ABSTRACT
Historically, the immune environment was not considered an important target for breast cancer treatment. However, the association of lymphocytic infiltrates in triple negative and HER-2 over-amplified breast cancer subtypes with better outcomes, has provoked interest in evaluating the role of the immune system in the luminal B subtype that accounts for 39% of breast cancers and has a poor patient prognosis. It is unknown which immunosuppressive cell types or molecules (e.g., checkpoint molecules) are relevant, or where measurement is most informative. We hypothesize that a profound immunosuppressive tumor and/or lymph node milieu is prognostic and impacts on responses to therapies.
Introduction
Breast cancer (BC) is the most common type of cancer, and the leading cause of cancer death, in women.Citation1 Despite a better understanding of BC aetiology and dramatic improvements in BC prognosis and treatment, BC is still associated with major morbidity and mortality, with more effective systemic treatment options needed. BC is recognized as a heterogeneous malignancy consisting of molecularly distinct neoplasms,Citation2 classified clinically into at least four subtypes based on receptor status and tumor proliferation rate.Citation3 Considerable efforts have focused on identifying reliable prognostic markers for BC that distinguish between the subtypes. For example, hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER-2) over-amplification and expression levels of the nuclear cell proliferation molecule, Ki67, have been shown to have prognostic value in BC.Citation4,5 However, few studies have focused on identifying prognostic markers for patients within a subtype.
One subtype is luminal B BC that is defined by positive estrogen receptor (ER) status and categorized into two subclasses, A and B. Luminal B BC has a higher tumor cell proliferation rate than luminal A BC,Citation6 which can be identified by quantification of mitotic activity through immunohistochemical staining for Ki67 expression. Luminal B disease was previously thought to have a better survival rate than other BC subtypes, however, the adjusted BC-specific mortality rate is twice as high in luminal B patients relative to luminal A patients. Moreover, survival rates in luminal B BC may be comparable to triple negative BC (TNBC).Citation7 Despite the advent of molecular BC profiling, there are no reliable prognostic markers to predict long-term outcomes in luminal B patients. As luminal B tumors account for up to 39% of BCs,Citation8 it is vital to improve the range of bio-indicators that identify patients at higher risk of relapse, and who would benefit from modifications to therapy.
The anticancer immune response
The immune system protects us from pathogens and cancerous cells by differentiating between healthy and damaged self, as well as between safe and pathogenic non-self, through surface and intra-cellular molecules termed pattern recognition receptors (PPRs). These receptors recognize endogenous damage (or danger)-associated molecular pattern molecules (DAMPs) and pathogen-associated molecular pattern molecules (PAMPs). Cells of the innate system, including macrophages and dendritic cells (DCs), use PPR-DAMP/PAMP interactions to prime the highly specific adaptive immune system. DCs play a pivotal role in priming antigen-specific T cells, including tumor-antigen-specific T cells; this process occurs in tumor-draining lymph nodes once DCs have trafficked through tumors and taken up tumor antigens ().Citation9 Macrophages mostly influence T cell function in peripheral tissues and tumors, although they may exert local effects in lymphoid tissues as well.Citation10 T cells can be differentiated into CD8+ cytotoxic T lymphocytes (CTLs) that lyse tumor cells, or CD4+ helper T (Th) cells, with the CD4+ Th-1 subset releasing cytokines that enable CTLs to kill tumor cells. As cancer cells are altered-self they express surface molecules similar to healthy cells making it difficult for the immune system to identify them as pathological. Nonetheless, as a result of their genetic instability, and increasing mutational load with disease progression, tumour cells may also express new molecules (neo antigens) that render them visible to the immune system, providing a window of oppourtunity for effective immune responses, including those driven by therapeutic intervention. However, cancerous cells can escape immune-mediated killing through evasion tactics, a major pathway being suppression of effector CTLs, whose cytolytic activity against tumor cells is essential to anticancer immunity.Citation11
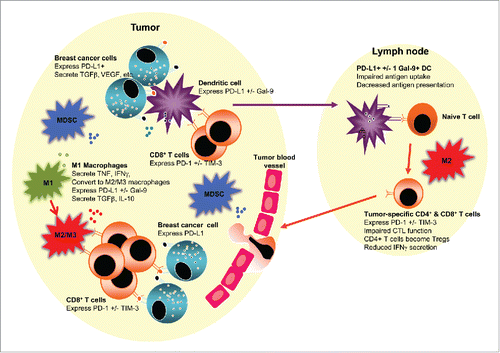
Figure 1. An immunosuppressive tumor and/or lymph node environment could be prognostic and impact responses to therapies in luminal B breast cancer. The figure depicts a tumor juxtaposed against a draining lymph node. The tumor milieu contains malignant cells which likely express checkpoint molecules such as PD-L1, and secrete suppressive molecules such as TGFβ and VEGF that limit T cell function and promote angiogenesis. Tumor-associated macrophages may initially represent pro-inflammatory, antitumor M1 macrophages (M1) that secrete TNF, IFNγ and other pro-inflammatory cytokines. However, in response to tumor-derived factors, M1 macrophages or newly recruited macrophages may polarize to anti-inflammatory, pro-tumor M2 macrophages (M2), or to an intermediate M3 macrophage (M3), both of which could express PD-L1 +/− Gal-9 or other checkpoint molecules, and secrete suppressive molecules such as TGFβ and IL-10, which together prevent effector CD8+ T cell function, particularly T cells expressing checkpoint molecules such as PD-1 and TIM-3. Myeloid-derived suppressor cells (MDSCs) augment the suppressive milieu by expressing and secreting similar immune suppressive molecules. Tumor-infiltrating dendritic cells (DCs) may take up whole tumor cells or tumor cell debris and traffic to lymph nodes. If, in response to the tumor microenvironment, DCs express checkpoint molecules such as PD-L1 and/or Gal-9, their ability to activate tumor-specific effector T cells is impaired, and they may instead induce Tregs. The presence of M2 macrophages in tumor-draining draining lymph nodes may further induce dysfunctional T cells expressing checkpoint inhibitors or Tregs.
Breast cancer and immunity
There is an increasing awareness of the relationship between the immune system and BC evolution, with the tumor microenvironment comprising of tumor and stromal cells. The latter includes immune cells, some of which inhibit or promote disease progression. Moreover, there is increasing recognition of a correlation between genetic instability and the BC immune landscape, with mutational load and heterogeneity leading to novel BC antigens. TNBC, luminal B and HER-2+ BC are reported to have a high mutational burden rendering them immunogenic, suggesting that immunotherapeutic approaches may be effective in these BC subtypes.Citation12 This is supported by database studies that describe immune benefit-enabled or disabled BC subtypes.Citation13 The former includes luminal B BC, which could be stratified by immune profile analysis into different prognostic groups, suggesting that the immune signature could be a useful prognostic indicator in luminal B BC. Gene network analysis predicted activation of TNF-α/IFNγ signaling pathways in immune-enabled tumors and activation of the transforming growth factor-β (TGF-β) pathway in immune-disabled tumors.Citation13 Gene expression studies of BC-adjacent tissues showed that the TNBC microenvironment had increased expression of genes involved in inflammationCitation14 and revealed further subtypes, some with a low immune response defined by a minimal T cell infiltrate and effector function, and high frequency of suppressive M2-like macrophages, and others with a high immune response and low frequency of M2-like macrophages.Citation15 These data imply that communication between tumor cells and other cells in cancer-adjacent tissue contributes to BC progression and/or metastasizing potential; the same is likely to be true for Luminal B BC ().
Several cell-intrinsic alterations are reported to be markers of poor prognosis in BC including reduced expression of the DAMP, high-mobility group protein (HMG)B1 by malignant cells, minimal CTL tumor infiltration, significant presence of immunosuppressive cells such as FOXP3+ regulatory T cells (Tregs) or CD68+ tumor-associated macrophages (TAMS).Citation16 Tumor-infiltrating T cells (TILs) are reported to be a useful positive biomarker in early BC in some, but not all, BC subtypes. A review of 15 studies showed that the magnitude of TIL varies within and between BC subtypesCitation17; TNBC, HER-2+ and HR+ BC contained the highest TIL levels, HER-2− BC had the lowest. The highest levels of FOXP3+ Tregs were in TNBC and HER-2+ BC, only a minority of HR+ BC demonstrated high levels of tumor-infiltrating FOXP3+ cells.Citation17
Breast cancer and chemotherapy
The realization that cancer progression depends on immune suppression suggests that the pre-treatment immune profile in tumors and lymph nodes may not only be prognostic, but also predictive of responses to therapies that engage the immune system, including standard chemotherapy. Once thought to be deleterious to immune cells, we now know that some chemotherapies stimulate beneficial changes in innate and adaptive antitumor immunity and render tumor cells accessible for immune destruction.Citation18,19 Chemotherapeutic and radiotherapeutic regimens commonly employed for the treatment of BC may induce immunogenic cell death in tumor cells, and affect TILs, however, clinical responses vary. Standard neoadjuvant chemotherapy for BC achieves complete pathologic responses in 10 to 20% of cases. While the biological factors that determine these responses are not well understood, the presence of TILs in pre-treatment tumors is believed to play a role.
Chemotherapy and the anticancer immune response
For BC, there is evidence that neoadjuvant chemotherapy is more efficient if tumors show a pre-existing or therapy-induced anticancer immune response, and it is now accepted that the success of anthracycline-based adjuvant chemotherapy is due to enhanced immunogenic tumor cell death, a cell death modality preceded by autophagy and followed by HMGB1 release. Some PPRs, such as Toll-like receptors (TLR) 3 and 4, sense HMGB1. Interaction of HMGB1 with TLR-4 on DCs upregulates tumor antigen cross-presentation by DCs and promotes induction of tumor-specific CD8+ CTLs in lymph nodes.Citation20 BC patients that have lost HMGB1 expression and/or harbor a loss-of-function mutation of TLR3 and TLR4 exhibit reduced metastasis-free and overall survival after treatment with anthracycline-based adjuvant chemotherapy.Citation21,22 These data support the concept that conventional anticancer treatments are only fully efficient once they have stimulated anticancer immune responses.
Cisplatin has been shown to induce necrotic cell death and inflammationCitation23, an environment known to stimulate DCs. This may be via expression of HMG proteins that bind platinum complexes to cause repair shielding and initiate apoptosis.Citation23 We have shown that while cisplatin only slightly enhances in vivo presentation of dominant tumor antigens to T cells in draining lymph nodes, both cisplatin and gemcitabine expand the CTL response to weaker subdominant tumor epitopesCitation24; a feature that may be key to tumor destruction as immune tolerance mechanisms are likely to delete responses to dominant epitopes. Moreover, immune-relevant metagene analyses have shown a significant positive correlation with response rates for BC to chemotherapy, in particular to a CXCL13-centered metagene signature reflecting the intratumoral presence of activated IFNγ-producing T cells.Citation25
Immune cells as prognostic and predictive markers of responses to therapy in BC
There is increasing evidence that pre-existing TILs and/or immune gene expression signatures indicate the magnitude of immune-suppression that confounds or supports different treatment strategies. Therefore, they may be predictive for responses to chemotherapies and other treatment modalities, including immunotherapy. This has been clearly shown for TNBC and HER-2+ BC wherein a strong association between higher lymphocytic infiltrations predicts a better outcome.Citation26,27
Histological studies have shown that co-localization of immune cells with cancer cells is significantly associated with a survival benefit for all BC subtypes.Citation28 It is not yet clear which immune cell types are useful as prognostic markers, and importantly whether they differ between BC subtypes. Gene expression studies revealed that differing levels of tumor-associated plasma B cells and myeloid-derived antigen-presenting cells (APCs), such as DCs and macrophages, contribute to varying pathologic responses to neoadjuvant chemotherapy.Citation29 Mouse studies of breast adenocarcinomas showed that doxorubicin treatment efficacy is dependent on CD8+ T cells and IFNγ production. Doxorubicin enhanced tumor antigen-specific CD8+ T cell proliferation in draining lymph nodes and promoted tumor infiltration of activated IFNγ+CD8+ T cells. A correlation between pre-treatment CD8+ and IFNγ gene expression levels in tumor samples from BC patients with clinical responses to anthracycline chemotherapy supported the pivotal contribution of innate and adaptive immunity in anthracycline treatment outcomes.Citation30 Targeted anticancer therapeutics, such as the anti-erb-b2 receptor monoclonal antibody, trastuzumab, also involved innate and adaptive immunity.Citation22 These data suggest that better prognostic markers, particularly immune prognostic markers, could assist management of BC. This area requires further study in BC in general, and particularly for luminal B BC.
An area that is poorly studied is whether immune responses occurring in tumor-draining lymph nodes affect patient outcomes. We hypothesize that tumor-draining lymph nodes will be impacted during disease progression and treatment, and that some intra-nodal changes will represent potential prognostic markers, as evidenced by the mouse studies in BC described above and our studies in lung cancerCitation9 and mesotheliomaCitation31; .
Negative immune regulators and breast cancer
Cancer cells modify their tissue environment by secreting factors and expressing molecules that suppress the function of effector immune cells, such as CD8+ CTLs and CD4+ Th-1 cells, and promote expansion of suppressive cells such as CD4+CD25+Foxp3+ TregsCitation32,33, myeloid-derived suppressor cells (MDSCs)Citation34 and macrophages skewed toward an anti-inflammtory, pro-wound healing phenotype, termed alternatively-activated or M2 macrophages.Citation35 Production of immunosuppressive molecules and overexpression of negative regulatory molecules by tumor cells, M2 TAMs, MDSCs and Tregs stimulate immune and other tissue cells to increase expression of inhibitory surface receptors such as, but not limited to, checkpoint molecules. Using mesothelioma as a model, we have shown that tumor-conditioned media generates M2-like macrophages that suppress T cell function. Our murine studies revealed that M2 macrophages dominate tumor-draining lymph nodes and that a mixed M1/M2 (termed M3) macrophage subset with suppressive function dominates the tumor microenvironment.Citation36 Thus, ultimately, tumors create a local and regional environment that is not permissive to effector T cell function ().
Negative immune regulatory molecules include a wide range of soluble factors, cell surface and intracellular molecules that in healthy people function to prevent excessive inflammation and dampen responses that could induce autoimmunity. Tumor cells can take advantage of these pathways thereby inactivating antitumor effector immune cells and allowing disease progression. Recently, a family of molecules referred to as immune checkpoint molecules has been described, with new members being reported. In cancer patients, some checkpoint molecules may be over-expressed on immune cells, tumor cells and other tissue cells. Clinical understanding and application is greatest for cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and programmed cell death (PD)-1 and its ligands, PD-L1 and PD-L2. Successful clinical trials using antagonist antibodies targeting these molecules has invigorated interest in the role of the immune system in cancer and in anticancer immunotherapies.
CTLA-4 on T cells ligates CD28, CD80 and/or CD86 on APCs such as DCs. Ligating CD28 stimulates effector T cells, which is crucial for eliminating cancerous cells. However, CTLA-4 has a higher affinity for CD80 and CD86, which outcompete CD28 thereby limiting T cell responses that could destroy cancerous tissue ().Citation37 Use of CTLA-4 checkpoint inhibitors, i.e., monoclonal antibodies that block CTLA-4 signaling (e.g., Ipilimumab) resulted in response rates of 11.1% to 28.5% in phase III clinical trialsCitation38,39. While tumor responses were exciting, with some patients showing complete tumor resolution for over 5 y, a significant number failed to respond, suggesting other immunosuppressive molecules may confound treatment outcomes.
Table 1. Checkpoint molecules and their ligands.
Ipilimumab has not yet been trialed in BC, however, CTLA-4 can be found at the protein and mRNA level in BC tumors, with weakly positive or negative expression in normal breast tissue.Citation40 Higher mRNA levels were seen in patients with axillary lymph node metastases and higher clinical stage disease, moreover CTLA-4+ T cells from these patients showed blunted responses to stimulation.Citation40 Responses in BC patients to anti-CTLA-4 antibody therapy may be determined by their pre-existing immunity, as mouse studies using the poorly immunogenic mouse 4T1 BC model, showed that anti-CTLA-4 alone had no effect on tumor growth or survival.Citation41 CTLA-4 expression may reflect significant immune suppression and therefore has potential as a marker of prognosis in luminal B BCCitation42 for anti-CTLA-4 treatment and other therapies that rely on a tumor-specific immune response for efficacy, including chemotherapy and newer targeted therapies.
PD-1 on T cells binds PD-L1 and PD-L2 on tumor and APCs causing increased apoptosis of effector CTLs and decreased apoptosis of Tregs resulting in a diminished antitumor T cell response. PD-1 inhibitors block PD-L1/L2-PD-1 interactions, restoring T cell effector function and increasing Treg apoptosis. Response rates of 25–43% for the FDA-approved anti-PD-1 monoclonal antibodies have been seen in metastatic melanoma ().Citation43-47
PD-1, PD-L1 and PD-L2 RNA and protein have been detected in BC tumors.Citation48 PD-1 expression is associated with poor prognosis in breast and epithelial-derived cancers.Citation49 However, likely due to differing techniques the data are inconsistent. PD-1+ TILs have been shown in 15.8% of tested BC cases with subset analyses showing PD-1+ TILs were associated with significantly worse overall survival in luminal B HER-2− and HER-2+ BC.Citation50 Others have shown that the concomitant presence of FOXP3+ Tregs, CD80+ and PD-1+ TILs correlates with high histological grade, ER− status and a severe lymphocytic infiltration.Citation51 Higher PD-1 and PD-L1 RNA levels were seen in TNBC and HER-2+ tumors compared with luminal A and luminal B HER-2− tumors; expression levels increased with tumor grade suggesting increasing immune suppression with disease progression.Citation52 Studies are currently ongoing to evaluate anti-PD-1 and anti-PD-L1 antibodies in metastatic BC.
Interestingly, PD-1− tumors have responded to PD-1/PD-L1 blockadeCitation53 implying that tumors may not be the key sites of PD-1/PD-L1 interactions, or that an unidentified mechanism is operating. PD-1/PD-L1 interactions are also likely to occur in tumor-draining lymph nodes that have not yet been extensively assessed in many cancers, including BC subtypes. Moreover, a threshold level has not been set as inclusion criteria for treatment with PD-1 or PD-L1 in any cancer, and even low levels as reported in luminal B BC might be sufficient justification for a clinical trial. Thus, expression of PD-1 and its ligands in tumor or lymph nodes may be useful prognostic/predictive markers in luminal B BC for PD-1/PD-L1/L2 treatment studies, and may reveal a suppressive factor that confounds the success of other therapies.
T cell immunoglobulin and mucin protein-3 (TIM-3) is found mainly on activated Th-1 cells that assist CTL activationCitation54,55 TIM-3s controversial putative ligand, Gal-9, is expressed on many cell types, including immune cells (). TIM-3/Gal-9 interactions, or TIM-3 interactions with other as yet unknown ligands, lead to Th-1 cell apoptosis preventing autoimmune responses in healthy people. However, in cancer patients Gal-9 and TIM-3 expression is increased, causing excessive Th-1 cell death and immunosuppression that prevents tumor cell elimination.Citation56 Gal-9 receptors can also mediate direct apoptotic activity as TIM-3 knockout mice exhibited Th-1 cell apoptosisCitation57,58, and no other ligand has been identified. The Gal-9-TIM-3 pathway also suppresses Th-17 cells, leading to decreased production of pro-inflammatory cytokines ().Citation59 Gal-9 expression by endothelial cells enables cytolysis of immune cells before they infiltrate tumors.Citation60 Moreover, Gal-9 promotes Treg proliferationCitation61 and tumor metastasis by facilitating tumor-endothelial cell interactionsCitation58. The role of members of the galectin family including Gal-9 in BC subtypes is unclear although, its expression on stromal cells has been reported in TNBC and HER-2+ subtypes.Citation62
Blocking TIM-3 and Gal-9 with antagonistic monoclonal antibodies may yield clinical outcomes similar or better than the anti-CTLA-4 or PD-1 antibodies, and the success of TIM-3 inhibitors in pre-clinical models is promising. For example, TIM-3 inhibitors induced tumor regression in a BC mouse model with improved responses when combined with PD-1 and CTLA-4 inhibitors.Citation63,64 No studies using Gal-9 antagonists have been yet been reported. Nonetheless, TIM-3 and Gal-9s immunosuppressive activity suggest their presence may indicate significant immune suppression in association with increased metastatic risk, and that expression of either or both molecules may be associated with a poorer antitumor immune response and poorer long-term prognosis in luminal B BC independent of any treatment given.
There are numerous other molecules including those secreted by tumor or myeloid cells that confound antitumor immunity and increase the risk of metastasis. TGF-β, IL-10 and VEGF are examples of molecules produced by tumor cells and TAMs in BCCitation65,66 that contribute to immune suppression. They stimulate MDSCs that promote Treg development and tumor angiogenesis, suppress APCs and produce reactive oxygen species that cause T cell apoptosis. IL-10 downregulates macrophage production of IL-12 an effector T cell-stimulating molecule, and decreases myeloid cell MHC class II expression leading to decreased antigen presentation that decreases T cell activity.Citation67 Expansion of MDSCs has been reported in BC and is associated with poorer prognosisCitation68, therefore identifying MDSCs and/or molecules responsible for MDSC expansion may be of prognostic value ().
The way ahead
Reliable prognostic markers for the luminal B subtype, which accounts for up to 39% of BC cases, are lacking. Gene expression profiles such as Oncotype DX and MammaprintCitation3 do not provide prognostic evaluation of the luminal B subtype. Further, there are no biomarkers available to predict treatment outcomes in luminal B patients. Thus, it is currently not possible to individualize choice of systemic therapy (chemotherapy and/or endocrine treatment) for these patients in the adjuvant setting. Many luminal B patients do not respond well to traditional chemotherapies and may have a higher 10-y mortality rate despite adjuvant treatment than luminal A or TNBC patients.Citation7,69 It is possible that immune suppression accounts for these outcomes and that suppressive immune cells and molecules represent new prognostic markers for this BC subtype.
Thus far, no checkpoint inhibitor clinical trial has been conducted for luminal B BC. Moreover, even in melanoma, believed to be a highly immunogenic cancer, only a proportion (20–40%) of melanoma patients responded to anti-CTLA-4 and/or anti-PD-1 therapyCitation38,44,70 indicating that a significant percentage of patients do not respond to immune therapies, or conversely, rapidly they acquire resistance. These data suggests that there are more immunosuppressive molecules with potential as prognostic markers for melanoma, and other cancers. Prognostic markers enabling selection of patients who experience durable immune responses to these and other therapies will likely improve therapeutic utility. CTLA-4, PD-1 and other inhibitory checkpoint molecules are therefore attractive study targets for BC. The cell types on which checkpoint molecules are expressed may be important when determining prognosis. For example, tumor-infiltrating CD4+ T cells express CTLA-4 and TIM-3Citation71, while CD8+ T cells express PD-1.Citation72 Tumor-infiltrating CD68+ macrophages may express PD-L1 and Gal-9, and TAMs have been associated with poor outcomes in TNBCCitation73,74, this is likely to be true for other BC subtypes. In contrast, the level of TILs, in particular CD8+ CTLs, has been shown to have prognostic value and potential for predictive value in TNBC and HER-2+ BC patients undergoing neoadjuvant chemotherapy. The localization of these markers can be examined through histochemical assessment. In the future, as costs reduce, molecular/genetic/multiplexed approaches appear promising because reproducibility of correlations among immune-relevant metagenes was highest in BC followed by colorectal cancer, non-small cell lung cancer and melanoma.Citation75
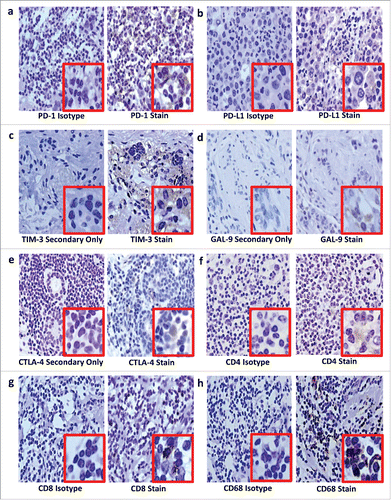
It is not yet clear if expression of immune suppressive markers is best measured in tumors and/or in regional lymph nodes for use as prognostic markers. Haematoxylin and eosin (H&E) and immunohistochemical analyses of tumor and lymph node samples can be used to determine which immune cells and their secreted factors are important in BC subtypes. However, to be meaningful, an internationally standardized methodology for examining immune cells in tumors and lymph nodes is required, although considerable efforts are underway.Citation76 Pitfalls include the following: subjectivity when selecting areas for analysis, a lack of standardization of the number of cells that should be counted per sample and even the thickness of sections, all of which could mean important areas are overlooked. Strict criteria are required to address these issues, as well as for selecting markers, determining threshold levels and standardizing staining protocols to determine which specific monoclonal antibodies and controls should be used. We are evaluating our staining protocol on Luminal B BC tissue and show detectable PD-1, PD-L1, TIM-3, Gal-9, CTLA-4, CD4+, CD8+ and CD68 (). Preliminary data in a small Luminal B patient cohort suggest PD-L1, Gal-9 and CD68 expression are associated with faster time to relapse, regardless of treatment (data not shown); further studies are required.
Figure 2. Immunohistochemical staining on luminal B breast cancer tissue for PD-1 (a), PD-L1 (b), TIM-3 (c), GAL-9 (d), CTLA-4 (e), CD4+ (f), CD8+ (g) and CD68 (h). After antigen retrieval on deparaffinized rehydrated sections, endogenous peroxidases were blocked using hydrogen peroxide (Riedel-de Haën, Seelze, Germany) while endogenous avidin and biotin were blocked using an avidin/biotin blocking kit (Invitrogen, Camarillo, USA). Sections were sequentially incubated with a protein block containing fetal calf serum, bovine serum albumin and human plasma to prevent background staining; then incubated with unconjugated primary antibodies or isotype controls; washed; linked first to biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, USA) or biotinylated goat anti-mouse IgG1 (Biolegend, San Diego, USA) secondary antibodies, then to streptavidin-conjugated horseradish peroxidase. Staining was visualized using hydrogen peroxide in 3,3-diaminobenzidine (Sigma Aldrich, St Louis, USA) counterstained with Harris' haematoxylin. Sections were dehydrated before mounting with Entellan mounting media (Merck Millipore, Darmstadt, Germany). In place of isotype controls for the polyclonal rabbit primary antibodies, TIM-3 (c), GAL-9 (d) and CTLA-4 (e), a secondary antibody-only stain was prepared. Curtin University (Perth Western Australia) and Bellberry (South Australia) Human Ethics Committees approved this study (approval numbers HR 107/2015 and 2015–03–151, respectively).
We found a lack of standardized staining and analysis for protocols, such as those for Ki67 used to distinguish luminal A and B BC; there is no standardized staining protocol or threshold point and no consensus regarding use of automated image analysis for diagnosis.Citation5,77,78 A similar situation exists for staining TILs in BCCitation76. Even TIL terminology needs to be standardized, and possibly re-evaluated, as intratumoral TILs are defined as lymphocytes in tumor nests that are in contact with each other and tumor cells without intervening stroma, while stromal TILs are dispersed between tumor cells that they do not directly contact.Citation76 Stromal TILs are reported to be a superior and more reproducible parameter despite recognition that stromal and intratumoral TILs are predictive of pathological response to neoadjuvant platinum-based chemotherapy.Citation79
Studies have scored TILs using a variety of semi-quantitative approaches, however, an international TILs working group for BCCitation76 reported that the validity of the modified approach based on a method by Denkert et al.Citation80 provides a superior framework for future standardization. Key points include
TILs should be reported for the stromal compartment.
All mononuclear cells should be scored with exception of polymorphonuclear leukocytes.
Full sections are preferred over biopsies whenever possible.
A full assessment of average TILs in the tumor area should be used. Do not focus on hotspots.
Note that no formal recommendation for a clinically relevant TIL threshold could be given.Citation76
In summary, we suggest that evaluation of markers of immune suppression has prognostic value in luminal B BC. To avoid confounding factors in assessment of the role of the immune system it is vital that the study cohort be carefully selected to minimize variation in Luminal B BC patients' immune systems, e.g., restricted age rangeCitation15 and absence of comorbid conditions which may affect immune status. Further, utilization of international standardized criteria for histological analysis is vital for interpretation of the results.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- IARC. In Stewart BW, Wild CP, eds. World Cancer Report 2014. Lyon, France: World Health Organisation 2014:16-27.
- Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med 2009; 360:790-800; PMID:19228622; http://dx.doi.org/10.1056/NEJMra0801289
- Perou CM, Borresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harbor Perspectives in Biol 2011; 3:1-17; http://dx.doi.org/10.1101/cshperspect.a003293
- Fisher ER, Fisher B, Sass R, Wickerham L. Pathologic findings from the national surgical adjuvant breast project (Protocol No. 4). XI. Bilateral breast cancer. Cancer 1984; 54:3002-11; PMID:6498774; http://dx.doi.org/10.1002/1097-0142(19841215)54:12%3c3002::AID-CNCR2820541231%3e3.0.CO;2-V
- de Azambuja E, Cardoso F, de Castro G, Jr., Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007; 96:1504-13; PMID:17453008; http://dx.doi.org/10.1038/sj.bjc.6603756
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98:10869-74; PMID:11553815; http://dx.doi.org/10.1073/pnas.191367098
- Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, Bernstein L, Enger SM, Press MF. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev 2012; 21:1848-55; PMID:22989461; http://dx.doi.org/10.1158/1055-9965.EPI-12-0474
- Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: Results from international breast cancer study group trials VIII and IX. J Clin Oncol 2013; 31:3083-90; PMID:23897954; http://dx.doi.org/10.1200/JCO.2012.46.1574
- Nelson DJ, Mukherjee S, Bundell C, Fisher S, van Hagen D, Robinson B. Tumor progression despite efficient tumor antigen cross-presentation and effective "arming" of tumor antigen-specific CTL. J Immunol 2001; 166:5557-66; PMID:11313395; http://dx.doi.org/10.4049/jimmunol.166.9.5557
- Jackaman C, Nelson DJ. Are macrophages, myeloid derived suppressor cells and neutrophils mediators of local suppression in healthy and cancerous tissues in aging hosts?. Exp Gerontol 2014; 54:53-7; PMID:24291067; http://dx.doi.org/10.1016/j.exger.2013.11.009
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Criscitiello C, Curigliano G. Immunotherapy of Breast Cancer. Prog Tumor Res 2015; 42:30-43; PMID:26377084; http://dx.doi.org/10.1159/000437183
- Miller LD, Chou JA, Black MA, Print C, Chifman J, Alistar A, Putti T, Zhou X, Bedognetti D, Hendrickx W et al. Immunogenic subtypes of breast cancer delineated by gene classifiers of immune responsiveness. Cancer Immunol Res 2016; 4:600-10; PMID:27197066; http://dx.doi.org/10.1158/2326-6066.CIR-15-0149
- Casbas-Hernandez P, Sun X, Roman-Perez E, D'Arcy M, Sandhu R, Hishida A, McNaughton KK, Yang XR, Makowski L, Sherman ME et al. Tumor intrinsic subtype is reflected in cancer-adjacent tissue. Cancer Epidemiol Biomarkers Prev 2015; 24:406-14; PMID:25465802; http://dx.doi.org/10.1158/1055-9965.EPI-14-0934
- Jezequel P, Loussouarn D, Guerin-Charbonnel C, Campion L, Vanier A, Gouraud W, Lasla H, Guette C, Valo I, Verrièle V et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: Importance of immune response. Breast Cancer Res 2015; 17:43; PMID:25887482; http://dx.doi.org/10.1186/s13058-015-0550-y
- Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, Semeraro M, Chaix M, Penault-Llorca F, Arnould L et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 2016; 12:864-75; PMID:26979828; http://dx.doi.org/10.1080/15548627.2016.1154244
- Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol 2016; 2:1354-60; PMID:27355489; http://dx.doi.org/10.1001/jamaoncol.2016.1061
- Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis 2009; 14:364-75; PMID:19145485; http://dx.doi.org/10.1007/s10495-008-0303-9
- Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol 2008; 20:545-57; PMID:18573339; http://dx.doi.org/10.1016/j.coi.2008.05.008
- McDonnell AM, Joost Lesterhuis W, Khong A, Nowak AK, Lake RA, Currie AJ, Robinson BW. Restoration of defective cross-presentation in tumors by gemcitabine. Oncoimmunology 2015; 4:e1005501; PMID:26155402; http://dx.doi.org/10.1080/2162402X.2015.1005501
- Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008; 68:4026-30; PMID:18519658; http://dx.doi.org/10.1158/0008-5472.CAN-08-0427
- Kroemer G, Piacentini M. Dying to survive - apoptosis, necroptosis, autophagy as supreme experiments of nature. Int J Dev Biol 2015; 59:5-9; PMID:26374520; http://dx.doi.org/10.1387/ijdb.150167mp
- Sharma A, Ramanjaneyulu A, Ray R, Rajeswari MR. Involvement of high mobility group B proteins in cisplatin-induced cytotoxicity in squamous cell carcinoma of skin. DNA Cell Biol 2009; 28:311-8; PMID:19435426; http://dx.doi.org/10.1089/dna.2009.0851
- Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8+ T cells in vivo. Cancer Immunol Immunother 2012; 61:2343-56; PMID:22714286; http://dx.doi.org/10.1007/s00262-012-1307-4
- Stoll G, Enot D, Mlecnik B, Galon J, Zitvogel L, Kroemer G. Immune-related gene signatures predict the outcome of neoadjuvant chemotherapy. Oncoimmunology 2014; 3:e27884; PMID:24790795; http://dx.doi.org/10.4161/onci.27884
- van Rooijen JM, Stutvoet TS, Schroder CP, de Vries EG. Immunotherapeutic options on the horizon in breast cancer treatment. Pharmacol Ther 2015; 156:90-101; PMID:26388292; http://dx.doi.org/10.1016/j.pharmthera.2015.09.003
- Karn T, Pusztai L, Rody A, Holtrich U, Becker S. The influence of host factors on the prognosis of breast cancer: Stroma and immune cell components as cancer biomarkers. Curr Cancer Drug Targets 2015; 15:652-64; PMID:26452382; http://dx.doi.org/10.2174/156800961508151001101209
- Maley CC, Koelble K, Natrajan R, Aktipis A, Yuan Y. An ecological measure of immune-cancer colocalization as a prognostic factor for breast cancer. Breast Cancer Res 2015; 17:131; PMID:26395345; http://dx.doi.org/10.1186/s13058-015-0638-4
- Alistar A, Chou JW, Nagalla S, Black MA, D'Agostino R, Jr., Miller LD. Dual roles for immune metagenes in breast cancer prognosis and therapy prediction. Genome Med 2014; 6:80; PMID:25419236; http://dx.doi.org/10.1186/s13073-014-0080-8
- Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011; 71:4809-20; PMID:21646474; http://dx.doi.org/10.1158/0008-5472.CAN-11-0753
- Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: A novel mechanism for IL-2. J Immunol 2003; 171:5051-63; PMID:14607902; http://dx.doi.org/10.4049/jimmunol.171.10.5051
- Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Seminars Cancer Biol 2006; 16:115-23; http://dx.doi.org/10.1016/j.semcancer.2005.11.005
- Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol 2015; 33:101-11; PMID:25728990; http://dx.doi.org/10.1016/j.coi.2015.02.003
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer 2006; 42:717-27; PMID:16520032; http://dx.doi.org/10.1016/j.ejca.2006.01.003
- Jackaman C, Yeoh TL, Acuil ML, Gardner JK, Nelson DJ. Murine mesothelioma induces locally-proliferating IL-10(+)TNF-alpha(+)CD206(-)CX3CR1(+) M3 macrophages that can be selectively depleted by chemotherapy or immunotherapy. Oncoimmunology 2016; 5:e1173299; PMID:27471652; http://dx.doi.org/10.1080/2162402X.2016.1173299
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001; 19:565-94; http://dx.doi.org/10.1146/annurev.immunol.19.1.565
- Fellner C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. P & T 2012; 37:503-30.
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11:155-64; PMID:20004617; http://dx.doi.org/10.1016/S1470-2045(09)70334-1
- Mao H, Zhang L, Yang Y, Zuo W, Bi Y, Gao W, Deng B, Sun J, Shao Q, Qu X. New insights of CTLA-4 into its biological function in breast cancer. Curr Cancer Drug Targets 2010; 10:728-36; PMID:20578982; http://dx.doi.org/10.2174/156800910793605811
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11:728-34; PMID:15701862
- Brown SD, Warren RL, Gibb EA, Martin SD, Spinelli JJ, Nelson BH, Holt RA. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res 2014; 24:743-50; PMID:24782321; http://dx.doi.org/10.1101/gr.165985.113
- Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016; 34:1510-7; PMID:26951310; http://dx.doi.org/10.1200/JCO.2015.64.0391
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Cha E, Wallin J, Kowanetz M. PD-L1 inhibition with MPDL3280A for solid tumors. Semin Oncol 2015; 42:484-7; PMID:25965367; http://dx.doi.org/10.1053/j.seminoncol.2015.02.002
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558-62; PMID:25428503; http://dx.doi.org/10.1038/nature13904
- Ibrahim R, Stewart R, Shalabi A. PD-L1 blockade for cancer treatment: MEDI4736. Semin Oncol 2015; 42:474-83; PMID:25965366; http://dx.doi.org/10.1053/j.seminoncol.2015.02.007
- Duchnowska R, Peksa R, Radecka B, Mandat T, Trojanowski T, Jarosz B, Czartoryska-Arłukowicz B, Olszewski WP, Och W, Kalinka-Warzocha E et al. Immune response in breast cancer brain metastases and their microenvironment: The role of the PD-1/PD-L axis. Breast Cancer Res 2016; 18:43; PMID:27117582; http://dx.doi.org/10.1186/s13058-016-0702-8
- Sun S, Fei X, Mao Y, Wang X, Garfield DH, Huang O, Wang J, Yuan F, Sun L, Yu Q et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother 2014; 63:395-406; PMID:24514954; http://dx.doi.org/10.1007/s00262-014-1519-x
- Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2013; 139:667-76; PMID:23756627; http://dx.doi.org/10.1007/s10549-013-2581-3
- Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer 2008; 8:57; PMID:18294387; http://dx.doi.org/10.1186/1471-2407-8-57
- Zawlik I, Gablo N, Szymanska B, Pawlowska Z, Chudobinski C, Chalubinska-Fendler J, Morawiec Z, Zielinska-Blizniewska H, Morawiec-Sztandera A, Kolacinska A. Immune checkpoints in aggressive breast cancer subtypes. Neoplasma 2016; 63:768-73; PMID:27468881; http://dx.doi.org/10.4149/neo_2016_514
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2016; 165:35-44; PMID:26997480; http://dx.doi.org/10.1016/j.cell.2016.02.065
- Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski MA. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J Immunol 2012; 188:3745-56; PMID:22422881; http://dx.doi.org/10.4049/jimmunol.1102609
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6:1245-52; PMID:16286920; http://dx.doi.org/10.1038/ni1271
- Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol 2010; 185:1383-92; PMID:20574007; http://dx.doi.org/10.4049/jimmunol.0903275
- Su EW, Bi S, Kane LP. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology 2011; 21:1258-65; PMID:21187321; http://dx.doi.org/10.1093/glycob/cwq214
- Heusschen R, Griffioen AW, Thijssen VL. Galectin-9 in tumor biology: a jack of multiple trades. Biochim Biophys Acta 2013; 1836:177-85; PMID:23648450; http://dx.doi.org/10.1016/j.bbcan.2013.04.006
- Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol 2009; 39:2492-501; PMID:19676072; http://dx.doi.org/10.1002/eji.200939274
- Griffioen AW, Thijssen VL. Galectins in tumor angiogenesis. Ann Transl Med 2014; 2:90; PMID:25405165; http://dx.doi.org/10.3978/j.issn.2305-5839.2014.09.01
- Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014; 41:270-82; PMID:25065622; http://dx.doi.org/10.1016/j.immuni.2014.06.011
- Grosset AA, Labrie M, Vladoiu MC, Yousef EM, Gaboury L, St-Pierre Y. Galectin signatures contribute to the heterogeneity of breast cancer and provide new prognostic information and therapeutic targets. Oncotarget 2016; 7:18183-203; PMID:26933916; http://dx.doi.org/10.18632/oncotarget.7784
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207:2187-94; PMID:20819927; http://dx.doi.org/10.1084/jem.20100643
- Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res 2011; 71:3540-51; PMID:21430066; http://dx.doi.org/10.1158/0008-5472.CAN-11-0096
- Kim OH, Kang GH, Noh H, Cha JY, Lee HJ, Yoon JH, Mamura M, Nam JS, Lee DH, Kim YA et al. Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes. Mol Cells 2013; 36:432-8; PMID:24158612; http://dx.doi.org/10.1007/s10059-013-0194-7
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett 2008; 267:204-15; PMID:18448242; http://dx.doi.org/10.1016/j.canlet.2008.03.028
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179:977-83; PMID:17617589; http://dx.doi.org/10.4049/jimmunol.179.2.977
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58:49-59; PMID:18446337; http://dx.doi.org/10.1007/s00262-008-0523-4
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A 2003; 100:10393-8; PMID:12917485; http://dx.doi.org/10.1073/pnas.1732912100
- Wolchok JD, Weber JS, Hamid O, Lebbe C, Maio M, Schadendorf D, de Pril V, Heller K, Chen TT, Ibrahim R et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer immun 2010; 10:9; PMID:20957980
- Jago CB, Yates J, Camara NO, Lechler RI, Lombardi G. Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol 2004; 136:463-71; PMID:15147348; http://dx.doi.org/10.1111/j.1365-2249.2004.02478.x
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537-44; PMID:19423728; http://dx.doi.org/10.1182/blood-2008-12-195792
- Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015; 7:1120-34; PMID:25959051; http://dx.doi.org/10.1039/C5IB00040H
- Carrio R, Koru-Sengul T, Miao F, Gluck S, Lopez O, Selman Y et al. Macrophages as independent prognostic factors in small T1 breast cancers. Oncol Rep 2013; 29:141-8; PMID:23076599; http://dx.doi.org/10.3892/or.2012.2088
- Stoll G, Zitvogel L, Kroemer G. Immune infiltrate in cancer. Aging 2015; 7:358-9; PMID:26143478; http://dx.doi.org/10.18632/aging.100770
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs working group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/10.1093/annonc/mdu450
- Harvey J, Thomas C, Wood B, Hardie M, Dessauvagie B, Combrinck M, Frost FA, Sterrett G. Practical issues concerning the implementation of Ki-67 proliferative index measurement in breast cancer reporting. Pathology 2015; 47:13-20; PMID:25474507; http://dx.doi.org/10.1097/PAT.0000000000000192
- Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: Analytical and critical review. Breast Cancer Res Treat 2012; 132:895-915; PMID:22048814; http://dx.doi.org/10.1007/s10549-011-1837-z
- Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 2013; 16:32-9; PMID:23593079; http://dx.doi.org/10.4048/jbc.2013.16.1.32
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/10.1200/JCO.2009.23.7370
