ABSTRACT
Background: To identify the optimal sequencing and timing of immunotherapy (IT) and stereotactic radiosurgery (SRS) for melanoma brain metastases (MBMs).
Methods: The elapsed days between IT and SRS were correlated with local control (LC), regional brain control (RBC), time to CNS progression (TTPCNS), overall survival (OS), and radiation necrosis (RN). Logistic regression and Cox proportional models were used for statistical analysis.
Results: Twenty-five patients with 58 MBMs underwent SRS and IT. Median follow-up was 22.7 mo (3.1–77.9 mo). A median of 2 SRS treatments of 21 Gy (range 16–24 Gy) and 4 cycles of Ipilimumab were delivered. SRS was delivered Before, After or Concurrently with IT in 9, 5, and 11 patients, respectively; 8/25 received SRS ≤30 d of IT and 17/25 were >30 d of IT. Median OS was 35.8 mo, 1- and 2-y OS was 83% and 64%, respectively, and LC was 94.8%. By timing, RBC and TTPCNS were significantly improved when SRS was delivered ≤30 d of IT (75% vs 23.5%, p = 0.03 and median not reached vs 5.7 mo, p = 0.02, respectively). By groups, Concurrent delivery improved TTPCNS (p = 0.04). The rate of RN was 20.7% (12/58 lesions) and RN was associated with improved OS (HR 0.21, p = 0.01).
Conclusions: High OS was found for MBM treated with SRS and IT compared to historical reports. A significant association for improved RBC and TTPCNS was found when SRS was delivered concurrently and within 30 d of IT. Occurrence of RN was higher than SRS alone series but significantly associated with improved OS.
Introduction
The development of melanoma brain metastases (MBMs) is a devastating complication of metastatic melanoma. MBMs are clinically evident in up to 40% of patients and increase up to 73% on autopsy studies.Citation1,2 Historically, median survival for these patients has been 6.7 mo (range 3–13 mo), varying with the number of cranial metastases and performance status.Citation3 Regional brain control (RBC) has been reported as approximately 35% at 1 y with a reported 3-mo time to progression in the brain.Citation4 Consequently, up to 54% of these patients succumb to their intracranial disease.Citation1
Local treatment of MBM includes surgery, whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS). SRS alone leads to high rate (70–80%) of local control (LC) for intracranial metastasesCitation5 depending on metastases size and number. However, even in selected patients managed with surgery (± postoperative SRS) or SRS alone, median overall survival (OS) still only ranges between 8 and 10 mo.Citation6
Recently, targeted therapy utilizing BRAF and MEK inhibitors or immunotherapy (IT) in the form of checkpoint inhibitors have become the current standard of care for systemic treatment of metastatic melanoma leading to improved survival. Immune checkpoint inhibitors (CTLA-4 inhibitor Ipilimumab; PD-1 inhibitors Pembrolizumab and Nivolumab) assist the patient's immune system in overcoming intrinsic immune-evasive tumor mechanisms, resulting in activation and proliferation of tumor-specific T cells. However, the prognosis of patients with MBM is so poor that most Phase III studies have excluded patients with active CNS disease due to their dismal prognosis and concerns that systemic treatments would not penetrate the blood brain barrier (BBB). Only one phase II trial has been published providing proof of principle of Ipilimumab activity in patients with MBM.Citation7 Indeed, activated T cells have now been proven to penetrate the BBB,Citation8 suggesting immunostimulatory treatments can be a potentially effective treatment against MBM.
Furthermore, standard fractionated radiotherapy (RT) does not appear to trigger antigenic signaling, but the combination of high dose per fraction RT (such as SRS or stereotactic body radiotherapy, SBRT) and IT results in increased antigen targeting, decreased numbers of inhibitory myeloid-derived suppressor cells, and increased levels circulating activated T cells.Citation9-12 Moreover, the phenomenon of distant tumor shrinkage in unirradiated sites after radiation, known as the abscopal effect, is modulated by the immune system, potentially via cytotoxic T lymphocytes (CTLs).Citation13,14 Although abscopal responses have been reported in metastatic melanoma for extracranial sites, it is unknown if privileged sites (e.g., within the BBB) can undergo “local” abscopal effects (i.e., long-term RBC improvement with optimized timing of SRS and IT).Citation9,15-19
Several retrospective studies have already reported that combining IT with high dose/fraction radiation for MBM improves outcomes particularly when SRS is delivered before or during IT thereby providing an antigenic “primer” for ctivated T cells.Citation4,20-25 Others suggest a range of time during which concurrent treatment may be helpful, such as ipilimumab infusion within 14 d or within 4 mo of SRS.Citation22 However, the exact sequencing and timing of the two modalities to achieve maximum benefit are still unclear. Therefore, in this investigation, we sought to identify if an optimal window of time for IT/SRS co-administration exists that leads to improved brain control. Thus we retrospectively evaluated patients with MBM treated with SRS and we analyzed these patients by groups (Before, After, Concurrent) and by a timing cutoff (≤ 30 d or > 30 d). To our knowledge, is the first study to analyze the timing effect based on specific groups and timing cut-points.
Methods
Patient selection and treatment
Between 2011 and 2015, 25 patients with MBM were treated with SRS and received anti-CTLA-4 agent (Ipilimumab, Ipi). These patients, harboring 58 MBM, were treated on a Novalis® linear accelerator platform (BrainLab; Munich, Germany). Lesions were treated with a SRS median dose of 21 Gy (16–24 Gy) to the planning target volume (PTV) following guidelines based on the Radiation Therapy Oncology Group trial 90-05 (). All 25 patients received Ipi at a median dose of 3 mg/kg (range 3–10 mg/kg) every 3 weeks for a median of four cycles (range 1–13). At the time of systemic disease progression, six patients received Pembrolizumab (Pembro) at 2–10 mg/kg every 2 or 3 weeks (median 13 cycles, range 3–37); no patients received Pembro alone, and as such, data analysis was not done in relation to Pembro and SRS timing ().
Table 1. Patient demographics and treatment characteristics.
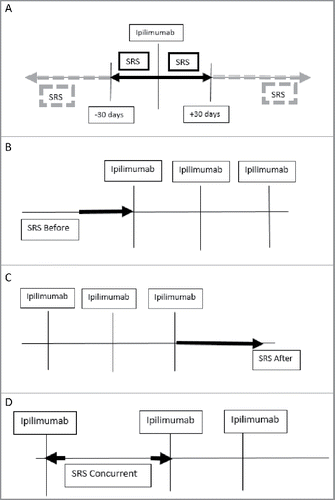
Patients were divided into groups based on the timing of SRS in relation to IT infusion (SRS Before IT vs After vs Concurrent). A secondary analysis was done based on the elapsed time between the two treatments with a cutoff of SRS occurring within ≤30 d of IT > 30 d of IT ().
Figure 1. Schema for grouping and timing. (A) Patients that had SRS ±30 d of Ipilimumab infusion (solid box) vs >30 d (dashed box) from Ipilimumab infusion were assessed. Additional analysis was conducted on patients who had (B) SRS Before (C) After or (D) Concurrently (interdigitated) with Ipilimumab.
At the time of failure (brain or systemic), patients received additional treatment. A small cohort (n = 6) received Pembro after systemic failure or additional radiation as detailed in : 7/25 patients received WBRT (5/7 as salvage therapy at a median of 4 mo after first SRS, and 2/7 received planned, up-front WBRT at diagnosis of MBM). Of note, 11 patients had received additional systemic therapy before SRS, including IL-2 (n = 1), Vemurafenib (n = 3), Temodar (n = 5), and IFN-alpha (n = 4).
Table 2. Characteristics of patients that received whole brain radiotherapy (WBRT).
Intracranial response assessment
T1-weighted, gadolinium-enhanced magnetic resonance images (MRI) with 1-mm slice thickness of the whole brain were obtained prior to SRS treatment and at each follow-up approximately every 6–12 weeks. The TTPCNS was defined as time to first intracranial progression of any kind. Radiation necrosis (RN) was defined based on neuroradiology interpretation of sequential scans with perfusion imaging and multidisciplinary review; time to RN was backdated from the date of consensus agreement of RN to date of first concerning finding. RECIST 1.1 criteria were used to score CNS and systemic imaging outcomes by CR (complete response), PR (partial response), SD (stable disease) or PD (progressive disease).Citation26
Statistical analysis
The impact of SRS groups (Before, After or Concurrent to IT) as well as timing (≤ 30 days, > 30 days) on four endpoints (RBC, TTPCNS, OS and RN) was assessed. For LC, RBC and RN, a Logistic Regression model was used to test the association of each of these parameters against time to infusion. The TTPCNS, RBC and OS were estimated using the Kaplan–Meier method. The association of OS with CNS progression and RN and the association of TTPCNS and RBC with timing of SRS and IT were assessed using a Cox proportional hazard model. The hazard ratio (HR) and 95% confidence interval (CI) were presented. The p-value < 0.05 was considered significant. All analyses were conducted using SAS V9.1.2 software (Cary, NC).
Results
Local control of SRS treated lesions
With a median follow up of 22.7 mo (range 3–78 mo), an LC of 94.8% (55/58) of the treated lesions was observed, with a CR rate of 38% in 22 lesions, occurring at a median of 5.4 mo (range 1.7–20.5 mo). A PR was seen in 18 (31%), SD in 15 (26%), while only 3 lesions (5%) had local failure at a median time of 9.1 mo after SRS (range 2–43 mo). LC was not, however, associated with improved OS (p = 0.54) based on subgroup analysis. Of note, 97% of lesions were in-tact and 3% were post-operative cavities.
Effect of sequencing and timing of SRS with IT on time to treatment progression and regional brain control
When evaluated by groups, 9 patients received SRS Before IT (median 119 d), 5 had SRS After IT (median 52 d), and 11 patients had SRS Concurrently with IT (median 8 d). When considering all 25 patients, the median shortest elapsed time between SRS administration and IT infusion was done 14 d prior to IT.
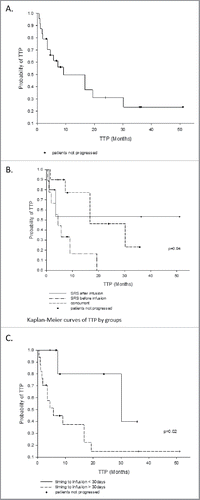
The median TTPCNS for all patients was 16.7 mo (). However, the concurrent delivery of SRS and ipilimumab was significantly associated with improved TTPCNS compared to the other two groups (p = 0.04) When comparing the Concurrent vs Before groups, a significant difference was noted with a median TTPCNS of 30.2 mo vs 4.5 mo, respectively (p = 0.02). Furthermore, TTPCNS for delivery of SRS within ≤30 d of Ipilimumab was significantly better than >30 d (p = 0.02, HR 5.09).
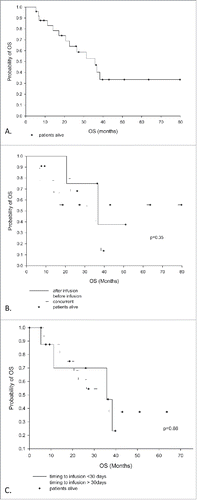
Figure 2. Time to progression in the CNS. (A) All patients, median time to progression = 16.7 mo. (B) By groups: the Concurrent group is statistically better than the Before and After groups, p = 0.04), and (C) by timing of SRS delivery: ≤ 30 d is statistically better than > 30 d, p = 0.02.
RBC was similar between the groups (p = 0.08) with a `1- and 2-y control rates of 52.2% and 34.8%, respectively. When analyzed by timing of administration, however, RBC was significantly improved when SRS occurred ≤ 30 d of IT (75% vs 23.5%, p = 0.03). The 1-y RBC for patients with SRS ≤ 30 d was 83.3% vs 37.4% if there were >30 d between SRS and IT; the 2-y RBC rates were, respectively, 83.3% vs 15%. At last follow up, 15/25 patients had failed intracranially after the initial SRS. Salvage treatment consisted of either repeat SRS to the new lesions (n = 8), WBRT (n = 5), new systemic therapy (n = 5), and one patient opted for hospice.
Overall survival and disease progression
At time of last follow up, 48% (12/25) patients were alive with a median OS of 35.8 mo from date of first SRS to last known follow up. The 1- and 2-y OS rates were 83% and 64%, respectively (). There was no effect of sequencing or timing of SRS/IT on overall survival. At last follow up, 44% of patients had stable systemic disease, 4% had a partial response, and 52% had progressive systemic disease. Six patients received Pembro after systemic progression on Ipilimumab. Despite failing extracranially, this cohort of six patients had an improved TTPCNS compared to those who never received Pembro (HR 0.21, p = 0.03). This group incidentally had a shorter median time between treatments (7.7 d before IT vs 19 d before IT in the non-Pembro group).
Overall survival and radiation necrosis
Radiographic RN was seen in 12/58 treated lesions (20.7%) in nine patients. The median dose for lesions with RN was 21 Gy (range 16 −24 Gy) and the median time to onset of RN was 14.7 mo (range 8.1–71.7 mo). Symptomatic RN occurred in only 5% of treated lesions, requiring treatment with bevacizumab (Avastin). The presence of RN, however, was associated with an improved survival with a HR of death of 0.21 (CI 0.05–0.77, p = 0.01). Patients without RN had a median OS of 22.6 mo, while the median OS of those with RN was not reached.
Discussion
This investigation has found that concurrent delivery of SRS within 1 mo of Ipi significantly improved the time to intracranial progression and thereby significantly improved regional brain control in patients with MBM. It also reports the longest median OS to date of 35.8 mo in these poor prognosis patients. Regarding timing, our data suggests an optimal window for the co-administration of SRS and IT, with SRS interdigitated between IT cycles by ≤30 d. We believe this result may be secondary to the synergy between SRS and IT, and mechanistically it could be explained by a “locoregional” abscopal effect in the brain leading to less intracranial recurrence and thus less neurologic death.
The mechanism of this synergy appears to be related to the high dose per fraction RT (SRS). This dosing is thought to be immunogenic and thus “primes” the immune system by improving antigen supply as a direct consequence of melanoma cell death.Citation4 Specifically, high-dose RT optimizes local tumor microenvironment by upregulating MHC class I molecules for antigen presentation to T cells and increases antigen supply via tumor ablation (in situ vaccination). This interaction unmasks previously occult cancer antigens to T cells, generates tumor specific CTLs and increases tumor infiltrating lymphocytes. We postulate that T cells, activated by IT drugs, then penetrate the BBB and neutralize microscopic deposits of tumors throughout the brain, thus increasing the TTPCNS, leading to improved RBC.Citation9-11,27-32
We, along with other authors, believe that this synergy is potentiated by a shorter interval between SRS and IT.Citation22,35 Mechanistic support for this theory comes from a recent study done in mice which demonstrated that when in vivo melanoma tumor cells were treated with Ipi and RT (10-20Gy/1 fraction) together, the mannose-6-phosphate receptor (MPR) was upregulated and tumor cell growth was significantly reduced compared to IT or RT alone (p = 0.0078).Citation33 When tumors expressed more MPR, then the effects of Ipi-induced CTLs came to fruition through the binding of their products (granzymes) to their receptors (MPR), thereby killing more tumor cells. This rapid and transient effect of MPR upregulation begins 24 h after SRS, peaks 3 d later, and normalizes to pre-treatment values by day 7, whereas the half-life of ipilimumab is 14.9 d.Citation34 This suggests that there may be an optimal window of time during which to combine the therapies with SRS hypothetically occurring within −6 days or +15 days after Ipi infusion. In our cohort, the median time between SRS and IT infusion was 14 d and this short interval could have driven our high LC and RBC. Other authors have also previously suggested the theory of an optimal window of co-administration as well. Several retrospective studies have shown that SRS occurring “within 4 mo,” “4 weeks” or “14 d” either before or after IT can reduce the volume of metastases and their results have trended toward improved overall survival; however, statistical significance for OS has been difficult to achieve with small, retrospective cohorts, similarly to our study.Citation22,35
Other potential explanations for the improved survival in this small cohort of patients compared to historic controls are: First, the LC and CR rates achieved were very high, 94.8% and 38%, respectively, which may have led to fewer CNS deaths. Second, additional RT using 5–8 Gy per fraction for extracranial cancer treatment (SBRT) may have played a role in ongoing antigenic priming, as eight of our patients received this modality during their entire treatment course for treatment of systemic disease. Finally, the majority of our patients (60%) had a low burden of extracranial disease at the time of diagnosis of brain metastases perhaps contributing to the lengthening of their OS.
The outcomes reported here are similar to other recent series, although our survival and intracranial control were somewhat higher (). For example, regarding timing, a recent study showed 1-y OS of 42.9% when Ipi was delivered within 14 d of SRS compared to our results of 83% when IT was given within 30 d of SRS.Citation22 Regarding grouping, our results compare favorably with those of Kiess et al.Citation4 who showed a 1-y OS of 65% and a median OS of 12.4 mo. Our data may have been an improvement on Kiess's results because we had a shorter time between treatments and lower extracranial disease burden. Therefore, the data presented in this paper suggests that closer timing of the two modalities potentially allowed for more potent synergy between treatments, leading to better brain control and less death from intracranial progression.
Table 3. Review of recent trials assessing SRS and immunotherapy for the treatment of melanoma brain metastases.
One concern of combined modality treatment is that it may lead to late toxicity in the form of RN. However, the rate of RN in this study was 20.7%, which falls within reported ranges of 6–24% with SRS alone.Citation37,38 In fact, it was lower than the RN rates reported by comparable recent series of GammaKnife Radiosurgery showing rates of 30–37.5%.Citation22,39 Furthermore, only three of our nine patients with RN were symptomatic (5% of lesions), and these patients were successfully treated with Avastin. Interestingly, RN was associated with improved OS in our study, as the patients with RN have not yet reached median OS while those with RN had a median OS of 22.6 mo (HR 0.21, p = 0.01). Our observation on the effect of RN on survival is in line with the results of the Colaco et al. study,Citation39 which also reported higher median OS in the RN group of 23.7 vs 9.9 mo. These are preliminary data that requires more dosimetric and radiographic analysis and will be reported separately.
Limitations of this study include its retrospective, non-randomized nature and small sample size containing biases inherent in retrospective analyses. However, we feel that our study still adds to the body of literature supporting ongoing clinical research investigating radiation and IT timing that maximizes the therapeutic ratio. Finally, systemic treatments for metastatic melanoma are evolving to include patients with brain metastases. Emerging phase II data on 18 MBM patients suggests that Pembro alone can produce a 22% response rate and is safe to use after Ipi failure.Citation40 In fact, PD-1 inhibition in combination with SRS may yield even better responses compared to anti-CTLA-4 inhibition in these poor prognosis patients, and drug choice should be taken into consideration when combining RT and IT as well.Citation36
Conclusions
The data presented here suggests that when SRS is delivered concurrently and within 30 d of Ipilimumab infusion, it leads to improved time to CNS progression and regional brain control in patients with MBMs leading to median survival of about 3 y in this group of poor prognosis patients. The incidence of RN appears similar to SRS alone historic controls, and seems to be associated with a low rate of symptomatic RN and surprisingly long overall survival.
Significant conclusions
When SRS is given concurrently with IT, and more specifically within a 30-d window on either side of IT, time to progression in the brain and therefore regional brain control are significantly improved and potentially contribute to the lengthened overall survival seen in this cohort of patients who historically have poor survival. RN may be higher than with SRS alone in this cohort, but it appears to be associated with improved overall survival.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Conceptualization: TS, SS, HC and BS; methodology: TS and BS; formal analysis: HC; investigation: TS; data curation: TS; writing original draft: TS; writing review and editing: TS, BS, SS and HC; visualization: TS, BS and HC; and supervision: BS.
References
- Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117(8):1687-96; PMID:20960525; http://dx.doi.org/10.1002/cncr.25634
- Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC, Besser M et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004; 22(7):1293-300; PMID:15051777; http://dx.doi.org/10.1200/JCO.2004.08.140
- Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010; 77(3):655-61; PMID:19942357; http://dx.doi.org/10.1016/j.ijrobp.2009.08.025
- Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, Chan TA, Yamada Y, Beal K. Stereotactic radiosurgery for melanoma brain metastases in patients receiving Ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015; 92(2)368-75; PMID:25754629; http://dx.doi.org/10.1016/j.ijrobp.2015.01.004
- Manon R, O'Neill A, Knisley J, Werner-Wasik M, Lazarus HM, Wagner H, Gilbert M, Mehta M, Eastern Cooperative Oncology Group. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an ECOG study (E 6397). J Clin Oncol 2005; 23(34):8870-6; PMID:16314647; http://dx.doi.org/10.1200/JCO.2005.01.8747
- Gibney GT, Forsyth PA, Sondak VK. Melanoma in the brain: biology and therapeutic options. Melanoma Res 2012; 22(3):177-183; PMID:22495668; http://dx.doi.org/10.1097/CMR.0b013e328352dbef
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF et al. Ipilimumab in patients with melanoma and brain metastases: an open-label phase 2 trial. Lancet Oncol 2012; 13(5):459-65; PMID:22456429; http://dx.doi.org/10.1016/S1470-2045(12)70090-6
- Wilson EH, Weninger W, Hunger CA. Trafficking of immune cells in the central nervous system. J Clin Invest 2010; 120(5):1368-79; PMID:20440079; http://dx.doi.org/10.1172/JCI41911
- Postow MA, Callahan MK, Barker CA, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366(10):925-31; PMID:22397654; http://dx.doi.org/10.1056/NEJMoa1112824
- Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85(2):293-5; PMID:22560555; http://dx.doi.org/10.1016/j.ijrobp.2012.03.017
- Twyman-Saint\sVictor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520(7547):373-7; PMID:25754329; http://dx.doi.org/10.1038/nature14292
- Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014; 2(9):831-8; PMID:25187273; http://dx.doi.org/10.1158/2326-6066.CIR-14-0069
- Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol 2015; 26:1813-23; http://dx.doi.org/10.1093/annonc/mdv209
- Wu L, Wu M, De la Maza L, Yun Z, Yu J, Zhao Y, Cho J, de Perrot M. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015; 6(14):12468-12480; PMID:25980578; http://dx.doi.org/10.18632/oncotarget.3487
- Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014; 3:e28780; PMID:25083318; http://dx.doi.org/10.4161/onci.28780
- Johnson CB, Jagsi R. The promise of the abscopal effect and the future of trials combining immunotherapy and radiation therapy. Int J Radiat Oncol Biol Phys 2016; 95(4):1254-56; PMID:27354132; http://dx.doi.org/10.1016/j.ijrobp.2016.02.067
- Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015; 41(6):503-10; PMID:25872878; http://dx.doi.org/10.1016/j.ctrv.2015.03.011
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016; 40(1):25-37; PMID:26582738; http://dx.doi.org/10.1016/j.currproblcancer.2015.10.001
- Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, Ng AK, Hodi FS, Schoenfeld JD. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with Ipilimumab. Oncoimmunology 2015; 4(11):e1046028; PMID:26451318; http://dx.doi.org/10.1080/2162402X.2015.1046028
- Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013; 2(6):899-906; PMID:24403263; http://dx.doi.org/10.1002/cam4.140
- Schoenfeld JD, Mahadevan A, Floyd SR, Dyer MA, Catalano PJ, Alexander BM, McDermott DF, Kaplan ID. Ipilimumab and cranial radiation in metastatic melanoma patients: a case series and review. J Immunother Cancer 2015; 3:50; PMID:26672895; http://dx.doi.org/10.1186/s40425-015-0095-8
- Patel KR, Shoukat S, Oliver DE, Chowdhary M, Rizzo M, Lawson DH, Khosa F, Liu Y, Khan MK. Ipilimumab and Stereotactic Radiosurgery Versus Stereotactic Radiosurgery Alone for Newly Diagnosed Melanoma Brain Metastases. Am J Clin Oncol. 2015 May 16. [Epub ahead of print]. PMID:26017484; http://dx.doi.org/10.1097/COC.0000000000000199
- Knisely JPS, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012; 117(2):227-33; PMID:22702482; http://dx.doi.org/10.3171/2012.5.JNS111929
- Tazi K, Hathaway A, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with Ipilimumab and stereotactic radiosurgery. Cancer Med. 2015; 4(1):1-6; PMID:25164960; http://dx.doi.org/10.1002/cam4.315
- Shoukat S, Marcus D, Rizzo M, Lawson D, Liu Y, Khan M. Outcome with stereotactic radiosurgery (SRS) and ipilimumab (Ipi) for malignant melanoma brain metastases.. J Clin Oncol; ASCO Annual Meeting; 2013. 2013. abstr 3032.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M et al. New response evaluation criteria in solid tumors: revised RECIST guideline (v1.1). Eur J Cancer 2009; 45(2):228-47; PMID:19097774; http://dx.doi.org/10.1016/j.ejca.2008.10.026
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105(4):256-65; PMID:23291374; http://dx.doi.org/10.1093/jnci/djs629
- Levy A, Chargari C, Cheminant M, Simon N, Bourgier C, Deutsch E. Radiation therapy and immunotherapy: implications for a combined cancer treatment. Crit Rev Oncol Hematol 2013; 85(3):278-87; PMID:23036459; http://dx.doi.org/10.1016/j.critrevonc.2012.09.001
- Shahabi V, Postow MA, Tuck D, Wolchok JD. Immune priming of the tumor microenvironment by radiotherapy. Rationale for combination with immunotherapy to improve anticancer efficacy. Am J Clin Oncol 2015; 38(1):90-7; PMID:25616204; http://dx.doi.org/10.1097/COC.0b013e3182868ec8
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58(3):862-70; PMID:14967443; http://dx.doi.org/10.1016/j.ijrobp.2003.09.012
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15(17):5379-88; PMID:19706802; http://dx.doi.org/10.1158/1078-0432.CCR-09-0265
- Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol 2012; 2:95; PMID:22937524; http://dx.doi.org/10.3389/fonc.2012.00095
- Kim S, Ramakrishnan R, Lavilla-Alonso S, Chinnaiyan P, Rao N, Fowler E, Heine J, Gabrilovich DI. Radiation induced autophagy potentiates immunotherapy of cancer via upregulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol Immunother 2014; 63:1009-1021; PMID:24943275; http://dx.doi.org/10.1007/s00262-014-1573-4
- Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008; 26:5950-5956; PMID:19018089; http://dx.doi.org/10.1200/JCO.2008.16.1927
- Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016. Oct;122(19):3051-8. Epub 2016 Jun 10; PMID:27285122; http://dx.doi.org/10.1002/cncr.30138.
- Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ, Yu HH, Etame AB, Weber JS, Gibney GT. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD1 therapy. Ann Oncol 2016; 27(3):434-41; PMID:26712903; http://dx.doi.org/10.1093/annonc/mdv622
- Sneed PK, Mendez J, Fogh SE, Barani IJ, Ma L, McDermott MW et al. Risk factors for radiation necrosis after radiosurgery for brain metastases. Int J Rad Onc Biol Phys 2012; 84(3):118-119; http://dx.doi.org/10.1016/j.ijrobp.2012.07.208
- Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011; 6:48; PMID:21575163; http://dx.doi.org/10.1186/1748-717X-6-48
- Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2015; 125(1):17-23; PMID:26544782; http://dx.doi.org/10.3171/2015.6.JNS142763
- Kluger HM, Goldberg SB, Sznol M, Choueiri TK, Powderly JD, Smith DC, Brahmer JR, Carvajal RD, Hammers HJ, Puzanov I et al. Safety and activity of Pembrolizumab in melanoma patients with untreated brain metastases. J Clin Oncol 2015; 33(18):2013-20; ( suppl; abstr 9009); PMID:25800770; http://dx.doi.org/10.1200/JCO.2014.58.1041