ABSTRACT
NK cells have shown promise in therapy of hematological cancers, in particular against acute myeloid leukemia. In contrast, the more NK cell-resistant acute lymphoblastic leukemia (ALL) is difficult to treat with NK-cell-based therapies, and we hypothesized that pre-activation of NK cells could overcome this resistance. We show in pediatric and adult patients with T-cell ALL (T-ALL) perturbed NK cell effector functions at diagnosis. Using an in vivo rat model for T-ALL, Roser leukemia (RL), suppressed NK cell effector functions were observed. NK cells from T-ALL patients had reduced expression of the activating receptors NKp46 and DNAM-1, but not NKG2D. In contrast to T-ALL patients, NKG2D but not NKp46 was downregulated on NK cells during rat RL. Decreased frequencies of terminally differentiated NKG2A+CD57−CD56dim NK cells in human T-ALL was paralleled in the rat by reduced frequencies of bone marrow NK cells expressing the maturation marker CD11b, possibly indicating impairment of differentiation during leukemia. RL was highly resistant to autologous NK cells, but this resistance was overcome upon pre-activation of NK cells with IL-12, IL-15, and IL-18, with concomitant upregulation of activation markers and activating receptors. Importantly, adoptive transfers of IL-12, IL-15, and IL-18 pre-activated NK cells significantly slowed progression of RL in vivo. The data thus shows that T-ALL blasts normally resistant to NK cells may be targeted by cytokine pre-activated autologous NK cells, and this approach could have potential implications for immunotherapeutic protocols using NK cells to more efficiently target leukemia.
Introduction
NK cells are increasingly attractive for immunotherapies toward cancers.Citation1-3 NK cell effector functions are induced when signals from one or more activating receptors override inhibition through inhibitory receptors binding to MHC class I.Citation4 Ligands for activating NK cell receptors, such as NKG2D, DNAX Accessory Molecule-1 (DNAM-1), and the natural cytotoxicity receptors (NCRs) NKp46 and NKp30 are variably upregulated on malignant cells, rendering them susceptible to NK cells.Citation5 NK cells have been successfully used in the treatment of haematological cancers. In particular, haploidentical KIR-mismatched bone marrow transplantations,Citation6 or infusions of haploidentical KIR-ligand mismatched NK cells promoted eradication of leukemic blasts in acute myeloid leukemia (AML) patients.Citation7 In contrast, there has been limited success in similar treatments of adult acute lymphoblastic leukemia (ALL) patients, while some success with pediatric patients is reported.Citation8 ALL blasts are generally resistant to NK cells, as they less frequently express ligands for activating NK cell receptors, in combination with normal MHC class I expression levels.Citation9,10
Reduced surface levels of DNAM-1, NKp46, NKp30, and/or NKG2D by NK cells are frequently observed in AML and variably in ALL patients.Citation9,11-14 Low surface expression of activating receptors at diagnosis is correlated with low NK cell activity, poor outcome, and increased risk of relapse.Citation15-19 Reduced NK cell activity is also reported in patients with other haematological disorders, such as chronic myeloid leukemia (CML) and myelodysplastic syndrome (MDS).Citation20,21 Reduced NKG2D expression is mediated by both TGF-β as well as soluble NKG2D ligands released from blasts,Citation22-24 but whether NKG2D is directly involved in targeting leukemic blasts is controversial.Citation25 In contrast, reduced expression of DNAM-1 has been directly correlated with inefficient NK cell-mediated killing of malignant cells.Citation26-28
Pre-activation of NK cells with cytokines before infusion has been attempted to enhance tumor clearance. While use of the classical NK-cell activating cytokine IL-2 alone has shown limited success,Citation29 combinatorial pre-activation of NK cells with IL-12, IL-15, and IL-18 has been shown to result in significant tumor regression in a mouse model of lymphoma,Citation30 suppression of graft-versus-host disease in the mouse,Citation31 and, importantly, in a clinical trial of relapsed or refractory AML.Citation32 Short-term pre-activation of human or mouse NK cells with IL-12, IL-15, and IL-18 has been shown to result in generation of NK cells with superior effector functions as compared with activation with IL-2 or IL-15 alone.Citation33 Re-stimulation of these cytokine-activated NK cells leads to enhanced functional activity; hence, NK cells differentiated after IL-12, IL-15, and IL-18 pre-activation was termed cytokine-induced memory-like cells.Citation33–36 Their potential utility in immunotherapy is thus obvious.
T-cell ALL (T-ALL) results from a malignancy of T-cell progenitors, representing 15% of pediatric and 25% of adult acute leukemia cases. Significant improvement in intensive risk-adapted chemotherapy protocols have yielded more than 80% 5-y overall survival for pediatric T-ALL patients. Compared to B-cell-derived acute leukemia (B-ALL), both pediatric and adult T-ALL patients remain at increased risk for induction failure and relapse.Citation37,38 Most studies investigating NK cell recognition of ALL have focused on B-ALL, while studies on NK cells and T-ALL are fragmentary. Here, we have addressed the interplay between NK cells and T-ALL, by phenotypic and functional characterization of NK cells in T-ALL patients at diagnosis, and complemented with a rat model for T-ALL, Roser leukemia (RL), to study NK-cell mediated targeting of T-ALL. RL is a radiation-induced leukemia from the PVG rat strain, with similar pathology to human T-ALL.Citation39 RL primarily infiltrates the spleen upon i.v. injection, but affects several organs at late stages such as the bone marrow, liver, lungs, thymus, testis, and meninges.
We show suppressed NK cell functions during acute T-ALL disease, both in human and in the rat, and show in the rat model that suppressed NK cell functions are extrinsic to the blasts, indicative of suppression mediated by other factors or cells in the environment. Pre-activation of autologous NK cells with IL-15, IL-12, and IL-18 sensitized NK cells to the otherwise highly resistant RL blasts, and upon adoptive transfer in vivo, pre-activated NK cells resulted in significant reductions in RL load. This implies that pre-activation of NK cells is of potential immunotherapeutic use against otherwise resistant T-ALL.
Results
Deficient NK cell functions and downregulated expression of DNAM-1 and NKp46 in T-ALL patients
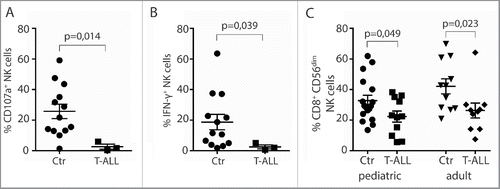
NK cell effector functions were tested in PBMC obtained at diagnosis from three pediatric T-ALL patients recruited in the period 2014–2016 at Oslo University Hospital, with PBMC from age-matched healthy children as controls. PBMC from another 10 pediatric and 9 adult T-ALL patients recruited at time of diagnosis in the period 1982–1997 were obtained from the Royal Victoria Infirmary in Newcastle, UK. NK cell numbers in the latter samples were too low for functional analysis. Low degranulation, as assessed by surface deposition of the granule marker CD107a, and low IFNγ production in response to the NK cell sensitive tumor target K562 was observed in CD3−CD56dim NK cells from pediatric T-ALL patients ( and ). We also observed a reduction in the proportion of CD56dim NK cells expressing CD8+ in both adult and pediatric patients (). CD8+ NK cells are suggested to represent more functionally active NK cells in terms of both cytotoxicity and cytokine production.Citation40,41
Figure 1. Diminished NK cell effector functions in T-ALL patients. PBMC from patients or healthy controls were co-incubated with K562 target cells in the presence of anti-CD107a mAb for 6 h. NK cells were gated as CD14/CD19−CD56dimCD3− cells, and assessed for (A) degranulation by measuring percentage CD107a+ NK cells, or (B) percent intracellular IFNγ expression (n = 3 T-ALL, n = 13 controls, ± SEM). Statistical significance was calculated using the non-parametrical Mann–Whitney test. (C) Frequencies of CD8+ CD3−CD56dim peripheral blood NK cells from healthy children or adult controls compared with pediatric or adult T-ALL patients. Data are presented as percentages ± SEM. Statistical significance was calculated using the non-parametrical Mann–Whitney test.
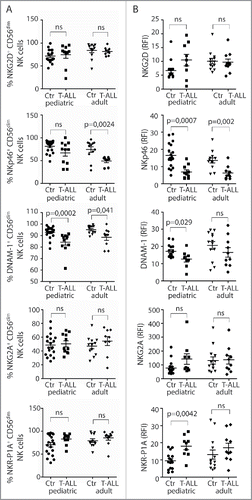
Next, expression of activating and inhibitory NK cell receptors by CD3−CD56dim NK cells in pediatric and adult T-ALL patients was compared with healthy children or adults. Reduced frequencies of NKp46+ NK cells were observed in the pediatric group, and of DNAM-1+ NK cells in both patient groups compared with healthy controls (). NKp46 expression levels were reduced in both adult and pediatric patients, while DNAM-1 expression levels were reduced in the pediatric patient group only (). Frequencies and expression levels of NKG2D in NK cells were similar between patients and controls ( and ). NK cells expressing the inhibitory receptors NKG2A and NKR-P1A were present at similar frequencies in patients and controls, but NKR-P1A was expressed at higher levels by NK cells from pediatric patients ( and ).
Figure 2. Reduced frequencies of NK cells with activating receptors in T-ALL patients. (A) Frequencies of CD56dimCD3− NK cells expressing NKG2D, NKp46, DNAM-1, NKG2A, and NKR-P1A were analyzed on CD3−CD56dim peripheral blood NK cells from healthy children or adults, and pediatric or adult T-ALL patients. Data are presented as percentages ± SEM. (B) Relative fluorescence index (RFI) of NKG2D, NKp46, DNAM-1, NKG2A, and NKR-P1A were calculated by dividing the median fluorescence intensity of each receptor on CD3−CD56dim peripheral blood NK cells to negatively stained populations with the same antibody. Data are presented as RFI values ± SEM. Statistical significance was calculated using the non-parametrical Mann–Whitney test.
NK cell receptor repertoires are skewed during T-ALL in the rat
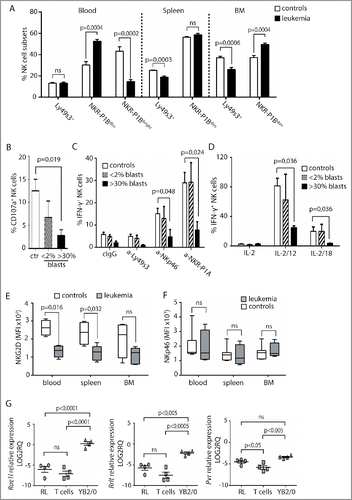
A rat model of T-ALL was used to investigate underlying mechanisms of how T-ALL affects NK cells. The leukemia, RL, was injected i.v. and animals killed when blasts reached >30% of PBMC. At this point, the spleen was grossly enlarged. The numbers of NK cells was comparable in blood and spleen in RL and healthy rats (Fig. S1A), but a skewing of NK cell subsets was observed during RL. In the rat, three major NK cell subsets are found. Two of the subsets are mature and fully functional, and express either Ly49s3 or the inhibitory NKR-P1B receptor,Citation42 while a third terminally differentiated NKR-P1Bbright subset is found in the blood and gut.Citation43 Higher frequencies of NKR-P1Bdim NK cells were found in both blood and bone marrow in rats with RL, accompanied by reduced proportions of NKR-P1Bbright cells in the blood (). The same pattern was observed for total numbers of each subset, though not to statistical significance (Figs. S1B and C). This skewing was not caused by preferential proliferation of NKR-P1Bdim NK cells (Fig. S1D), suggesting differentiation could be affected. In the mouse, CD11b and CD27 distinguish immature and mature NK cells. This is not as clear in the rat,Citation43 but a skewing toward more CD27+ and fewer CD11b+ NKR-P1Bdim cells was observed in the bone marrow (Fig. S1E). Human NK cells differentiate from CD56bright to CD56dim NK cells, and further along a pathway with increasing CD57 and decreasing NKG2A frequencies.Citation44,45 A tendency for lower frequencies of CD57+NKG2A− NK cells was found in pediatric T-ALL patients (p = 0.085), with a similar trend for adult T-ALL patients (Fig. S1F), indicating that, as in the rat, NK cell differentiation could be affected.
Figure 3. Low NK-cell responses and skewed receptor repertoires in rats with RL. (A) Flow cytometric analysis of the distribution of Ly49s3+, NKR-P1Bdim, or NKR-P1Bbright NK cells in blood, spleen, and bone marrow isolated from control rats (n = 9) or rats with RL (n = 10). Data represent the average of six independent experiments ± SEM. (B) Degranulation of NK cells from healthy rats (n = 6), rats with blast load <2% of PBMC (n = 3), or >30% of PBMC (n = 4) in response to YAC-1. NK cells were gated as NKR-P1A+CD3− cells. Data represent the average of three independent experiments ± SEM. Intracellular IFNγ production by NKR-P1A+CD3− NK cells was analyzed by flow cytometry in samples stimulated for 6 h by (C) the indicated plate-bound antibodies or (D) IL-2 alone or in combination with IL-12 or IL-18 using healthy control rats (n = 6), rats with blast load <2% of PBMC (n = 3), rats with blast load >30% of PBMC (n = 3). Values represent the average of three independent experiments ± SEM. MFI analysis of (E) NKG2D or (F) NKp46 expression on NKR-P1A+CD3− NK cells from control rats (n = 4) or rats with RL (n = 5). Values represent the average of three independent experiments. (G) qRT-PCR analysis of RL (n = 4), primary T cells (n = 4), and YB2/0 cells (n = 4). Statistical significance was calculated using the non-parametrical Mann–Whitney test.
Reduced NK cell functions and skewing of NK cell receptor repertoire in rats with T-ALL
Similarly to human patients, NK cells from rats with RL showed low degranulation against an NK cell sensitive tumor target (), and reduced production of IFNγ in response to stimulation of activating receptors NKp46, Ly49s3, or NKR-P1A, or in response to IL-12 or IL-18 in combination with IL-2 ( and ). Reduced NK cell functions were not observed at earlier time points when the blast burden was below 2% ( and ).
In contrast to human patients, NKG2D expression was lower in NK cells from spleen, blood, and bone marrow from rats with RL (), accompanied by reduced frequencies in in the spleen (Fig. S2A). Expression levels and frequencies of NKp46+, Ly49s3+, or NKR-P1A+ NK cells were similar in healthy and RL rats (, Fig. S2B, and data not shown). Lack of antibodies toward rat DNAM-1 prevented testing its surface expression. Dnam1 was similarly expressed in NK cells purified from RL or healthy rats, but this was also observed for Nkg2d, indicating post-transcriptional regulation (Fig. S2C). Further analysis of RL indicated limited expression of the NKG2D ligands Rae1l or Rrlt, while these were expressed by the NK cell sensitive rat tumor cell line YB2/0 (). RL expressed Pvr (CD155), a ligand for DNAM-1, at higher levels than primary T cells ().
Reduced NK cell functionality and downmodulation of NKG2D in the rat was not directly mediated by the RL blasts. In vitro overnight co-cultures of enriched, autologous splenic NK cells from healthy rats with RL did not affect either degranulation toward YAC-1 or IFNγ production in response to IL-12 (Figs. S3A and B), nor NKG2D surface expression upon overnight co-culture of enriched NK cells with RL (Fig. S3C). Moreover, serum concentration of TGF-β was similar in healthy and sick rats (Fig. S3D), and although RL expressed Tgfb at higher levels than primary T cells (Fig. S3E), we did not detect elevated TGF-β levels in in vitro cultures of RL alone or with enriched NK cells (Fig. S3F). Moreover, overnight or 5-d cultures of NK cells with serum from rats with RL did not affect IFNγ production or NKG2D expression levels (Figs. S3G and H, and data not shown). This indicates that soluble serum factors are unlikely to directly affect NK cells, although exogenous TGF-β reduced NKG2D expression in vitro (Fig. S3I).
NK cells pre-activated with IL-15, IL-12, and IL-18 potently kill the resistant RL blasts
RL are resistant to lysis by autologous, IL-2-activated NK cells.Citation46 Here, poor degranulation and low cytotoxicity by resting, autologous NK cells in response to RL is shown ( and ). Extending the killing assay from 4 to 20 h led to increased specific lysis of RL independent of Fas/Fas-L (). Also, conjugate formation between NK cells and RL increased over time (Fig. S4A). Compared to primary T cells, expression of Trailr2, Fas, and adhesion molecules CD2, CD48, and ICAM-1 was low on RL, while MHC class I was similarly expressed, indicating reduced potential for interaction of NK cells with RL (Figs. S4B and C).
Figure 4. Cytokine pre-activation upregulates activating receptors and promotes NK cell lysis of RL. (A) Degranulation of primary enriched splenic NK cells in response to YAC-1 or RL. Values represent data from three independent experiments (n = 9) ± SEM. (B) Specific lysis of YAC-1 or RL target cells measured by 4 or 20 h 51Cr release assay using enriched, splenic NK cells. Values represent the average of triplicates of one representative experiment out of four independent experiments. (C) qRT-PCR analysis of Dnam1 expression in primary NK cells (n = 4), NK cells pre-cultured overnight in medium with IL-15 (n = 3), or in medium with IL-15, IL12, and IL-18 (n = 3). Statistical significance was calculated using the Kruskal–Wallis test. (D) MFI analysis of CD25, NKG2D, Ly49s3, FasL, and NKp46 on NK cells after overnight culture in medium alone (n = 3), with IL-15 alone (n = 3), or with IL-15, IL12, and IL-18 (n = 3). Data represent the average of three independent experiments ± SEM. Statistical significance was calculated using the non-parametrical Mann–Whitney test. (E) Specific lysis of YB2/0 or RL measured by 51Cr release assay with enriched splenic NK cells pre-cultured overnight in medium alone, with IL-15 alone, or with IL-15, IL12, and IL-18. NKG2D antibody or isotype control was pre-incubated with NK cells 30 min before assay. Data represent average of triplicates of one representative experiment of three independent experiments.
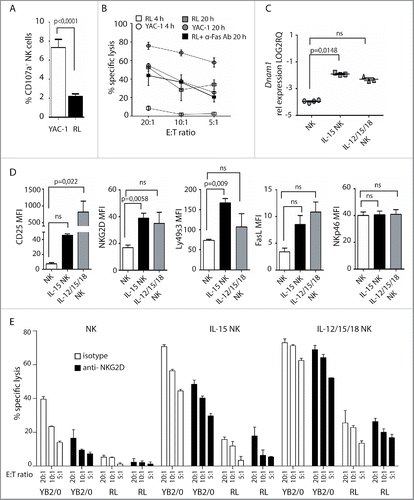
IL-15 in combination with IL-12 and IL-18 is shown to enhance NK cell effector functions and tumor recognition.Citation30,32 We therefore tested targeting of RL by autologous, enriched NK cells from healthy rats pre-treated with either IL-15 alone or in combination with IL-12 and IL-18. Heightened activity of NK cells was observed with increased expression of CD25 and CD69, in particular after culture with all three cytokines ( and ). Moreover, increased expression levels of NKG2D, Ly49s3, and FasL, but not NKp46, was observed after pre-activation with IL-15 alone or with IL-12 and IL-18 (). qRT-PCR analysis showed upregulation of Dnam1 (). IL-15 pre-activated NK cells demonstrated higher cytolytic activity toward the resistant RL compared with untreated NK cells, which was further enhanced by IL-12, IL-15, and IL-18 pre-activation reaching levels comparable to killing of the sensitive YB2/0 targets by untreated NK cells (). While lysis of YB2/0 targets was partially reduced by NKG2D antibody blockade, lysis of RL appeared to be independent of NKG2D. This correlates with the expression of NKG2D ligands by YB2/0 but not by RL (), and suggests that killing of RL by cytokine pre-activated NK cells is mediated by other activating receptor(s), possibly via DNAM-1/CD155.
IL-15/12/18 pre-activated NK cells slow RL development in vivo
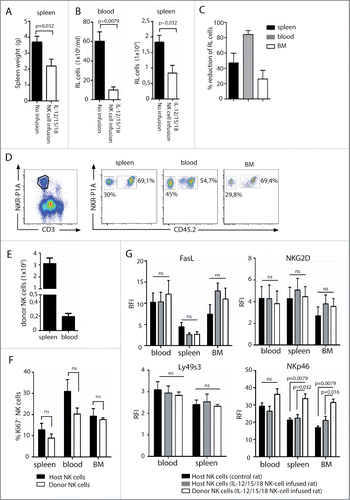
To test whether IL-12, IL-15, and IL-18 pre-activated NK cells target RL also in vivo, we established a model where rats were initially sub-lethally irradiated at 4 Gy to facilitate engraftment of transferred NK cells. Also, irradiation was previously shown to be necessary for effective tumor clearance by cytokine pre-activated NK cells in the mouse.Citation30 RL was injected 24 h post-irradiation, followed by adoptive transfer of 4–6 × 106 IL-12, IL-15, and IL-18 pre-activated CD45.1+ NK cells at days 3, 6, and 9. Control rats were irradiated and injected with RL only. All rats were killed after 3–4 weeks when blast numbers exceeded 30% of PBMC of control rats. A striking reduction in spleen weight (), and total numbers of RL in spleen and blood was observed in rats infused with pre-activated NK cells (). The reduction of RL load was most striking in the blood (). Donor NK cells were clearly detectable in blood, spleen, and BM at sacrifice ( and ). Although donor NK cells were pre-activated before adoptive transfer, they showed similar proliferative activity to host NK cells (CD45.2+) at sacrifice (). Also, CD25, FasL, and activating receptors NKG2D and Ly49s3 were similarly expressed between donor and host NK cells ( and ), indicating that the heightened activation status achieved after overnight pre-activation with IL-12, IL-15, and IL-18 is lost after adoptive transfer. This loss occurs within days after transfer and independently of whether rats are injected with RL or not (data not shown). Interestingly, NKp46 was consistently expressed at higher levels by donor NK cells at sacrifice ().
Figure 5. Adoptive transfer of cytokine pre-activated NK cells reduces RL load. Rats (n = 10) were sub-lethally irradiated at 4 Gy and injected with RL 24 h later. IL-12, IL-15, and IL-18 pre-activated NK cells were adoptively transferred to rats (n = 5) at days 3, 6, and 9. Control rats received no NK cells (n = 5). (A) Spleen weights of rats having RL with (white bars, n = 5) or without (black bars, n = 5) infusion of pre-activated NK cells. (B) Total numbers of RL per mL blood or total numbers of RL per spleen in rats with (white bars, n = 5) or without (black bars, n = 5) infusion of pre-activated NK cells. (C) Percent reduction of RL blasts in spleen, blood, or BM in rats receiving pre-activated NK cells. (D) Donor NK cells (CD45.2neg) are readily detected in spleen, blood, and BM at sacrifice 4 weeks after RL injection. (E) Total numbers of donor CD45.2neg NK cells per spleen or per mL blood at sacrifice 4 weeks after RL injection. (F) Percent proliferating Ki67+ host or donor NK cells at sacrifice 4 weeks after RL injection. (G) Expression of FasL, NKG2D, Ly49s3, and NKp46 on host and donor NK cells at sacrifice 4 weeks after RL injection, presented as RFI values ± SEM. Statistical significance was calculated using the non-parametrical Mann–Whitney test. Data represents two individual experiments with a total five rats in each group.
Discussion
Immunotherapy with different NK-cell-based strategies is showing promise in the clinic, in particular against AML.Citation6,7,32 Similar treatment of ALL has been less successful, likely due to their higher resistance toward NK cells.Citation8 T-ALL blasts are particularly resistant to NK cells, but studies investigating NK cells in context of T-ALL are fragmentary. Here, we examined NK cell phenotypes and functions during T-ALL, and show that pre-activation of NK cells with IL-12, IL-15, and IL-18 lead to targeting of T-ALL both in vitro and in vivo.
We observed diminished NK cell effector functions in pediatric T-ALL patients and in rats with RL. These results are in line with previous studies reporting defective NK cell functions in patients with other haematological cancers,Citation15,16,28 which has been correlated to decreased expression levels of activating NK cell receptors,Citation14,28 and increased risk of relapse.Citation15,16,18 In the rat model, serum or RL blasts does not affect NK cell functionality, suggesting that factors in tissues or suppressor cells, such as myeloid-derived suppressor cells (MDSC) may be involved.
NK cells from pediatric T-ALL patients expressed reduced surface levels of DNAM-1 and NKp46 but not NKG2D. This is in contrast to data from B-ALL and AML patients where NKG2D is generally downregulated,Citation14 as it is during rat T-ALL. The discrepancy between rat and human T-ALL with respect to NKG2D is not likely mediated by differential ligand expression, as neither RL nor human T-ALL expresses NKG2D ligands.Citation9 Instead, downregulation of NKG2D has been attributed to soluble NKG2D ligands or TGF-β in serum, but serum from RL rats had no effect on NKG2D expression. Possibly other cells that are expanded in context of cancers, such as MDSC, are involved.Citation47,48 It is currently unclear how NKp46 is downregulated in the human T-ALL patients. NKp46 downregulation observed in AML patients is independent of TGF-β,Citation12 and AML blasts lack expression of NKp46 ligands.Citation49 Potentially, soluble ligands or ligands expressed by extracellular vesicles could mediate NKp46 downregulation. In contrast, the DNAM-1 ligands CD155 and CD112 are frequently found expressed by malignant cells, and directly linked to receptor downregulation.Citation26,27 DNAM-1 surface expression was not tested in the rat due to unavailability of antibodies, but as CD155 was detected at the mRNA level in RL, we hypothesize that DNAM-1 may be regulated similarly in human and rat T-ALL. We have also observed variable expression of CD155 and CD112 by human T-ALL blasts (unpublished observations). While this points to potential involvement of DNAM-1 in recognition of T-ALL, CD155 also interacts with the inhibitory receptor TIGIT expressed by human and rat NK cells.
The inhibitory NKR-P1A receptor was expressed at higher levels in the pediatric T-ALL patient group. In parallel, increased frequencies NK cells expressing the inhibitory NKR-P1B receptor were observed in rats with RL. The implication of enhanced expression of inhibitory NKR-P1s in unclear at the moment, as is the relationship between human NKR-P1A and the four NKR-P1 members in the rat; activating NKR-P1A and -F, and inhibitory NKR-P1B and -G. The ligand for human NKR-P1A, LLT1, or ligands for rat NKR-P1 receptors—the Clr molecules—are not expressed by T-cell leukemias.Citation50,51 In rats with RL, NKR-P1Bdim bone marrow NK cells contained more CD27+CD11b− cells than in healthy controls. While CD27+CD11b− NK cells represent less differentiated cells in mice, CD27 and CD11b are not good maturation markers in the rat. However, bone marrow rat NK cells express less CD11b than peripheral NK cells, indicating that CD11b to a certain extent may predict maturation.Citation43 Also, in T-ALL patients, reduced frequencies of the most differentiated NK cells were observed. The human and rat data could suggest an impairment of NK cell differentiation, or alternatively, exhaustion of mature, effector NK cells.
We show that rat autologous NK cells poorly target T-ALL blasts. Previous studies have shown that pre-activating NK cells with combinations of IL-12, IL-15, and IL-18 upregulate activating NK cell receptors, enhance expression of the cytolytic molecules FasL, TRAIL, perforin, and granzyme B,Citation31 and induce a memory-like phenotype.Citation30,33,35,36 Further, NK cells upregulate CD25, rendering NK cells more sensitive to IL-2 driven activationCitation52 and expansion.Citation53 Accordingly, rat NK cells upregulated CD25 and CD69 in response to IL-12, IL-15, and IL-18, and upregulated surface expression of NKG2D and Ly49s3 and of DNAM-1 mRNA. Importantly, cytokine pre-activated NK cells displayed increased cytotoxicity toward the resistant RL. Blocking NKG2D minimally influenced killing of RL, corroborating the low levels of NKG2D ligands found in RL. Recognition of RL must therefore rely on other activating receptors. Expression of the DNAM-1 ligand CD155 by RL could possibly mediate such recognition. The heightened activity status of NK cells induced by the cytokines may be as important as the increased expression of activation receptors. IL-15 alone or in combination with IL-12 and IL-18 yielded comparable upregulation of activating receptors, but only IL-15, IL-12, and IL-18 pre-activation led to superior NK cell cytotoxicity toward the resistant RL blasts. Cytokine pre-activation could also lead to increased sensitivity by promoting an override of inhibitory input via MHC class I.
Our data show the potential of using pre-activated NK cells for eradication of normally resistant cancers, as reduced burdens of RL was observed in vivo after adoptive transfer of IL-12, IL-15, and IL-18 pre-activated NK cells. Previously, tumor regression after transfer of IL-12, IL-15, and IL-18 pre-activated NK cells was demonstrated in a mouse lymphoma model and in human refractory AML.Citation30,32 Although there is a clear effect of infusing pre-activated NK cells, there is an obvious need to improve their efficiency. NK cells persist in vivo, but the heightened expression levels of activation markers and activating receptors are lost within days of transfer. Also, the transferred NK cells delay the disease with only a few days, and improved protocols for pre-activation and for maintaining heightened activity of NK cells in vivo are needed. The latter has been tackled by infusion of low-dose IL-2,Citation32 but this may be a futile strategy for T-ALL. Instead IL-15 could represent a more optimal strategy. Obtaining sufficient numbers of expanded pre-activated autologous NK cells from cancer patients in a clinical setting is at present technically challenging. These NK cells are also potentially functionally impaired, and also face inhibition through self MHC class I expressed by leukemic blasts, although we clearly demonstrate that this latter problem is partly overcome by cytokine pre-activation of NK cells. Alternatively, cytokine pre-activated allogeneic haploidentical NK cells could represent a more feasible approach, as recently demonstrated in a clinical trial of relapsed/refractory AML by Fehniger and colleagues.Citation32 The use of haploidentical NK cells is safe and more feasible, and not associated with any detrimental graft-vs.-host reactions.Citation7
In summary, we show that NK cells are functionally suppressed in T-ALL patients at diagnosis and in rats having RL. We show in the rat model that the suppressed NK functions were not directly mediated by the blasts themselves, or by soluble serum factors, suggesting factors extrinsic to the blasts are responsible. Pre-activating NK cells with IL-15, IL-12, and IL-18 led to efficient targeting and killing of the normally resistant RL both in vitro and in vivo. Further improvements of protocols for NK cell pre-activation could lead to enhanced in vivo activity and improved therapeutic protocols for T-ALL and other haematological cancers.
Materials
Patients and healthy controls
PBMCs were collected at diagnosis and before therapy initiation from 19 patients admitted between 1982 and 1997 at the Royal Victoria Infirmary in Newcastle, UK, and from three patients admitted between 2014 and 2016 at Oslo University Hospital, Oslo, Norway. All were diagnosed with T-ALL. The pediatric group consisted of 13 patients (1–18 y, median age 5.5 y), and the adult group of nine patients (25–48 y, median age 37 y). As controls, PBMCs were obtained from 13 healthy adult controls and 20 healthy children (1–16 y, median age 9.5 y) undergoing elective surgery at Oslo University Hospital. All samples were obtained after informed consent according to the Declaration of Helsinki, and anonymized. The study was approved approval by the regional ethical committees. For all samples, peripheral venous blood was immediately processed to separate PBMCs.
Animals
Eight to twelve-week-old PVG-RT7b (PVG.7B) rats were used. The rats have been maintained at the Department of Comparative Medicine, Institute of Basic Medical Sciences, University of Oslo for more than 20 generations. The Department of Comparative Medicines institutional veterinarian has established the rules for feeding, monitoring, handling, and sacrifice of animals in compliance with regulations set by the Ministry of Agriculture of Norway and “The European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes.” The institutional veterinarian has delegated authority from the Norwegian Animal Research Authority (NARA). The laboratory animal facilities are subject to a routine health-monitoring program and tested for infectious organisms according to a modification of Federation of European Laboratory Animal Science Associations (FELASA) recommendations. The use of animals for this study was approved by NARA, license number 6010 (in vitro) and 6060 (in vivo). Rats were killed by asphyxiation with CO2 in a chamber allowing controlled input of gas, to reduce suffering.
Antibodies and flow cytometry
Antibodies used were from BD Biosciences anti-rat CD3 (G4.18-FITC (cat.no 554832) or-PE (554833)), anti-rat NKR-P1A (10/78-PE, cat.no. 555009), anti-mouse CD27-PE (cat.no. 558754), anti-mouse CD11b (WT.5-biotin, cat.no.554981), anti-rat CD54 (1A29-PE, cat.no. 554970), anti-human CD7 (M-T701-PerCP-Cy5.5, cat.no. 561602), anti-human CD8 (SK1-FITC, cat.no. 345772), anti-human CD56 (B159-Alexa647, cat.no. 557711), and Streptavidin-PerCP (cat.no. 554064) from eBiosciences anti-human CD3 (OKT3-Alexa700, cat.no. 56–0037–42), anti-human CD14 (M5E2-PerCP-Cy5.5, cat.no. 45–0149–42), anti-human CD19 (1D3-PerCP-Cy5.5, cat.no. 45–0199–42), and anti-human NKG2D (A10-biotin, cat.no. 13–5872); from R&D Systems anti-rat Fas (goat IgG, cat.no. AF2159) and anti-human DNAM-1 (#102511-PE, cat.no. FAB666P); from BioLegend anti-human NKp46 (9E2-BV650, cat.no. 331927); from LifeSpan Biosciences anti-human CD161 (B199.2-biotin, cat.no. LS-C35855); from Beckman Coulter anti-human NKG2A (Z199-PE-Cy7, cat.no. B10246); from Thermo Fisher Scientific Streptavidin-QDot605 (cat.no. Q10101MP). mAbs to rat NKR-P1A (3.2.3), rat Ly49s3/i3/s4/i4 (DAR13), rat NKp46 (Wen23), rat NKR-P1BPVG (STOK27), rat CD2 (OX34), rat CD48 (OX45), rat MHC-1 (OX18), and rat CD45.2 (His41) were generated from hybridomas and conjugated according to standard protocols. Anti-rat NKG2D antibody was a kind gift from Dr S. Krams.Citation54 Cells were labeled with relevant antibodies and analyzed by Fortessa, LSRII, or FACSCalibur (BD Biosciences). Frozen, human samples were thawed immediately before use, and dead cells were excluded from analysis using the Fixable Viability Dye eFluor780 (eBiosciences, cat.no. 65–0865–14). Lymphocytes (singlets) were gated by forward and side scatter. Rat NK cells were gated as NKR-P1A+CD3−, and human NK cells as CD56+CD3−CD14−CD19−. Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Cells and cell cultures
Human PBMC were processed by either Lymphoprep (Axis-Shield, cat.no. 1114544) or Ficoll (GE Healthcare, cat.no. 17–1440–02), and viably frozen in liquid nitrogen. Rat mononuclear cells (MNC) from spleen, blood, and bone marrow were prepared by Lymphoprep separation from both rats with end-stage leukemia and from healthy rats. This resulted in 50–60% NKR-P1A+CD3− NK cells. The mouse T cell lymphoma cell line YAC-1 (ATCC TIB-160), the rat B lymphoblastic cell line YB2/0 (ATCC® CRL1662™), and the human CML cell line K562 (ATCC® CCL−243™) were maintained in complete RPMI (cRPMI; RPMI supplemented with 10% FBS, 2-ME, sodium pyruvate, and penicillin/streptomycin).
Enrichment of NK cells and cytokine pre-activation
Rat NK cells were enriched from mononuclear spleen cells by incubation in nylon wool (PolySciences, cat.no. 18369–50) at 37°C for 45 min, followed by negative selection using a mixture of antibodies toward T cells (R73 and OX19), B cells (OX12 and OX33), monocytes (ED1), and macrophages/granulocytes (OX41), and Pan mouse IgG Dynabeads (Thermo Fisher Scientific, cat.no. 11041). Cytokine pre-activated NK cells were generated by overnight cultures of enriched NK cells with 10 ng/mL IL-12 (Sigma-Aldrich, cat.no. SRP4163), 10 ng/mL IL-15 (PeproTech, cat.no. 400–24), and 50 ng/mL IL-18 (R&D Systems, cat.no. 521-RL-025)).
Roser leukemia (RL) model
The radiation-induced T cell lymphoblastic leukemia RL (originating from the PVG strain; CD45.1 allotype) was a gift from Dr Roser, Cambridge, UK. Leukemia was induced by i.v. injection of 4 × 104 RL into the tail vein of rats from the PVG.7B strain (CD45.2 allotype). Propagation of RL injected i.v. occurs primarily in the spleen, but are detected in high numbers in blood and bone marrow during late stages of the disease.Citation39 The disease was monitored by blood samplings from the tail vein, and RL identified as CD45.1−CD3+ cells by flow cytometry. The end point was set to 30% RL among PBMCs. Blood, spleen, and bone marrow was collected for analysis at sacrifice. For in vivo targeting by NK cells, rats were sub-lethally irradiated at 4 Gy from an X-ray source (RS320, Xstrahl Life Sciences, UK), followed by injection of RL 24 h later. IL-12, IL-15, and IL-18 pre-activated NK cells were washed four times in PBS, and 4–6 × 106 cells were injected per rat at days 3, 6, and 9. Control rats were irradiated and injected with RL only. All rats within an experimental series were terminated when blasts reached >30% of PBMC in control rats.
CD107a degranulation and 51Cr release assays
Degranulation by rat NK cells was measured using a hamster anti-rat CD107a antibody generated in our laboratory as described previously. With this antibody 10–15% degranulation is measured against potent target cells.Citation55 Degranulation by human NK cells were measured by incubating 100 µL PBMC (2 × 106 cells/mL) with 100 µL K562 target cells (2 × 106 cells/mL) in the presence of anti-human CD107a-BV510 (BD Biosciences, cat.no. 563078) for 6 h, with Brefeldin A (Sigma-Aldrich, B7651) added at 5 µg/mL for the last 5 h. NK cell cytotoxicity was measured with a standard 51Cr release assay, using enriched NK cells as effector cells and 51Cr-labeled YAC-1, YB2/0, or RL as target cells as described previously.Citation56 For blocking experiments, blocking antibodies were added at 5 µg/mL. Results are presented as mean values from triplicates for each E:T cell ratio.
Analysis of intracellular IFNγ and Ki67 expression
Intracellular IFNγ expression in human NK cells was measured after 6 h of culture of PBMC in the presence of K562 target cells as described above for the degranulation assay. Cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.5% saponin in PBS and 2% FBS for 20 min, and stained with anti-IFNγ (BD Biosciences, cat.no. 554552) for 30 min in permeabilization buffer. Intracellular IFNγ expression in rat NK cells was investigated by flow cytometry after 6 h of incubation as described previously.Citation43 Briefly, enriched NK cells were seeded at 3 × 105 cells/well in 96-well plates in the presence of IL-2 (from a CHO cell line stably transfected with a rat IL-2 expression construct), and indicated concentrations of IL-12 (ThermoFischer) or IL-18 (50 ng/mL, R&D Systems), or in wells pre-coated with 10 µg/mL of indicated mAbs. Where indicated, RL were co-incubated with the enriched NK cells at indicated ratios. GolgiStop (BD Biosciences, cat.no. 554715) was added for the last 5 h of culture. Proliferative activity was measured by intracellular staining of Ki67 using anti-mouse/rat Ki67 (SolA15-PE-Cy7, eBiosciences, cat.no. 14–5698–80) after fixation and permeabilization using the Foxp3/Transcription Factor Staining Buffer Set from eBiosciences (cat.no. 00–5523–00) according to manufacturer's instruction.
RNA extraction, cDNA synthesis, and real-time PCR
NK cells were positively selected from spleen of rats with leukemia or from control rats using Pan Mouse IgG Dynabeads coupled to anti-NKR-P1A antibodies. Total RNA was isolated using the mirVana miRNA Isolation Kit (Thermo Fisher Scientific, cat.no. AM1560) in accordance with the manufacturer's instructions using RNase-free equipment. RNA concentration and purity was measured by 260:280 and 260:230 ratios (purity measured between 1.8 and 2.0) using NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). To generate cDNA, 2 µg of RNA was mixed with 2 µL random hexamer primers (Thermo Fisher Scientific, cat.no. S0142) and ddH2O up to 15 µL volume and run 10 min at 70°C. One microliter of M-MLV reverse transcriptase (Promega, cat.no. M1701), 5 µL of M-MLV 5x buffer (Thermo Fisher Scientific, cat.no. 18057018), 1 µL RNase inhibitor (Promega, cat.no. N2615), 0.01 M DTT, and 2 µL of 10 mM dNTPs were added for total volume 25 µL and run for 1 h at 37°C. cDNA was stored at −20°C until use. Real-time PCR was performed with Power SYBR® Green PCR Master Mix (Thermo Fisher Scientific, cat.no. 4367659), and run using the 7900HT Fast Real-Time PCR System (ThermoFischer), with initial run for 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of denaturation at 95°C (15 sec) and amplification at 60°C (1 min). Melting curves were performed at 95°C (15 sec) followed by 60°C (20 sec) and 95°C (20 sec). Primers were designed using NCBI PrimerBlast, and specificity tested in silico by BLAST. Primers are listed in . All samples were normalized to Cd45 expression. Expression levels are reported as log transformation of 2^−ΔCt (Log2RQ).
Table 1. List of primers.
Conjugate assay
Enriched splenic NK cells were labeled with 0.5 µM CFSE (Thermo Fisher Scientific, cat.no. C1157) at 3 × 106 cells/mL in PBS and 2% FBS for 10 min at 37°C, and anti-NKR-P1A Alexa647. Target cells (YAC-1 or RL) were stained with seminaphtharhodafluor (SNARF-1) (Thermo Fisher Scientific, cat.no. C1270) according to the manufacturer's protocol. 100 µL enriched NK cells (3 × 105 cells) were mixed with 100 µL target cells (3 × 105 cells) with an effector to target ratio 1:1, spun for 30 sec at 300 g, and incubated at 37°C for 2, 10, 60, or 120 min. Reactions were stopped by addition of 2% paraformaldehyde. As negative control target and effector cells were mixed after fixation. Conjugate formation was analyzed by flow cytometry, and percentage of NKR-P1A+ NK cells in conjugates with target cells calculated.
ELISA
Serum samples were obtained at sacrifice and stored at −80°C until use. Supernatants were obtained after overnight co-culture of RL with enriched splenic NK cells at different RL:NK cell ratios, and stored at −20°C until use. Concentrations of TGF-β in serum and cell culture supernatants were measured in duplicates using the Quantikine ELISA kit for Mouse/Rat/Porcine/Canine TGF-β1 (R&D Systems, cat.no. MB100B) following the manufacturer's instructions. Samples were activated for 10 min with 1N HCl and neutralized with 1.2N NaOH/0.5M HEPES. Serum samples were diluted 1:60 before assay. Absorbance was read at 450 nm, and concentrations interpolated from a standard curve.
Statistical analysis
Graphics and statistical analysis were performed with the GraphPad Prism software. Data are presented as the mean ± standard error of the mean (SEM). Statistical significance was calculated using the non-parametrical Mann–Whitney U test or the Kruskal–Wallis test. p values less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1274478_s02.docx
Download MS Word (5.3 MB)Funding
This work was supported by The South-Eastern Norway Regional Health Authority under grant 2013068, the European Union under grant FP7-PEOPLE-2012-ITN-315963 (CELLEUROPE), and Anders Jahres fond til vitenskapens fremme. The authors thank Dr Ke-Zheng Dai and Ulla Heggelund for technical assistance.
References
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329-39; PMID:17438573; http://dx.doi.org/10.1038/nri2073
- Davis ZB, Felices M, Verneris MR, Miller JS. Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense Against Cancer. Cancer J 2015; 21:486-91; PMID:26588681; PMID:26264743; http://dx.doi.org/10.1097/PPO.0000000000000156
- Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol 2016; 17:1025-36; PMID:27540992; http://dx.doi.org/10.1038/ni.3518
- Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31:227-58; PMID:23516982; http://dx.doi.org/10.1146/annurev-immunol-020711-075005
- Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 2017; 222:11-20; PMID:26264743; http://dx.doi.org/10.1016/j.imbio.2015.07.012
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295:2097-100; PMID:11896281; http://dx.doi.org/10.1126/science.1068440
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105:3051-7; PMID:15632206; http://dx.doi.org/10.1182/blood-2004-07-2974
- Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, Romeo E, Cognet C, Martinetti M, Maccario R et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009; 113:3119-29; PMID:18945967; http://dx.doi.org/10.1182/blood-2008-06-164103
- Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 2005; 105:2066-73; PMID:15536144; http://dx.doi.org/10.1182/blood-2004-09-3548
- Romanski A, Bug G, Becker S, Kampfmann M, Seifried E, Hoelzer D, Ottmann OG, Tonn T. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp Hematol 2005; 33:344-52; PMID:15730858; http://dx.doi.org/10.1016/j.exphem.2004.11.006
- Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, Nanni M, Biassoni R, Bottino C, Moretta A et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol 2001; 31:1076-86; PMID:11298332; http://dx.doi.org/10.1002/1521-4141(200104)31:4%3c1076::AID-IMMU1076%3e3.0.CO;2-Y
- Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 2007; 109:323-30; PMID:16940427; http://dx.doi.org/10.1182/blood-2005-08-027979
- Sanchez-Correa B, Morgado S, Gayoso I, Bergua JM, Casado JG, Arcos MJ, Bengochea ML, Duran E, Solana R, Tarazona R. Human NK cells in acute myeloid leukaemia patients: analysis of NK cell-activating receptors and their ligands. Cancer Immunol Immunother 2011; 60:1195-205; PMID:21644031; http://dx.doi.org/10.1007/s00262-011-1050-2
- Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol 2012; 189:1360-71; PMID:22730533; http://dx.doi.org/10.4049/jimmunol.1200796
- Sørskaar D, Lie SO, Førre O. Natural killer cell activity of peripheral blood and bone marrow mononuclear cells from patients with childhood acute lymphoblastic leukemia. Acta Paediatr Scand 1985; 74:433-7; PMID: 3859177; http://dx.doi.org/10.1111/j.1651-2227.1985.tb10998.x
- Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 2002; 99:3661-7; PMID:11986221; http://dx.doi.org/10.1182/blood.V99.10.3661
- Tajima F, Kawatani T, Endo A, Kawasaki H. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia 1996; 10:478-82; PMID: 8642865
- Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de LH, Sarvaria A et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014; 99:836-47; PMID:24488563; http://dx.doi.org/10.3324/haematol.2013.087536
- Lowdell MW, Craston R, Samuel D, Wood ME, O'Neill E, Saha V, Prentice HG. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol 2002; 117:821-7; PMID:12060116; http://dx.doi.org/10.1046/j.1365-2141.2002.03495.x
- Boissel N, Rea D, Tieng V, Dulphy N, Brun M, Cayuela JM, Rousselot P, Tamouza R, Le Bouteiller P, Mahon FX et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol 2006; 176:5108-16; PMID:16585609; http://dx.doi.org/10.4049/jimmunol.176.8.5108
- Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, Ku E, Zhong B, Boulware D et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007; 109:4816-24; PMID:17341666; http://dx.doi.org/10.1182/blood-2006-07-035519
- Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 2004; 172:7335-40; PMID:15187109; http://dx.doi.org/10.4049/jimmunol.172.12.7335
- Dasgupta S, Bhattacharya-Chatterjee M, O'Malley BW, Jr., Chatterjee SK. Inhibition of NK cell activity through TGF-β 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol 2005; 175:5541-50; PMID:16210663; http://dx.doi.org/10.4049/jimmunol.175.8.5541
- Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-β downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol 2010; 12:7-13; PMID:20150362; http://dx.doi.org/10.1093/neuonc/nop009
- Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 2003; 102:1389-96; PMID:12714493; http://dx.doi.org/10.1182/blood-2003-01-0019
- Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol 2012; 90:109-15; PMID:21383766; http://dx.doi.org/10.1038/icb.2011.15
- Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, Kiessling R, Malmberg KJ. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol 2009; 183:4921-30; PMID:19801517; http://dx.doi.org/10.4049/jimmunol.0901226
- Carlsten M, Baumann BC, Simonsson M, Jädersten M, Forsblom AM, Hammarstedt C, Bryceson YT, Ljunggren HG, Hellström-Lindberg E, Malmberg KJ. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia 2010; 24:1607-16; PMID:20613786; http://dx.doi.org/10.1038/leu.2010.149
- Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011; 17:6287-97; PMID:21844012; http://dx.doi.org/10.1158/1078-0432.CCR-11-1347
- Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012; 209:2351-65; PMID:23209317; http://dx.doi.org/10.1084/jem.20120944
- Hüber CM, Doisne JM, Colucci F. IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation. Eur J Immunol 2015; 45:1727-35; PMID:25778912; http://dx.doi.org/10.1002/eji.201445200
- Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016; 8:357ra123; PMID:27655849; http://dx.doi.org/10.1126/scitranslmed.aaf2341
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 2009; 106:1915-9; PMID:19181844; http://dx.doi.org/10.1073/pnas.0813192106
- Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant 2014; 20:463-73; PMID:24434782; http://dx.doi.org/10.1016/j.bbmt.2014.01.006
- Berrien-Elliott MM, Wagner JA, Fehniger TA. Human Cytokine-Induced Memory-Like Natural Killer Cells. J Innate Immun 2015; 7:563-71; PMID:25924651; http://dx.doi.org/10.1159/000382019
- Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood 2012; 120:4751-60; PMID:22983442; http://dx.doi.org/10.1182/blood-2012-04-419283
- Onciu M. Acute lymphoblastic leukemia. Hematol Oncol Clin North Am 2009; 23:655-74; PMID:19577163; http://dx.doi.org/10.1016/j.hoc.2009.04.009
- Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program 2006; 1:133-41; PMID:17124052; http://dx.doi.org/10.1182/asheducation-2006.1.133
- Dibley M, Dorsch S, Roser B. T cell leukaemia in the rat: the pathophysiology. Pathology 1975, 7:219-35; PMID: 1081666; http://dx.doi.org/10.3109/00313027509094412
- Ahmad F, Hong HS, Jäckel M, Jablonka A, Lu IN, Bhatnagar N, Eberhard JM, Bollmann BA, Ballmaier M, Zielinska-Skowronek M et al. High frequencies of polyfunctional CD8+ NK cells in chronic HIV-1 infection are associated with slower disease progression. J. Virol 2014; 88:12397-408; PMID:25122796; http://dx.doi.org/10.1128/JVI.01420-14
- Gibbings D, Befus AD. CD4 and CD8: an inside-out coreceptor model for innate immune cells. J Leukoc Biol 2009; 86:251-9; PMID:19401396; http://dx.doi.org/10.1189/jlb.0109040
- Kveberg L, Jiménez-Royo P, Naper C, Rolstad B, Butcher GW, Vaage JT, Inngjerdingen M. Two complementary rat NK cell subsets, Ly49s3+ and NKR-P1B+, differ in phenotypic characteristics and responsiveness to cytokines. J Leukoc Biol 2010; 88:87-93; PMID:20395458; http://dx.doi.org/10.1189/jlb.0110039
- Inngjerdingen M, Kveberg L, Vaage JT. A novel NKR-P1Bbright NK cell subset expresses an activated CD25+CX3CR1+CD62L−CD11b−CD27− phenotype and is prevalent in blood, liver, and gut-associated lymphoid organs of rats. J Immunol 2012; 188:2499-508; PMID:22308308; http://dx.doi.org/10.4049/jimmunol.1003939
- Björkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björklund AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010; 116:3853-64; PMID:20696944; http://dx.doi.org/10.1182/blood-2010-04-281675
- Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010; 116:3865-74; PMID:20733159; http://dx.doi.org/10.1182/blood-2010-04-282301
- Nestvold J, Stokland A, Naper C, Rolstad B. Phenotype and natural killer cell sensitivity of a radiation-induced acute T-cell leukaemia (Roser leukaemia) in PVG rats. Scand J Immunol 2004; 60:153-8; PMID:15238084; http://dx.doi.org/10.1111/j.0300-9475.2004.01436.x
- De Veirman K, Van Valckenborgh E, Lahmar Q, Geeraerts X, De Bruyne E, Menu E, Van Riet I, Vanderkerken K, Van Ginderachter JA. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol 2014; 4:349; PMID:25538893; http://dx.doi.org/10.3389/fonc.2014.00349
- Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β 1. J Immunol 2009; 182:240-9; PMID:19109155; http://dx.doi.org/10.4049/jimmunol.182.1.240
- Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, Cosman D, De Libero G, Wodnar-Filipowicz A. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood 2005; 105:3615-22; PMID:15657183; http://dx.doi.org/10.1182/blood-2004-07-2585
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol 2009; 10:48-57; PMID:19011627; http://dx.doi.org/10.1038/ni.1674
- Germain C, Guillaudeux T, Galsgaard ED, Hervouet C, Tekaya N, Gallouet AS, Fassy J, Bihl F, Poupon G, Lazzari A et al. Lectin-like transcript 1 is a marker of germinal center-derived B-cell non-Hodgkin's lymphomas dampening natural killer cell functions. Oncoimmunology 2015; 4:e1026503; PMID:26405582; http://dx.doi.org/10.1080/2162402X.2015.1026503
- Stegmann KA, De Souza JB, Riley EM. IL-18-induced expression of high-affinity IL-2R on murine NK cells is essential for NK-cell IFN-γ production during murine Plasmodium yoelii infection. Eur J Immunol 2015; 45:3431-40; PMID:26420375; http://dx.doi.org/10.1002/eji.201546018
- Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 2012; 209:947-54; PMID:22493516; http://dx.doi.org/10.1084/jem.20111760
- Zhuo M, Fujiki M, Wang M, Piard-Ruster K, Wai LE, Wei L, Martinez OM, Krams SM. Identification of the rat NKG2D ligands, RAE1L and RRLT, and their role in allograft rejection. Eur J Immunol 2010; 40:1748-57; PMID:20306467; http://dx.doi.org/10.1002/eji.200939779
- Sudworth AD, Ke-Zheng D, Vaage JT, Kveberg L. Degranulation response in cytotoxic rat lymphocytes measured with a novel CD107a antibody. Front Immunol 2016; 7:572; PMID: 28003815; http://dx.doi.org/10.3389/fimmu.2016.00572
- Vaage JT, Naper C, Løvik G, Lambracht D, Rehm A, Hedrich HJ, Wonigeit K, Rolstad B. Control of rat natural killer cell-mediated allorecognition by a major histocompatibility complex region encoding nonclassical class I antigens. J Exp Med 1994; 180:641-51; PMID:8046337; http://dx.doi.org/10.1084/jem.180.2.641
