ABSTRACT
The B7 family and tumor necrosis factor receptor (TNFR) superfamily play a vital role in the T-cell co-stimulatory and co-inhibitory pathways, regulating T-cell activation, tolerance, and exhaustion; therapeutic modulation of these pathways is translated into effective new cancer treatments. Better understanding of the immune molecular landscapes of the B7 and TNFR families would guide head and neck immuno-oncology clinical research. We performed comprehensive molecular profiling of 10 B7 and 6 TNFR family members in head and neck cancer. Over 20% of patients had B7 and TNFR gene alterations. B7 gene amplifications were relatively more common (3–11%) than TNFR gene amplifications (0–5%). Analysis of 496 sequenced samples revealed that all genes were upregulated: B7 and TNFR mRNA were upregulated in 158 cases (> 30%) and 83 cases (∼15%), respectively. B7-H1 (PD-L1) mRNA upregulation was the most common (∼10%). Promoter methylation analysis indicated an epigenetic basis for B7 and TNFR gene regulation (especially B7-H1, which was relatively strongly correlated with promoter methylation). B7-H1 expression was significantly associated with worse overall survival, and its expression was increased in cases with gene amplifications. Human papillomavirus (HPV) status correlated significantly with B7-H1 alterations at genetic level. Almost half (47.1%) of HPV-negative patients had deep or shallow B7-H1 deletion; >90% of HPV-positive patients had diploid, copy number gain, or amplification of B7-H1. This is the first study elucidating the immune molecular landscapes of the B7 and TNFR families in head and neck cancer, providing a potential novel rationale for clinical investigations.
Introduction
T-cell co-stimulation plays a vital role in regulating immune responses to promote protective immunity and to prevent autoimmunity. T cells are activated by two classic signals: antigen recognition (signal one), in which T-cell receptors (TCRs) recognize the peptides presented by the major histocompatibility complex (MHC); and co-stimulation (signal 2), involving a combination of co-regulators. These co-regulators consist of both co-stimulatory and co-inhibitory molecules expressed on antigen-presenting cells (APC) binding to their corresponding TCRs.Citation1 The T-cell co-stimulatory and co-inhibitory pathways are key regulators of T-cell activation, tolerance, and exhaustion; therapeutic modulation of these pathways is now a research hotspot, and has been translated into effective new strategies for treating cancer.Citation2 Currently, the co-stimulatory and co-inhibitory pathways mainly involve two major families: the B7 family of immunoglobulinsCitation3 and the tumor necrosis factor receptor (TNFR) superfamily.Citation4
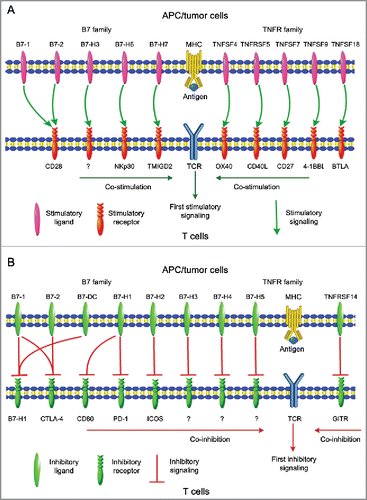
The B7 family has 10 reported members. It has been proven that the well-known co-inhibitory signaling pathways, including CD80 (B7-1) or CD86 (B7-2)/CTLA-4 and PD-L1 (B7-H1)/PD-1, are promising targets in cancer immune checkpoint therapy over the past decade.Citation3 B7-1 and B7-2 also bind to CD28 to provide stimulatory signals.Citation5 Other B7 family members include B7-H3 (provides both stimulatory and inhibitory signals);Citation6 B7-DC, B7-H4, and B7-H5 (inhibit T-cell responses);Citation7,8 and B7-H2, B7-H6, and B7-H7 (activate naive T cells).Citation9-11 At present, it has been determined that six TNFR superfamily proteins expressed by APC or tumor cells function as important secondary signals and may serve as new targets in cancer immunotherapy: OX40L (TNFSF4), CD40 (TNFRSF5), CD70 (TNFSF7), CD137L (TNFSF9), HVEM (TNFRSF14), and GITRL (TNFSF18).Citation2,4,12 With the exception of HVEM, which provides inhibitory signals, the TNFR family members aid T-cell response activation. summarizes the T-cell co-stimulatory and co-inhibitory molecules of the abovementioned members of B7 and TNFR families.
Figure 1. Summary representation of (A) T-cell co-stimulatory and (B) co-inhibitory molecules of the B7 and TNFR family members.
Head and neck squamous cell carcinoma is the sixth most common cancer worldwide; about 80% of cases originate in the oropharynx or oral cavity.Citation13 It has a relatively poor prognosis, and only 60% of patients are expected to survive 5 y.Citation14 Smoking and alcohol drinking are the major risk factors for head and neck cancer, and it was recently shown that human papillomavirus (HPV) infection is an important risk factor and a favorable prognostic factor compared with HPV-negative status in head and neck cancer.Citation15 The complex mutational landscape of head and neck cancer has limited the application of targeted therapies,Citation16 and there is a dire need for more effective therapies. As head and neck cancer is also known for its immunosuppressive character,Citation17 immunotherapy may be a promising strategy; preliminary data from trials on certain immune checkpoint inhibitors (nivolumab and pembrolizumab, which target PD-L1) for treating head and neck cancer are encouraging.Citation18,19
The B7 and TNFR family members and their receptors have great potential as therapeutic targets in head and neck cancer immunotherapy. Therefore, the aim of this study was to analyze the mutation and copy number data, mRNA expression and DNA methylation, and the relevant clinical profiles to elucidate the molecular landscape of the B7 and TNFR families in head and neck cancer using existing, publically available data from cBioPortal for Cancer Genomics.Citation20,21 A better understanding of this immune landscape may aid the development of the rationale for, and thereby guide ongoing clinical research on head and neck immuno-oncology.
Results
Co-signaling molecules of B7 and TNFR families
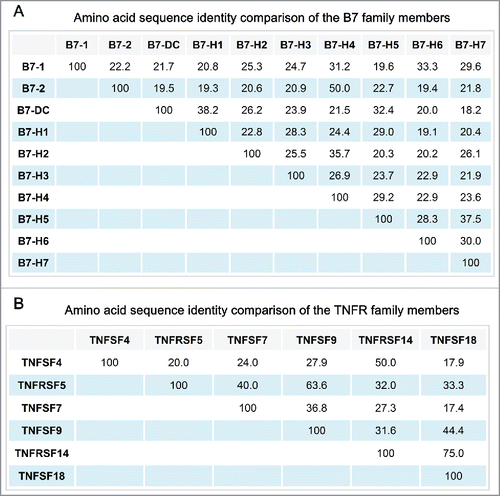
To date, 10 B7 and 6 TNFR family proteins mainly expressed by APC and tumor cells have been identified to play important roles in immunoregulation. We propose a unified nomenclature for each of these proteins by using the gene names as provided by the National Center for Biotechnology Information (NCBI) (Supplementary materials). The 10 B7 family members are B7-1, B7-2, B7-DC, B7-H1, B7-H2, B7-H3, B7-H4, B7-H5, B7-H6, and B7-H7; the 6 TNFR family members are TNFSF4, TNFRSF5, TNFSF7, TNFSF9, TNFRSF14, and TNFSF18. Alignment of B7 protein amino acid sequences indicated that each of the 10 B7 proteins shares about 20% identity, at least, with other B7 family members (); the 6 TNFR proteins also share about 20% identity, at least, with other TNFR family members ().
Determination of B7 and TNFR gene alterations across head and neck cancer studies
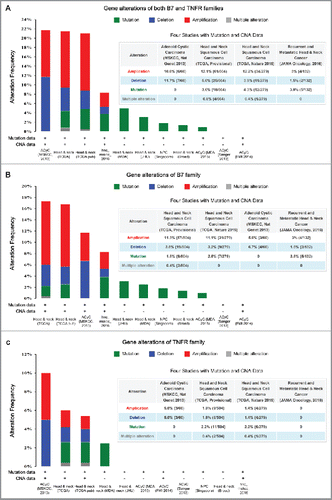
The frequency of B7 and TNFR gene alterations (including mutations, deletions, and amplifications) across 11 studies on head and neck cancer are shown in . In two studies, more than 20% of patients had B7 and TNFR gene alterations (). Generally, the B7 and TNFR mutation rates were both about 2%. B7 amplifications were relatively more common (3–11%) than that of TNFR (0–5%) ( and ). B7 amplifications were more prominent in head and neck squamous cell carcinoma, being amplified in 57 of 504 patients (11.3%) ().
Expression of B7 and TNFR proteins in head and neck cancer
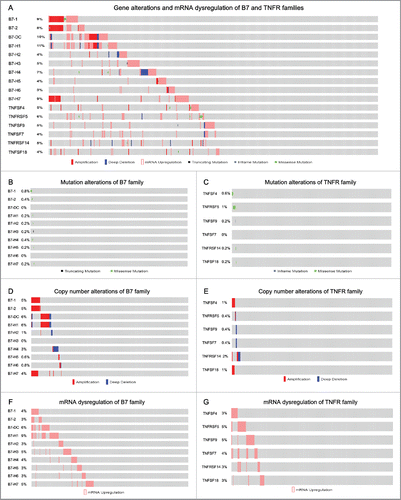
Given the high frequency of B7 and TNFR gene alterations, their expression may also be dysregulated. Therefore, we assessed the mRNA dysregulation of each member of both families across 496 sequenced head and neck cancer samples with complete data from The Cancer Genome Atlas (TCGA), as queried with cBioPortal.Citation20,21 depicts the gene alterations and mRNA dysregulation of the B7 and TNFR family members. For each of the 10 B7 and 6 TNFR genes, mutations were either not observed, or present in about less than 1% of patients ( and ). The frequency of B7-1, B7-2, B7-DC and B7-H1 copy number alterations (CNA) was about 5–6%, while CNA were less common in the TNFR genes ( and ). Interestingly, B7 mRNA levels were upregulated in more than 30% of cases (158/496), while TNFR mRNA was upregulated in about 15% of cases (83/496). It should be noted that all genes were exclusively upregulated; there was no downregulation, and B7-H1 (PD-L1) mRNA was the most frequently upregulated (∼10%), which suggests its important role in head and neck cancer ( and ). Fig. S1 shows the alterations and mRNA dysregulation of each gene from the two families as grouped according to the T-cell regulatory functions.
Figure 4. Gene alterations and mRNA dysregulation of B7 and TNFR family members in 496 head and neck cancer samples. (A) B7 and TNFR gene alterations and mRNA dysregulation. (B) B7 family member mutation alterations. (C) TNFR family member mutation alterations. (D) B7 family member CNA. (E) TNFR family member CNA. (F) B7 family member mRNA dysregulation. (G) TNFR family member mRNA dysregulation. Each gray box along the X-axis represents each individual.
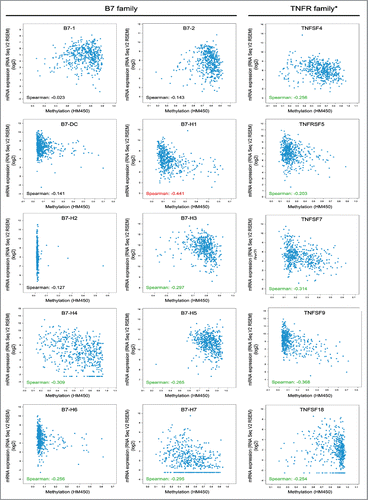
As B7 and TNFR proteins (especially B7 proteins) are constitutively expressed in head and neck cancer, we therefore investigated the correlation between promoter DNA methylation with the mRNA expression level. The mRNA levels of B7-H1, B7-H3, B7-H4, B7-H5, B7-H6, B7-H7, TNFSF4, TNFRSF5, TNFSF7, TNFSF9, and TNFSF18 were negatively correlated with promoter methylation status (). Notably, B7-H1 mRNA level was relatively strongly correlated with promoter methylation (Spearman: −0.441). These results indicate that the expression of most B7 and TNFR family members, especially B7-H1 (PD-L1), may be epigenetically regulated in head and neck cancer.
Figure 5. Correlation between the promoter methylation status and mRNA expression level of the B7 and TNFR families in 496 head and neck cancer samples. Spearman coefficient in red, relatively strong correlation; Spearman coefficient in green, moderate correlation; Spearman coefficient in black, poor correlation. B7-H1 mRNA levels were negatively correlated with promoter methylation status relatively strongly. B7-H3, B7-H4, B7-H5, B7-H6, B7-H7, TNFSF4, TNFRSF5, TNFSF7, TNFSF9, and TNFSF18 mRNA levels were moderately negatively correlated with their promoter methylation status. Promoter methylation status data for TNFRSF14 were unavailable.
B7-H1 as a potential prognostic biomarker in head and neck cancer
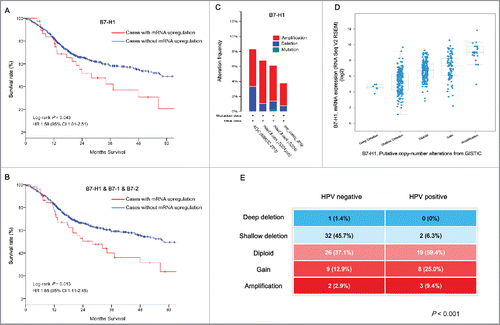
Overall survival was compared between tumor samples with or without mRNA upregulation in each B7 and TNFR family member. Patients with upregulated B7-H1 had significantly poor prognosis (p = 0.043) (); those with upregulated B7-1 and B7-2 tended to have worse overall survival (p = 0.098). Other B7 family members did not show obvious correlations with survival. B7-H1 mainly binds to PD-1, and B7-1 and B7-2 mainly bind to CTLA-4, inhibiting immune responses. Our findings show that B7-H1 mRNA upregulation aided in predicting prognosis in head and neck cancer; B7-1 and B7-2 upregulation were potential prognostic biomarkers. Next, we combined B7-H1, B7-1, and B7-2 as a prognostic factor, and found that they aided better separation of patients with poor treatment outcomes (p= 0.013) (). We also examined whether B7-H1 was altered in four different data sets with cBioPortal CNA data, and found that B7-H1 amplification was common in head and neck cancer, albeit at varying frequencies (). We also found that the levels of B7-H1 mRNA (which were negatively correlated with promoter methylation) were increased gradually in cases with gene alterations (deep and shallow deletions, diploid, copy number gains and amplifications), indicating that it may also be regulated by gene amplification (). Meanwhile, there were no significant differences in B7-H1 mRNA levels between patients with negative and positive HPV status, probably due to the lack of samples with complete data; it is interesting that HPV status significantly correlated with B7-H1 CNA. Almost half (47.1%) of HPV-negative patients had deep or shallow deletion, while more than 90% of HPV-positive patients had diploid, copy number gain, or amplification of B7-H1 (). B7-H1 was altered at the genetic level in HPV-infected patients; B7-H1 expression tended to be higher in HPV-positive tumors, which may account for its evasion of immune recognition. These results suggest that B7-H1 may be a promising target for head and neck cancer immunotherapy.
Figure 6. B7-H1 is a potential biomarker of head and neck cancer. (A) Overall survival of patients with head and neck cancer with upregulated B7-H1 mRNA. (B) Overall survival of patients with head and neck cancer with upregulated B7-H1, B7-1, or B7-2 mRNA. (C) B7-H1 genetic alteration in four studies from cBioPortal. (D) Increased B7-H1 mRNA in head and neck cancer tissues with B7-H1 amplification. (E) Significant correlation between HPV status and B7-H1 CNA.
Discussion
Given the encouraging results of clinical trials evaluating the treatment of advanced cancer with second-generation antibodies such as anti-PD-L1, the B7 and TNFR family members are being closely monitored as potential immunotherapeutic targets in cancer. Currently, head and neck cancer represents one of the most promising areas of immunotherapy research; a rational approach to advancing clinical investigation requires a deeper understanding of the immune landscape of potential novel immune checkpoints. Here, we provide an overview of 10 and 6 members of the B7 and TNFR families, respectively, that function as important secondary signals in a large cohort of head and neck tumors.
We found relatively higher levels of amplification of B7 family members in head and neck cancer. We therefore assessed their genomic alterations; consistent with the amplification results, all B7 family members had increased levels of mRNA expression at varying frequencies, in particular, B7-H1 (PD-L1) mRNA was upregulated most frequently (∼10%). Interestingly, the DNA methylation analysis showed that B7-H1 expression correlated negatively with methylation of its promoter in a relatively strong manner. Taken together with the CNA results, we speculate that both gene amplification and promoter methylation regulate B7-H1 in head and neck cancer. Other B7 family members only had poor or moderate correlation with DNA methylation.
Together with the clinical data, we found that of the B7 family, only B7-H1 (PD-L1) mRNA upregulation was significantly associated with worse overall survival in head and neck cancer, demonstrating its potential role as a predictive biomarker of immunotherapy regimens. PD-L1/PD-1 interaction induces T-cell tolerance,Citation22 and PD-L1 expressed on tumor cells delivers an anti-apoptotic signal that results in resistance to cytotoxic lymphocytes.Citation23 Although the prognostic effect might be less prominent than that of B7-H1, patients with upregulated B7-1 and B7-2 (both bind to CTLA-4 to provide inhibitory signals) also tended to have poor prognosis. Interestingly, combining B7-H1, B7-1, and B7-2 aided in improving the predictive ability. These results indicate that PD-L1 may play a pivotal role in the immunosuppression of head and neck cancer; the effects of CTLA-4 in head and neck cancer should be further explored, and it has the potential to supplement PD-L1 for prognostication and treatment. Some studies have also reported that B7-H1 expression determined by immunohistochemistry may serve as a prognostic marker in head and neck tumors.Citation24,25 Lin et al. found that high PD-L1 expression correlates with distant metastasis and poor survival in oral squamous cell carcinoma;Citation24 Mukaigawa et al. determined that positive PD-L1 expression is significantly associated with poor disease-free survival of salivary gland carcinomas.Citation25 Together, our results support an important role of B7-H1 in the progression of head and neck cancer.
Interestingly, it is worth noting that B7-H1 is more likely to be amplified in HPV-positive patients. Lyford-Pike et al. found that the PD-1/PD-L1 pathway plays an important role in HPV-positive head and neck cancer immune resistance; high PD-L1 expression in tumor cells creates an immune-privileged site for initial viral infection and subsequent adaptive immune resistance.Citation26 Other studies have also observed higher PD-L1 expression in HPV-positive tumor cells.Citation27,28 We demonstrated that B7-H1 is altered at genetic level in HPV-positive patients, supporting the rationale for administering therapies targeting PD-1 or PD-L1 to patients with head and neck cancer. In fact, the most recent trial evaluating pembrolizumab (targets PD-L1) in head and neck cancer reported 25% overall response in HPV-positive patients but only 14% overall response in HPV-negative patients.Citation19 Future studies are needed to elucidate the underlying mechanisms of this phenomenon.
In addition to the B7 family, the pathways controlled by the TNFR superfamily modulate both innate and adaptive immunity, co-stimulating or restricting immune responses. Translation of the fundamental mechanisms of the TNFR family members has led to the designing of new therapeutic agents beyond CTLA-4 and PD-L1/PD-1, such as MEDI6383 (targets OX40), dacetuzumab (targets CD40), and urelumab (targets CD137).Citation2 The TNFR superfamily members are studied less frequently in head and neck cancer. Previous studies have indicated that combining CD137 agonist monoclonal antibody (mAb) and cetuximab may promote more beneficial effects in head and neck cancer treatment;Citation29 the synergy between anti-OX40 and anti-PD-L1 or anti-CTLA-4 is also being tested.Citation17 Here, we found that alteration in the 6 TNFR family members was relatively less common and was not significantly correlated with survival. The role of the TNFR family in immunoregulation in head and neck cancer may be less prominent than that of the B7 family; further studies are warranted to confirm the therapeutic value of the TNFR family members.
The diversity among different data collections, which may be attributed to the differing head and neck cancer subtypes studied, is interesting. Across the 11 data collections, we found that B7 and TNFR mutation rates were relatively higher in studies on head and neck squamous cell carcinoma (∼3% to 5%), but were quite low in studies on adenoid cystic carcinoma (< 1%). The B7 and TNFR mutation rates in adenoid cystic carcinoma were generally low as compared with other adult solid tumors;Citation30 we also observed low mutation frequencies of the B7 and TNFR families, indicating that they may play less prominent roles in the oncogenesis of adenoid cystic carcinoma. Furthermore, it should be noted that there were high frequencies of copy number alterations (amplification and deletion) of the B7 and TNFR families in head and neck squamous cell carcinoma and adenoid cystic carcinoma (∼20%), suggesting their potential roles in affecting disease prognosis by regulating the immune response. The rate of amplifications and deletions in the data collection of recurrent and metastatic head and neck cancers was relatively low (∼5%), probably due to the various cancer subtypes included (e.g., head and neck squamous cell carcinoma, salivary gland carcinoma, and cutaneous carcinoma);Citation31 the change in immune status in recurrent/metastatic head and neck cancers may also contribute to this. In this study, samples with complete TCGA data, which mainly included head and neck squamous cell carcinoma, were queried for the further analyses. Future studies are warranted to confirm our findings in more of the less common subtypes of head and neck cancer, and to explore the more specific roles and underlying mechanisms of the B7 and TNFR families.
In this study, we demonstrated that the B7 family, especially B7-H1, is the major driving force of T-cell co-stimulation and co-inhibition in head and neck cancer. Currently, numerous trials are evaluating immune checkpoint therapy with mAbs directed against PD-1/PD-L1 or CTLA-4 in head and neck cancer. In a recent phase III trial, treatment with nivolumab resulted in longer survival than standard therapy did in patients with platinum-refractory recurrent squamous cell carcinoma of the head and neck.Citation18 A phase Ib trial found that pembrolizumab was well tolerated and demonstrated clinically meaningful antitumor activity in head and neck cancer.Citation19 Furthermore, several trials are being conducted to assess the efficacy of ipilimumab (targets CTLA-4) in head and neck cancer (e.g., NCT01860430, NCT02741570), and the results are expected to be promising. As shown in the present study, HPV-positive patients were likely B7-H1 alteration; accordingly, clinical trials should further explore whether patients with different HPV status might derive different benefits from immune checkpoint therapy. Other B7 family ligands or receptors have subsequently been investigated in various cancers, indicating a bright future for the discovery of more immunotherapeutic agents against head and neck cancer.
The limitation of this study should be acknowledged. We used mainly the cBioPortal for Cancer Genomics database to construct the landscape of the B7 and TNFR families in head and neck cancer. Although the database contains unique data integration issues from large-scale cancer genomic projects, future studies exploring the role of these gene families or other immune-related genes in more datasets or other cancer types are warranted. Nevertheless, this study represents an important step toward forming a comprehensive landscape of the B7 and TNFR families in head and neck cancer, and these findings could help provide indicators for future research.
conclusion, all 10 B7 and 6 TNFR family members are overexpressed in head and neck cancer at varying frequencies. Importantly, B7-H1 mRNA upregulation is significantly associated with worse overall survival, and its upregulation is plausibly related to both gene amplification and DNA methylation. B7-H1 tended to be amplified at genetic level in HPV-positive patients, presenting the possibility that HPV-infected patients with head and neck cancer may derive more benefit from immunotherapies. To our knowledge, this is the first study on the immune molecular landscape of the pivotal B7 and TNFR families in head and neck cancer, providing a potential novel rationale for ongoing clinical investigations.
Methods
Bioinformatics analyses
We queried the NCBI database for the 10 human B7 (B7-1, B7-2, B7-DC, B7-H1, B7-H2, B7-H3, B7-H4, B7-H5, B7-H6, an dB7-H7) and 6 TNFR (TNFSF4, TNFRSF5, TNFSF7, TNFSF9, TNFRSF14, and TNFSF18) proteins (Supplementary materials); their amino acid sequences were analyzed for similarity with other known sequences using Clustal: Multiple Sequence Alignment (http://www.ebi.ac.uk/Tools/msa/clustalo/). Clustal uses seeded guide trees and Hidden Markov Model (HMM) profile–profile techniques to generate alignments between three or more sequences, which enable multithreaded computation of pairwise distances and alignment match states.Citation32-34
Determination of B7 and TNFR family member alterations in head and neck cancer
The frequency of gene alterations (including mutations, deletions, and amplifications) of the B7 and TNFR families was assessed across 11 studies on head and neck cancer using the cBioPortal for Cancer Genomics database and TCGA,Citation20,21 which included the details of more than 1,000 patients. Further, we assessed the genomic alterations (including mRNA dysregulation and promoter methylation) of both families across 496 sequenced head and neck cancer samples with complete TCGA data, as queried with cBioPortal. In this study, mutations were identified by whole-exome sequencing and were validated by targeted re-sequencing and interrogation of RNA for expression of the mutated alleles,Citation35 which included truncating, in-frame, and missense mutations. Truncating mutations are point mutations in the genetic code that generate one stop codon, thereby interrupting protein translation. Any insertion or deletion that is evenly divisible by three is termed an in-frame mutation, which would not disrupt the reading frame, considering the triplet nature of gene expression by codons. Missense mutations are point mutations where a single nucleotide is changed to result in the substitution of a different amino acid and a nonfunctional protein.
Prognostic significance of B7 and TNFR families in head and neck cancer
We obtained the clinical profiles, including the survival data, of all head and neck cancer samples from TCGA. Then, we evaluated the prognostic effects of B7 and TNFR family members in patients with head and neck cancer (excluding those with prior cancers).
Statistical analyses
STATA version 12.0 (Stata Corporation, College Station, TX) and GraphPad Prism 5 (GraphPad Software, La Jolla, CA) were used for statistical analyses. The Pearson chi-square test was used to compare categorical variables, and Fisher's exact test was used if indicated. The Spearman correlation test was conducted to assess the relationship between promoter methylation and mRNA expression. The Spearman coefficient was considered to indicate poor correlation if < 0.2, moderate if < 0.4, relatively strong if < 0.6, strong if < 0.8, and very strong if > 0.8. Kaplan–Meier survival curves were used to estimate the actuarial rates; log-rank tests were used for comparisons. The unadjusted Cox proportional hazards model was used to calculate the hazard ratio. The mRNA expression data are presented as the mean ± SD. Two-tailed p-values < 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1288329_supplementary_data.zip
Download Zip (1.2 MB)Acknowledgments
We would like to thank the staff members of the Cancer Genome Atlas for their involvement in the cBioPortal for Cancer Genomics Program. We also thank the anonymous editors and reviewers for their insightful comments and great efforts to improve this manuscript.
Funding
This work was supported by grants from the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B10); the Health & Medical Collaborative Innovation Project of Guangzhou City, China (201400000001); the Planned Science and Technology Project of Guangdong Province (2013B020400004); and the Science and Technology Project of Guangzhou City, China (14570006).
References
- Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci 1975; 53:27-42; PMID:238498; http://dx.doi.org/10.1038/icb.1975.3
- Assal A, Kaner J, Pendurti G, Zang X. Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy 2015; 7:1169-86; PMID:26567614; http://dx.doi.org/10.2217/imt.15.78
- Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 2016; 44:955-72; PMID:27192563; http://dx.doi.org/10.1016/j.immuni.2016.05.002
- Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 2016; 44:1005-19; PMID:27192566; http://dx.doi.org/10.1016/j.immuni.2016.04.019
- Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest 2013; 123:580-93; PMID:23281398; http://dx.doi.org/10.1172/JCI65013
- Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer 2014; 134:2764-71; PMID:24013874; http://dx.doi.org/10.1002/ijc.28474
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001; 193:839-46; PMID:11283156; http://dx.doi.org/10.1084/jem.193.7.839
- Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol 2013; 34:556-63; PMID:23954143; http://dx.doi.org/10.1016/j.it.2013.07.003
- Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H et al. B7-H2 is a costimulatory ligand for CD28 in human. Immunity 2011; 34:729-40; PMID:21530327; http://dx.doi.org/10.1016/j.immuni.2011.03.014
- Textor S, Bossler F, Henrich KO, Gartlgruber M, Pollmann J, Fiegler N, Arnold A, Westermann F, Waldburger N, Breuhahn K et al. The proto-oncogene Myc drives expression of the NK cell-activating NKp30 ligand B7-H6 in tumor cells. Oncoimmunology 2016; 5:e1116674; PMID:27622013; http://dx.doi.org/10.1080/2162402X.2015.1116674
- Jung K, Choi I. Emerging Co-signaling networks in T cell immune regulation. Immune Netw 2013; 13:184-93; PMID:24198743; http://dx.doi.org/10.4110/in.2013.13.5.184
- Clouthier DL, Watts TH. TNFRs and control of chronic LCMV infection: implications for therapy. Trends Immunol 2015; 36:697-708; PMID:26481667; http://dx.doi.org/10.1016/j.it.2015.09.005
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin 2015; 65:87-108; PMID:25651787; http://dx.doi.org/10.3322/caac.21262
- Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med 2008; 359:1143-54; PMID:18784104; http://dx.doi.org/10.1056/NEJMra0707975
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363:24-35; PMID:20530316; http://dx.doi.org/10.1056/NEJMoa0912217
- Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016; 1:e89829; PMID:27777979; http://dx.doi.org/10.1172/jci.insight.89829
- Bell RB, Leidner RS, Crittenden MR, Curti BD, Feng Z, Montler R, Gough MJ, Fox BA, Weinberg AD, Urba WJ. OX40 signaling in head and neck squamous cell carcinoma: overcoming immunosuppression in the tumor microenvironment. Oral Oncol 2016; 52:1-10; PMID:26614363; http://dx.doi.org/10.1016/j.oraloncology.2015.11.009
- Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375(19):1856-1867; PMID:27718784; http://dx.doi.org/10.1056/NEJMoa1602252
- Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17:956-65; PMID:27247226; http://dx.doi.org/10.1016/S1470-2045(16)30066-3
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2:401-4; PMID:22588877; http://dx.doi.org/10.1158/2159-8290.CD-12-0095
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6:pl1; PMID:23550210; http://dx.doi.org/10.1126/scisignal.2004088
- Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood 2007; 110:180-5; PMID:17289811; http://dx.doi.org/10.1182/blood-2006-11-060087
- Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 2008; 111:3635-43; PMID:18223165; http://dx.doi.org/10.1182/blood-2007-11-123141
- Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT et al. High PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinoma. PLoS One 2015; 10:e0142656; PMID:26562534; http://dx.doi.org/10.1371/journal.pone.0142656
- Mukaigawa T, Hayashi R, Hashimoto K, Ugumori T, Hato N, Fujii S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol 2016; 114:36-43; PMID:27111278; http://dx.doi.org/10.1002/jso.24266
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013; 73:1733-41; PMID:23288508; http://dx.doi.org/10.1158/0008-5472.CAN-12-2384
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013; 73:128-38; PMID:23135914; http://dx.doi.org/10.1158/0008-5472.CAN-12-2606
- Partlova S, Boucek J, Kloudova K, Lukesova E, Zabrodsky M, Grega M, Fučíková J, Truxová I, Tachezy R, Špíšek R et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015; 4:e965570; PMID:25949860;http://dx.doi.org/10.4161/21624011.2014.965570
- Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, Ferris RL. CD137 stimulation enhances cetuximab induced natural killer (NK): dendritic cell (DC) priming of anti-tumor T cell immunity in head and neck cancer patients. Clin Cancer Res 2016; 23(3):707-716; PMID:27496866; http://dx.doi.org/10.1158/1078-0432.CCR-16-0879
- Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet 2013; 45:791-8; PMID:23685749; http://dx.doi.org/10.1038/ng.2643
- Morris LG, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, Wong RJ, Lee NY, Sherman EJ, Baxi SS et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol 2016; 3(2):244-255; PMID:27442865; http://dx.doi.org/10.1001/jamaoncol.2016.1790
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011; 7:539; PMID:21988835; http://dx.doi.org/10.1038/msb.2011.75
- Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res 2015; 43:W580-4; PMID:25845596; http://dx.doi.org/10.1093/nar/gkv279
- McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res 2013; 41:W597-600; PMID:23671338; http://dx.doi.org/10.1093/nar/gkt376
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517:576-82; PMID:25631445; http://dx.doi.org/10.1038/nature14129