Whitney non-parametric statistical test as described in legend Univariate analyses of the prognostic factors for OS and DFS were performed with the Cox proportional hazards model (SPSS Statistics Software 22.0, IBM, United States). Kaplan–Meier estimates of survival were used to illustrate the survival curves and to obtain estimates of the median rates of OS and DFS.
Results
Increased NKG2A expression in NK cells from the intratumor tissues of HCC patients
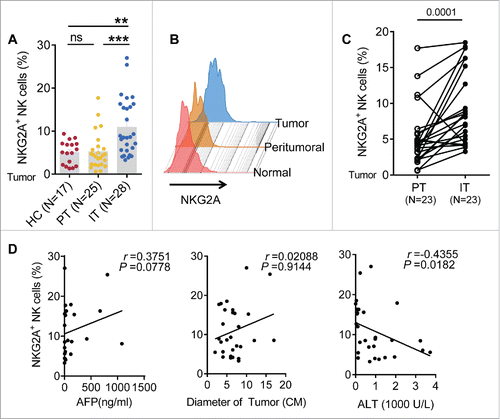
To investigate the expression of NKG2A in intrahepatic NK cells, we analyzed TIL from healthy livers, IT and peritumor tissues (PT). As shown in and , the percentage of NKG2A+ NK cells in the central tumor region was significantly higher than in the PT and healthy livers, which became significantly evident when we compared the paired central tumor tissues and PT of each patient individually (). The absolute number of total NKG2A-expressing NK cells in IT (59.0 ± 108.5 × 103/g) was slightly higher than that in PT (27.7 ± 37.5 × 103/g) regions, but no significant difference has been found (Fig. S2D). Furthermore, it was noted that increased percentages of NKG2A+ NK cells were highly positively associated with a large tumor diameter (), suggesting that the percentage of NKG2A+ NK cells was related to certain clinical characteristics of HCC patients. Conversely, the percentage of NKG2A+ NK cells was negatively associated with alanine aminotransferase (ALT) levels (), indicating the possibility that liver injury was decreased due to high expression of NKG2A. Although there was a high r value (r = 0.3751) between NKG2A expression and high α-fetoprotein (AFP) levels, we did not observe a clear positive linear correlations in (left). We also analyzed the expression of other NK cell receptors (NKG2D, NKG2C, NKp44, NKp46, CD44, LAG3, CD200R, CD158b and Siglec-7) on NK cells from PT and IT tissues when sufficient amount of cells is available. We observed a decreased expression of activation receptors (NKG2D and CD44) and increased expression of inhibitory receptors (Siglec-7, CD200R and LAG3) on NK cells from IT regions compared with NK cells from PT regions, suggesting an immune-suppressed status of intra-hepatic NK cells from tumor nest. But there were no differences in NKp44, NKp46, NKG2C and CD158b expression levels on NK cells between PT and IT regions (Fig. S1A). Interestingly, as previously reported,Citation12 the expression of NKG2A in peripheral NK cells was increased in patients with CHB; however, the expression level in the HC and patients with LC or even HCC was not increased (Fig. S2A and B). Additionally, no significant association was observed between the percentage of circulating NKG2A+ NK cells and clinical parameters such as ALT and AST levels (Fig. S2C).
Figure 1. The frequency of intratumor NKG2A+ NK cells is increased in HCC. (A, B) The percentage of NKG2A-expressing NK cells in healthy livers (N = 17) and IT (N = 28) and PT (N = 25) from HCC patients. The differences in the cumulative data were calculated using a Kruskal–Wallis ANOVA followed by Dunn's multiple comparisons test. (C) NKG2A expression in NK cells from the paired IT and PT from each HCC patient (N = 23, Wilcoxon non-parametric statistical test). (D) The correlation between NKG2A expression in hepatic NK cells from IT and the serum AFP and ALT levels and tumor diameters of HCC patients. Spearman's correlation coefficients are shown.
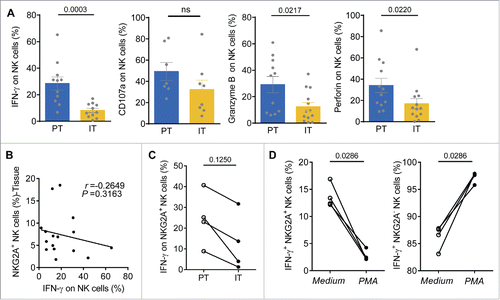
The activation of NK cells is tempered by the combined signals from activating receptors and inhibitory receptors; therefore, we hypothesized that these NKG2A-induced phenotypic changes might be paralleled by functional alterations in NK cells. To evaluate the potential functional changes in NK cells from HCC patients, we examined their capacity to produce IFNγ following stimulation with IL-12, as previously described.Citation14 There was a significant reduction of IFNγ, Granzyme B and perforin production in NK cells from IT compared with PT from HCC patients (). However, there was no significant difference in CD107a expression (). A negative correlation between intracellular IFNγ levels and NKG2A expression in NK cells from tissue samples was also noted (). Moreover, the capacity of NKG2A+ NK cells from intratumor regions to produce IFNγ in response to PMA/ionomycin stimulation was also significantly decreased, indicating that the NKG2A+ NK cells in cancer nests were exhausted (). In addition, when NK cells from the blood of HC were activated by PMA/ionomycin for 4 h in vitro, the ability of the NKG2A+ NK cells to secrete IFNγ was, surprisingly, significantly decreased to less than 5%, which was even lower than that observed under stimulation with medium alone (, left). In contrast to the NKG2A+ NK cells, NKG2A− NK cells produced much higher IFNγ levels, of up to 98% (, right).
Figure 2. Functional impairment of intratumor NK cells from HCC patients. (A) TIL from PT (blue) and IT (yellow) were stimulated with IL-12, as described in the Materials and Methods. Intracellular IFNγ, CD107a, Granzyme B and perforin levels were determined using flow cytometry by gating on NK cells (CD3−CD56+) (Mann–Whitney non-parametric statistical test). (B) Analysis of the correlation between IFNγ levels and the percentage of NKG2A-expressing NK cells in the tissue samples. Each dot represents a single region (PT/IT) from an HCC patient (Spearman's correlation test). (C) The levels of IFNγ produced by NKG2A+ NK cells in IT and PT regions in paired HCC patients (Wilcoxon non-parametric statistical test). (D) Blood NK cells from the healthy controls were pre-incubated with medium alone or with PMA/ionomycin for 4 h. Intracellular IFNγ levels in NKG2A+ NK cells (Left) and NKG2A− NK cells (Right) were monitored via flow cytometry.
Higher HLA-E (NKG2A ligand) expression is correlated with a poor prognosis of HCC patients
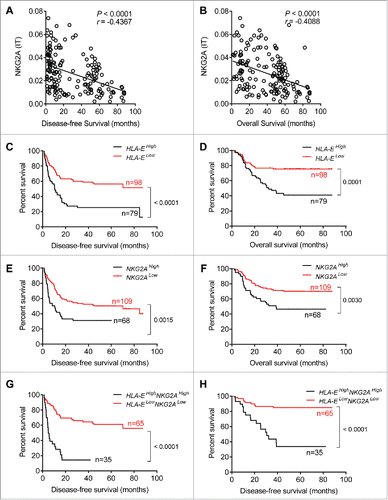
The NKG2A inhibitory signaling pathway requires the interaction of NKG2A with its ligand, HLA-E. After examining tissues from 177 HCC patients, our results showed that either HLA-E or NKG2A expression were significantly increased in IT ( and ), and a positive correlation existed between NKG2A expression and the density of HLA-E in IT (). On the one hand, the statistical analysis showed significant negative correlations of the integrated optical density (IOD)/area of HLA-E in intratumor regions with disease-free survival (DFS) or OS (r = −0.4826 for DFS, r = −0.3676 for OS; p < 0.0001 in all comparisons), but there was no a clear linear correlations observed (Fig. S3B and C). On the other hand, a significant linear negative correlations of IOD/area of NKG2A in IT regions with DFS or OS (r = −0.4367 for DFS, r = −0.4088 for OS; p < 0.0001 in all comparisons) have been observed. To further assess the predictive potential of HLA-E+ or NKG2A+ cells in cancer nests, the patients were divided into two groups using the minimum p-value cut-off values for their densities. The survival curves generated by subgroup showed that lower HLA-expression in intratumor regions was correlated with longer OS and DFS (). Similarly, patients with higher NKG2A expression in IT regions were associated with a shorter OS (p = 0.0030) and DFS (p = 0.0015) (). Further, a comprehensive analysis showed that patients with lower HLA-E and lower NKG2A had significantly longer OS and DFS (both p < 0.0001) (). The Cox regression and time-to-event outcome analyses indicated that TNM staging and tumor thrombus significantly influenced DFS and OS (p < 0.05 for all comparisons in Table S2). However, among these indicators, HLA-E and NKG2A expression in IT more strongly influenced DFS and OS than other clinical parameters (p < 0.0001, HR = 4.72 for DFS; p < 0.0001, HR = 5.61 for OS; Table S2). Taken together, these results suggest that the presence of HLA-E+ and NKG2A+ cells in central tumor regions exhibits predictive value for HCC patients. Additionally, we assessed the expression of the NKG2C receptor, which binds HLA-E shared with NKG2A, and no significant differences were observed in the blood of the CHB, LC and HCC patients or in the PT and IT (Fig. S1A and B).
Figure 3. The density of NKG2A and its ligand are increased in the intratumoral regions from HCC patients. (A) Representative micrographs showing NKG2A+ (up) and HLA-E+ (down) cells in the peritumor tissue (right) and intratumor regions (left) of HCC patients. Original magnifications: 10×, 40×, bar = 100 μm. (B, C) Cumulative data are shown (Mann–Whitney non-parametric statistical tests). (D) A positive correlation was observed between integrated optical density (IOD)/area HLA-E+ cells and NKG2A expression (percentage by FACS) in intratumor NK cells from HCC patients (N = 11). Spearman's correlation coefficients (r) and p values are shown.
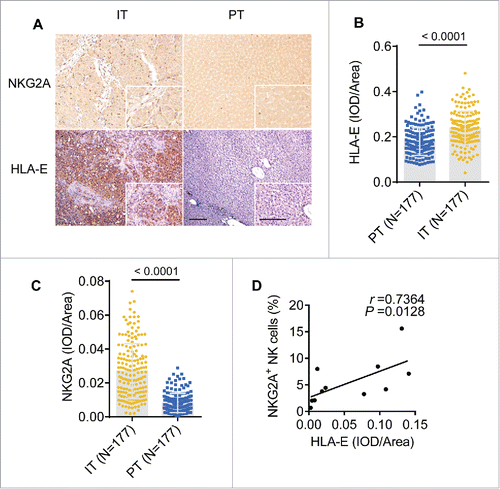
Figure 4. Shorter survival of patients with higher densities of HLA-E+ and NKG2A+ cells within tumors. (A, B) Correlations between DFS or OS and integrated optical density (IOD)/area of NKG2A+ cells in the IT regions of HCC patients (N = 177). Spearman's correlation coefficients (r) and p values are shown. (C–H) Kaplan–Meier survival curves for the duration of DFS (left) and OS (right) in months, according to the HLA-E+ cells density (C, D), NKG2A+ cells density (E, F) or both NKG2A+ and HLA-E+ cells density (G, H) in IT (low densities, red line; high densities, black line) (log-rank test).
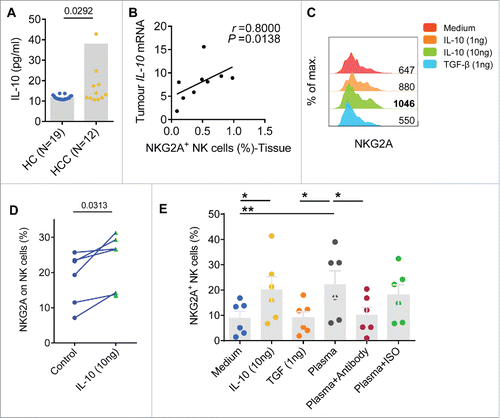
Expression of IL-10 in tumors increases NKG2A expression
Previously, we showed that IL-10 upregulates NKG2A expression in NK cells in CHB patients.Citation12 Here, we found that the plasma concentrations of IL-10 in HCC patients were significantly higher than the IL-10 levels recorded in HC (). In addition, a significant positive linear relationship was identified between IL-10 expression in tumor tissues and the proportion of NKG2A-expressing NK cells in the tumor (). To evaluate the possibility that the altered expression of NKG2A was induced by IL-10, we pre-incubated NK cells from healthy individuals with different concentrations of IL-10 (10 ng/mL and 1 ng/mL) for 72 h. We added TGF-β, which is another cytokine showing highly elevated levels in HCC patients, to the same culture system to test the specific role of IL-10.Citation14 We observed that the percentage and MFI of NKG2A on NK cells were markedly upregulated by the presence of exogenous IL-10 ( and ). Furthermore, after 72 h of co-culture, NKG2A expression was higher on NK cells that had been pre-incubated with the plasma of HCC patients than that on NK cells that were cultured with medium alone or with plasma from the HC. Treatment with an anti-IL-10 antibody partially inhibited NKG2A overexpression in the plasma of HCC patients (), indicating that exogenous IL-10 or IL-10 from HCC patient plasma specifically increased NKG2A expression.
Figure 5. Soluble plasma IL-10 levels are associated with increased NKG2A expression in NK cells. (A) The plasma concentrations of IL-10 were detected in samples taken from healthy controls (N = 19) and HCC patients (N = 12) (Mann–Whitney non-parametric test). (B) Correlation between IL-10 expression and the percentage of NK cells expressing NKG2A in tissue samples from HCC patients (N = 9). Spearman's correlation coefficients are shown. (C) Blood NK cells from the healthy controls were pre-incubated with TGF-β or IL-10. NKG2A expression in NK cells was monitored at 72 h via flow cytometry. The histograms correspond to the NK cells from one representative donor treated with medium alone (red lines), TGF-β (1 ng/mL, blue lines) or IL-10 (1 ng/mL, orange lines; 10 ng/mL, green lines). (D) NKG2A expression on NK cells from each healthy controls pre-incubated with or without IL-10 (10 ng/mL) was monitored at 72 h by flow cytometry (Wilcoxon non-parametric statistical test). (E) NK cells from the healthy controls were cultured with medium alone, TGF-β, IL-10, plasma from HCC patients alone or an anti-IL-10 Ab or isotype control Ab for 3 d. NKG2A expression levels were analyzed via FACS. The differences in the cumulative data were calculated using a Friedman ANOVA followed by Dunn's multiple comparisons test.
NKG2A expression is predominantly increased on CD56dim NK cells compared with CD56bright NK cells
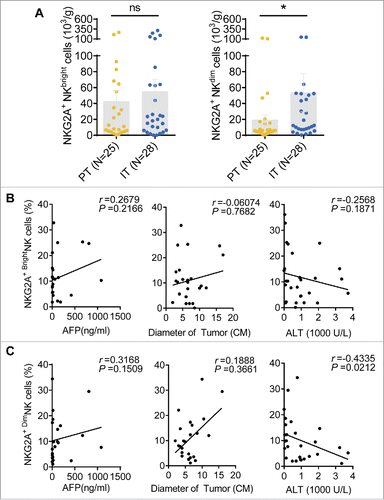
NK cells are divided into CD56bright and CD56dim NK subsets. CD56bright NK cells are mainly responsible for cytokine secretion, whereas CD56dim NK cells are primarily responsible for cytotoxicity. We analyzed the percentage of NKG2A expression in different subsets of NK cells in HCC patients. The gating strategy used to separate the CD56bright NK cells and CD56dim NK cells is shown in . The cumulative data demonstrated that the number of CD56Dim NK cells was markedly increased in IT regions, whereas the number of CD56bright NK cells was decreased; there was no change in HC and PT (). Interestingly, although NKG2A expression was significantly increased on both the total NK cells and CD56dim NK cells in intratumor regions compared with the peritumor region in HCC patients, while NKG2A expression in the CD56bright NK subset did not differ between these regions (), suggesting that increased expression of NKG2A predominantly occurs in intratumor rather, than peritumor CD56dim NK cells, and not in the CD56bright NK subset. Notably, a significant increasing trend was observed when the paired central tumor and peritumor regions of each tissue sample were compared individually (). As summarized in , the NKG2A+ NK cells from different tumor regions were divided into NKG2A+ CD56bright NK cells and NKG2A+ CD56dim NK cells by percentage. We found that the proportion of NKG2A+ CD56dim NK cells gradually increased in intratumor regions compared with PT and healthy livers (); in contrast, the proportion of NKG2A+ CD56bright NK cells decreased (). As expected, we did not observe any significant change in the number of NKG2A+ cells among the circulating CD56bright and CD56dim NK cells from HCC patients compared with the HC (Fig. S3). Interestingly, the absolute number of NKG2A-expressing CD56dim NK cells in IT (53.7 ± 127.4 × 103/g) was significant higher than that in PT (19.3 ± 31.7 × 103/g) regions, which was not been observed for total NK cells (Fig. S2B) or CD56bright NK cells (). Furthermore, we observed a correlation between NKG2A expression and the clinical characteristics of CD56bright NK cells and CD56dim NK cells from tumor tissues. A similar trend was observed that high percentages of NKG2A+ CD56bright NK and NKG2A+ CD56dim NK cells were positively associated with low ALT levels (). Most importantly, only the percentage of NKG2A+ CD56dim NK cells has the most obvious linear relationship with massive tumor size (r = 0.1888), which was not observed for total NK cells (r = 0.02088) or CD56bright NK cells (r = −0.06074) (), suggesting that CD56dim NK cells are associated with tumor features in the cancer nest.
Figure 6. NKG2A expression is mainly increased in intratumor CD56dim NK cells from HCC patients. (A) The gating strategy for TIL used to analyze the CD56bright NK cells and CD56dim NK cells via flow cytometry. (B) The percentages of CD56bright NK cells and CD56dim NK cells from healthy livers (N = 17) and the IT (N = 28) and PT (N = 25) regions of HCC patients (Kruskal–Wallis ANOVA followed by Dunn's multiple comparisons test). (C) Representative NKG2A expression in different liver NK cell subsets from healthy controls (left) and intratumor (right) and PT from HCC patients (middle). (D) Cumulative data on the percentages of NKG2A+ CD56bright NK cells (left) and NKG2A+ CD56dim NK cells (right) from healthy livers (N = 17) and intratumor (N = 28) and PT (N = 25) from HCC patients (Kruskal–Wallis ANOVA followed by Dunn's multiple comparisons test). (E) The percentages of NKG2A+ CD56bright NK cells (left) and NKG2A+ CD56dim NK cells (right) in paired central tumor and PT from each HCC patient. (F) The proportion of NKG2A+ CD56bright NK cells (yellow) and NKG2A+ CD56dim NK cells (blue) among the total NKG2A+ NK cells from intratumor regions (right), PT (middle) and healthy livers (left).
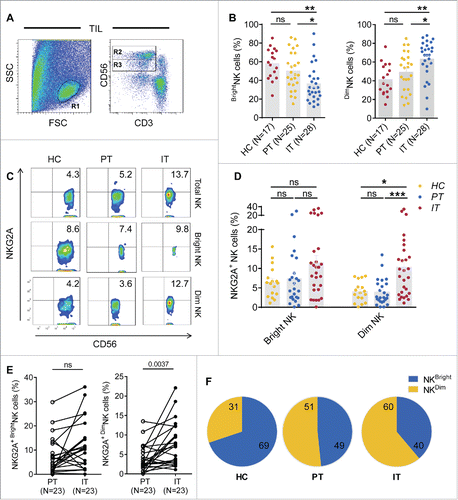
Figure 7. Correlations between the percentages of different NKG2A+ NK cell subsets and the clinical characteristics of patients with HCC. (A) The absolute number of NKG2A+CD56bright NK cells (left) or NKG2A+CD56dim NK cells (right) in per gram IT tissues (N = 28) and PT tissues (N = 25) from HCC patients. Cumulative data are shown (Mann–Whitney non-parametric test). Correlations between NKG2A expressions in CD56bright NK cells (B) or CD56dim NK cells (C) and AFP and ALT levels and tumor diameters. Each dot represents a single patient. Spearman's correlation coefficients are shown.
Discussion
Accumulating data suggest dysfunction of NK cells in the cancer nest in HCC patients and mouse models of liver cancer. However, the underlying mechanism is not fully understood. In the present study, we determined the expression of NKG2A in NK cells that were freshly isolated from the surgical tissues of HCC patients and analyzed its association with clinical parameters. Our results indicated that NKG2A expression was significantly increased in NK cells from IT compared with healthy liver tissues and PT. This increased number of NKG2A+ NK cells display decreased intracellular IFNγ production. Importantly, the expression of the NKG2A and NKG2A ligand (HLA-E) was strongly associated with a shorter survival time of HCC patients, implying that the NK cells in the IT of the patients were exhausted. Furthermore, we found that increased IL-10 levels in HCC patients could upregulate NKG2A expression, and anti-IL-10 antibodies could restore normal NKG2A expression levels, indicating a possible intervention approach.
To our knowledge, this is the first study to show increased NKG2A expression in the NK cells of HCC patients. Previous studies have demonstrated that the upregulation of NKG2A expression in NK cells from various solid tumors allows the tumor cells to escape immunological surveillance. For instance, in patients with lung cancer, it has been shown that significantly upregulated NKG2A expression in NK cells in the tumor microenvironment facilitates metastasis.Citation17 Immunohistochemical staining revealed an increased numbers of NKG2A+ infiltrating lymphoid cells in colorectal carcinoma patients. Furthermore, these high absolute numbers of NKG2A+ TIL are highly correlated with increased levels of the specific NKG2A ligand HLA-E.Citation18 Similar phenotypic changes and relationships with poor prognosis have been reported in breast cancer patients.Citation19 In addition to the increase in NKG2A expression detected on NK cells, increased NKG2A expression has been observed on CD8+ T cells, which downregulates their cytotoxicity.Citation20 Gooden et al. reported a high frequency of NKG2A expression and high HLA-E expression in the IT of ovarian cancer patients. In contrast to the characteristics observed in the liver, ovarian tumor tissue exhibits a very small number of infiltrating NK cells. Moreover, increased NKG2A expression is mainly observed in intraepithelial CD8+ T lymphocytes in patients with ovarian cancer.Citation21 Similar findings have been reported in patients with cervical carcinoma. Sheu et al. showed that cervical cancer cells expressed abundant IL-15 and TGF-β, which promoted NKG2A expression in CD8+ T cells in a kinetic co-culture assay.Citation22 An anti-NKG2A checkpoint inhibitor called monalizumab/IPH2201 is currently under clinical phase I/II trial of head and neck cancer (ClinicalTrials.gov: NCT02331875) and ovarian cancer (ClinicalTrials.gov: NCT02459301).Citation23,24 Ruggeri et al. recently observed that adoptive transfer of NKG2A+ NK cells was hard to treat human primary leukemia or Epstein–Barr virus cell lines bearing immune-deficient mice (NSG); however, pre-treatment with monalizumab can rescued these NSG mice from disease progression, suggesting that anti-NKG2A checkpoint antibody is also efficient as a therapeutic approach to malignant hematologic diseases.Citation25 In agreement with the previously reported data from other tumor types, our data emphasize the relevance of elevated NKG2A expression in NK cells, and NKG2A may serve as an indicator of survival and a therapeutic target in immunotherapy escape strategies for tumor cells.
Increasing experimental evidence has shown a reduced percentage and antitumor function of NK cells in HCC patients. However, due to the limited supply of fresh liver tissues, the vast majority of these studies focused on circulating NK cells in the peripheral blood of HCC patients, and only a few papers have reported decreased infiltration and functional impairment of NK cells in IT compared with peritumor and non-tumor tissues from patients with advanced stages of HCC.Citation1,3-6,26 These pervious results were consistent with our findings. However, the available research data on NK cell dysfunction are mainly focused on the impairment of activation of NK cell receptors, such as NKG2D, NKp30, CD69 and CD244.Citation5-7 There is very little direct evidence showing changes in the expression of inhibitory receptors on hepatic NK cells from patients with HCC.Citation1 As a high-incidence population that could develop liver cancer in the future, patients with persistent HBV or HCV infections have been shown by both our group and other investigators to display significantly increased expression of inhibitory receptors, such as NKG2A and Tim-3, compared with HC.Citation12,27,28 Based on these studies, we show for the first time that NKG2A expression in intratumor CD56dim NK cells plays a critical role in the immune escape of HCC from NK cell attack.
Upregulated HLA-E expression has been observed in HCC tissues compared with adjacent non-tumor liver tissues and normal livers from HC.Citation29 The importance of HLA-E expression in the clinical outcomes of tumor patients has been addressed in only a few studies.Citation30 It seems that the prognostic performance of HLA-E depends on the balance between the activating and inhibitory functions of HLA-E, which may be utterly different in diverse tumor types. An association between high HLA-E expression and a poor prognosis has been reported in colorectal carcinoma, ovarian cancer and breast carcinoma.Citation21,31-34 Interestingly, Benevolo et al. reported that a favorable outcome was associated with high expression of HLA-E in patients with colorectal carcinoma.Citation18 Hence, the predictive utility for colorectal cancer still requires additional work due to these paradoxical results.Citation32-34 A positive correlation between HLA-E expression and survival time has been observed in patients with glioblastoma.Citation35 In this study, we found that high NKG2A expression was associated with high HLA-E expression in IT. There were no differences in NKG2C expression in NK cells from intratumor, peritumor and normal liver tissues, indicating that HLA-E mainly exerts an inhibitory function in HCC patients by interacting with the inhibitory receptor NKG2A. IL-10 plays an important role in maintaining normal NKG2A expression in the healthy liver.Citation36 Data related to HBV infections reported in previous studies indicate that regulatory CD4+CD25+ T cells secrete a significantly higher amount of IL-10 in the livers of chronic HBV patients than in HC.Citation37,38 In addition to regulatory T cells, myeloid-derived suppressor cells might be another source of IL-10 in HCC patients.Citation39 A similar mechanism could occur in HCC patients, and further research is still required to identify the local source of the increased IL-10 levels.
It is commonly recognized that CD56brightCD16− NK cells are mainly responsible for cytokine production, whereas CD56dimCD16+ NK cells are mostly cytolytic. However, some studies have revealed CD56dim NK cells to be a major source of cytokine production.Citation40-42 In the present study, we identified a significant reduction in the percentage of CD56bright NK cells in IT (). Furthermore, we found that the increase in both percentage and absolute number of NKG2A was primarily occurred in CD56dim NK cells. These data support the notion that hepatic CD56dim NK cells may undergo functional suppression in the tumor microenvironment, resulting in NK cell exhaustion and tumor escape. Therapeutic strategies aimed at inducing NK cell activation and reversing NK cell dysfunction have displayed promising effects in metastatic melanoma, kidney cancer, lung cancer and hematological malignancies.Citation1 Our results revealed that anti-IL-10 antibodies could restore the normal expression of NKG2A (). Similar mechanisms have been reported in patients with chronic HBV or HCV infections.Citation12,13 Taken together, our results imply that NKG2A functions as an important checkpoint inhibitor with potential value related to re-establishing the antitumor response mediated by NK cells and even CD8+ T lymphocytes. Accordingly, it will be useful to further identify the antitumor effects of blocking the binding between NKG2A and HLA-E in vivo.
In summary, we observed increased NKG2A expression in NK cells from the IT of HCC patients. This upregulation of NKG2A was associated with upregulation of its ligand, HLA-E, and NK cell exhaustion. Reduced HLA-E expression in intratumor regions was correlated with longer OS and DFS in HCC patients, suggesting that NKG2A blockade has the potential to restore immunity against a tumor by reversing NK cell exhaustion. In addition, we observed that high plasma levels of IL-10 in HCC patients can increase NKG2A expression in NK cells, and blocking the effects of IL-10 may inhibit NKG2A expression in NK cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
ZGT and CS designed the study. CS, JYW, JXS, and HYS performed the experiments and analyses. CS and ZGT wrote the manuscript. ZGT, HMW, WHX, and RS supervised the study and critically reviewed the manuscript. CS, JX, QH, MH, HW, CSZ, and MJZ were involved in the collection of clinical samples.
KONI_A_1264562_supplementary_data.zip
Download Zip (3.4 MB)Funding
This work was supported by the Ministry of Science & Technology of China (# 2013CB944901) and National Science Foundation China (#81302599, #91442112, #31670908, #31390433 and #81472646).
References
- Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin 2015; 36:1191-9; PMID:26073325; http://dx.doi.org/10.1038/aps.2015.41
- Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61:427-38; PMID:21930732; http://dx.doi.org/10.1136/gutjnl-2011-300509
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129:428-37; PMID:18824414; http://dx.doi.org/10.1016/j.clim.2008.08.012
- Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol 2015; 12:292-302; PMID:25308752; http://dx.doi.org/10.1038/cmi.2014.91
- Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57:1107-16; PMID:23225218; http://dx.doi.org/10.1002/hep.26192
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799-807; PMID:19551844; http://dx.doi.org/10.1002/hep.23054
- Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43:1013-20; PMID:16168521; http://dx.doi.org/10.1016/j.jhep.2005.05.026
- Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol 2013; 34:182-91; PMID:23414611; http://dx.doi.org/10.1016/j.it.2013.01.003
- Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol Immunol 2016; 13:328-36; PMID:26639736; http://dx.doi.org/10.1038/cmi.2015.96
- Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell Mol Immunol 2016; 13:628-39; PMID:26277898; http://dx.doi.org/10.1038/cmi.2015.42
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/10.1038/nature10673
- Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, Sun R, Tian Z. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology 2013; 144:392-401; PMID:23103614; http://dx.doi.org/10.1053/j.gastro.2012.10.039
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol 2004; 173:6072-81; PMID:15528343; http://dx.doi.org/10.4049/jimmunol.173.10.6072
- Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 2012; 8:e1002594; PMID:22438812; http://dx.doi.org/10.1371/journal.ppat.1002594
- Kuang DM, Xiao X, Zhao Q, Chen MM, Li XF, Liu RX, Wei Y, Ouyang FZ, Chen DP, Wu Y et al. B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets. J Clin Invest 2014; 124:4657-67; PMID:25244097; http://dx.doi.org/10.1172/JCI74381
- Sun C, Xu J, Song JX, Liu CQ, Wang JY, Weng CC, Sun H, Wei H, Xiao W, Sun R et al. The predictive value of centre tumour CD8(+) T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget 2015; 6:35602-15; PMID:26415232
- Jin S, Deng Y, Hao JW, Li Y, Liu B, Yu Y, Shi FD, Zhou QH. NK cell phenotypic modulation in lung cancer environment. PLoS One 2014; 9:e109976; PMID:25299645; http://dx.doi.org/10.1371/journal.pone.0109976
- Benevolo M, Mottolese M, Tremante E, Rollo F, Diodoro MG, Ercolani C, Sperduti I, Lo Monaco E, Cosimelli M, Giacomini P. High expression of HLA-E in colorectal carcinoma is associated with a favorable prognosis. J Transl Med 2011; 9:184; PMID:22032294; http://dx.doi.org/10.1186/1479-5876-9-184
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/10.1172/JCI45816
- Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol 2002; 3:189-95; PMID:11812997; http://dx.doi.org/10.1038/ni757
- Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, Nijman H, van Hall T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8(+) T lymphocytes. Proc Natl Acad Sci USA 2011; 108:10656-61; PMID:21670276; http://dx.doi.org/10.1073/pnas.1100354108
- Sheu BC, Chiou SH, Lin HH, Chow SN, Huang SC, Ho HN, Hsu SM. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8+ T lymphocytes in human cervical carcinoma. Cancer Res 2005; 65:2921-9; PMID:15805295; http://dx.doi.org/10.1158/0008-5472.CAN-04-2108
- Carotta S, Targeting NK Cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol 2016; 7:152; PMID:27148271; http://dx.doi.org/10.3389/fimmu.2016.00152
- Chester C, Fritsch K, Kohrt HE. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol 2015; 6:601; PMID:26697006; http://dx.doi.org/10.3389/fimmu.2015.00601
- Ruggeri L, Urbani E, Andre P, Mancusi A, Tosti A, Topini F, Bléry M, Animobono L, Romagné F, Wagtmann N et al. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica 2016; 101:626-33; PMID:26721894; http://dx.doi.org/10.3324/haematol.2015.135301
- Guo CL, Yang HC, Yang XH, Cheng W, Dong TX, Zhu WJ, Xu Z, Zhao L. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev 2012; 13:5909-13; PMID:23317279; http://dx.doi.org/10.7314/APJCP.2012.13.11.5909
- Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 2010; 52:322-9; PMID:20133006; http://dx.doi.org/10.1016/j.jhep.2009.12.005
- Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol 2005; 79:12365-74; PMID:16160163; http://dx.doi.org/10.1128/JVI.79.19.12365-12374.2005
- Cheung PF, Yip CW, Wong NC, Fong DY, Ng LW, Wan AM, Wong CK, Cheung TT, Ng IO, Poon RT et al. Granulin-epithelin precursor renders hepatocellular carcinoma cells resistant to natural killer cytotoxicity. Cancer Immunol Res 2014; 2:1209-19; PMID:25315249; http://dx.doi.org/10.1158/2326-6066.CIR-14-0096
- Wieten L, Mahaweni NM, Voorter CE, Bos GM, Tilanus MG. Clinical and immunological significance of HLA-E in stem cell transplantation and cancer. Tissue Antigens 2014; 84:523-35; PMID:25413103; http://dx.doi.org/10.1111/tan.12478
- de Kruijf EM, Sajet A, van Nes JG, Natanov R, Putter H, Smit VT, Liefers GJ, van den Elsen PJ, van de Velde CJ, Kuppen PJ. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol 2010; 185:7452-9; PMID:21057081; http://dx.doi.org/10.4049/jimmunol.1002629
- Levy EM, Bianchini M, Von Euw EM, Barrio MM, Bravo AI, Furman D, Domenichini E, Macagno C, Pinsky V, Zucchini C et al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol 2008; 32:633-41; PMID:18292941; http://dx.doi.org/10.3892/ijo.32.3.633
- Bossard C, Bezieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F, Mosnier JF. HLA-E/beta2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int J Cancer 2012; 131:855-63; PMID:21953582; http://dx.doi.org/10.1002/ijc.26453
- Zeestraten EC, Reimers MS, Saadatmand S, Goossens-Beumer IJ, Dekker JW, Liefers GJ, van den Elsen PJ, van de Velde CJ, Kuppen PJ. Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer 2014; 110:459-68; PMID:24196788; http://dx.doi.org/10.1038/bjc.2013.696
- Kren L, Slaby O, Muckova K, Lzicarova E, Sova M, Vybihal V, Svoboda T, Fadrus P, Lakomy R, Vanhara P et al. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: an unexpected prognostic significance? Neuropathology 2011; 31:129-34; PMID:20667016; http://dx.doi.org/10.1111/j.1440-1789.2010.01149.x
- Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, Ellis G, Khanna P, Dusheiko G et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog 2010; 6:e1001227; PMID:21187913; http://dx.doi.org/10.1371/journal.ppat.1001227
- Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology 2008; 123:57-65; PMID:17764450; http://dx.doi.org/10.1111/j.1365-2567.2007.02691.x
- Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol 2006; 177:739-47; http://dx.doi.org/10.4049/jimmunol.177.1.739
- Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol 2011; 46:156-64; PMID:20822377; http://dx.doi.org/10.3109/00365521.2010.516450
- Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood 2010; 116:1299-307; PMID:20505160; http://dx.doi.org/10.1182/blood-2009-11-253286
- Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010; 115:2167-76; PMID:19965656; http://dx.doi.org/10.1182/blood-2009-08-238469
- Moretta L. Dissecting CD56dim human NK cells. Blood 2010; 116:3689-91; PMID:21071612; http://dx.doi.org/10.1182/blood-2010-09-303057
