ABSTRACT
Evidence of tumor-resident mature B cell and antibody compartments and reports of associations with favorable prognosis in malignant melanoma suggest that humoral immunity could participate in antitumor defense. Likely striving to confer immunological protection while being subjected to tumor-promoting immune tolerance, B cells may engender multiple functions, including antigen processing and presentation, cytokine-mediated signaling, antibody class switching, expression and secretion. We review key evidence in support of multifaceted immunological mechanisms by which B cells may counter or contribute to malignant melanoma, and we discuss their potential translational implications. Dissecting the contributions of tumor-associated humoral responses can inform future treatment avenues.
Malignant melanoma and immune responses in the clinical landscape
Rising incidence (global incidence reported in 2013 in 15–25 individuals in every 100,000) and the worst patient survival rates of all skin tumors continue to make malignant melanoma treatment clinically challenging, despite recent breakthroughs in targeted therapies.Citation1 Multiple moles, family history of melanoma and unprotected or intense exposure to UV light,Citation2 especially UVB-induced somatic DNA mutations, such as cytidine to thymidine (C>T) transitions,Citation3 are among the risk factors. Mutation of genes such as BRAF and NRAS involved in the MAPK kinase pathway are present in more than 50% of the melanoma tumors.Citation4-6 Until 5 y ago, development of distant metastases was generally associated with a historic median survival of less than one year.Citation7 Recent breakthroughs in the understanding of the molecular and immunological basis of melanoma have contributed to the development of new MAPK pathway inhibitors, small molecule inhibitor drugs and checkpoint blockade antibody treatments, improving clinical outcomes in subsets of patients.Citation8
Reports of tumor-resident and systemic immune responses in melanoma patients, clinical observations of partial lesion regressions and spontaneous remissions, increased rates of malignant melanoma in immunosuppressed patients (organ transplant recipients and HIV-infected individuals),Citation9,Citation10 as well as partial successes of early immunostimulating treatments such as interleukin-2 (IL-2) and interferon-α2b (IFNα-2b) reported over many years, together support the presence of an active immune surveillance in patients with melanoma.Citation11,Citation12 Investigations into the drivers of immune responses to melanoma elucidated not only a set of tumor-specific melanoma-antigen-encoding gene families (MAGE, BAGE, GAGE), but also several antigenic epitopes derived from human melanocyte lineage-specific proteins (MART-l/Melan-A, gpl00, gp75 and tyrosinase) recognized by CD8+ and CD4+ T cells.Citation13-15 Various peptide-based vaccination therapy approaches have been trialed in patients using these antigens, often in conjunction with cytokines, toll-like receptor (TLR) agonists and adjuvants, some demonstrating circumscribed success.Citation16 Evidence for a correlation between antitumor T cell responsesCitation17-19 and heightened lymphocytic infiltrates within melanoma lesionsCitation20 with longer patient survival have maintained interest in the search for therapies based on counteracting peripheral tolerance. Several personalized therapeutic approaches have been developed for melanoma involving adoptive cell therapy (ACT) with T cells.Citation21-23 Some promising outcomes have been reported in small-scale studies of patients with malignant melanoma treated with autologous tumor-infiltrating lymphocyte (TIL)-based ACT, with larger, randomized phase III clinical trials to ascertain broader clinical benefits still required.
More recently, immunotherapeutic antibodies that block immune checkpoint molecules have led to the regulatory approval of the anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody ipilimumab for the treatment of metastatic melanoma,Citation24,Citation25 followed by the anti-programmed cell death protein 1 (PD1) receptor antibodies nivolumab and pembrolizumab.Citation26,Citation27 These agents function through blocking inhibitory molecules on the T cell surface, thereby counteracting immune suppressive signals.Citation28,Citation29 In 2016, the FDA approved the drug atezolizumab for bladder and lung cancers. Atezolizumab is an inhibitor of the PD1 ligand (PD-L1), expressed on tumor cells that is thought to restrict T cell activation through recognition and engagement with PD1 on T cells.Citation30 This has opened the way for several phase I clinical trials currently looking at efficacy of this class of agents in patients with melanoma. The emergence of these checkpoint inhibitor antibodies has been an important clinical breakthrough, bringing cancer immunotherapy to the forefront of clinical oncology. Numerous reports have drawn correlations between patient responses to checkpoint inhibitors and the presence and nature of tumor-infiltrating T lymphocytes and T cell responses to melanoma tumor antigens.Citation31
Although insights into the roles of T cells in antitumor responses have been widely studied and accepted as an essential immunological dimension in anti-cancer immunity, the roles of B cells and of the humoral response remain insufficiently elucidated. B cells confer a broad array of functions, which include antigen processing and presentation, cytokine-mediated signaling, immune regulation, expression and secretion of antibodies. These attributes can contribute to antitumor immunity and to treatment responses or, on the other hand, tumors can co-opt inhibitory immune pathways aimed at maintaining B cell immune tolerance. The characteristics affecting these opposite outcomes still need to be fully uncovered. Here, we review evidence from human clinical investigations and from murine cancer models in support of multifaceted immunological mechanisms by which B cells may respond or contribute to melanoma.
B cells in melanoma tumor inflammation
B cell infiltration
Lymphocyte populations are found in and around many solid tumor lesions including melanomas. While T cells are the most prominent, infiltrating human B cells are increasingly being reported in melanomas and other tumor types.Citation32-37
In an immunohistochemical study of 106 primary human melanoma samples, the majority of tumor tissues contained significant amounts of infiltrating CD20+ cells, thought to be B lymphocytes, dispersed in the stroma immediately around the tumor.Citation37 Denser B cell infiltration correlated with activated T cells, which could imply potential activation of an antitumor response. The percentage of both intra-tumoral and peri-tumoral B cell infiltration also positively correlated with patient survival, since patients' samples with significantly higher levels of B cell infiltration failed to develop visceral metastasis over the subsequent 5-y window of observation.Citation37 In concordance, a study following a patient cohort over 5-y since primary melanoma tumor resection, found that initial larger B cell infiltration in primary tumors of patients positively correlated with subsequent improved disease-free survival after cancer vaccine therapy.Citation38 A similar correlation was found in a study on two cohorts of 57 and 41 patients with primary cutaneous melanoma.Citation39 An earlier report, however, found no significant correlation between B cell infiltration and survival, although there was a significant association between total infiltrating TILs and disease progression in a cohort of 58 patients with malignant melanoma.Citation40 A study from a cohort of 91 patients with a clinical follow up of at least 10 y found instead an increased tumor progression and decreased overall survival in those patients with more than 15% B cell density among TILs in primary cutaneous melanoma.Citation41 Possible explanations for these discrepancies among studies could include the different tumor locations analyzed (primary cutaneous melanomas, distal or lymph node metastases), the way data were analyzed (cell frequencies reported as absolute numbers or as a proportion of TILs) and B cell detection markers (e.g., the pan-B cell marker CD20 alone may not be sufficient). The latter aspect may be crucial toward building meaningful correlations and a wider understanding of the B cell subsets and their markers would probably be required.
Although these observations support the idea that the level of B cell infiltration may, overall, be representative of the host's potential to develop antitumor responses, it remains to be determined whether higher lymphocyte infiltration is a result of a tumor antigen-specific immune response or merely recruitment of lymphocytes to tumor microenvironments in response to inflammation. Such contrasting findings point to a complex relationship between humoral responses and tumors.
B cells in human melanoma-associated tertiary lymphoid structures (TLS)
Activation of lymphocytes during adaptive immune responses typically occurs in secondary lymphoid structures such as lymph nodes, spleen and mucosal-associated lymphoid tissues. Chronic inflammation, however, can be accompanied by the formation of tertiary lymphoid structures (TLS) at lesion sites in both mouse models and humans.Citation42 These structures can vary in size and organizational structure, from disordered mixtures of dendritic cells (DCs), T and B cells to highly ordered structures bearing striking resemblance to germinal centers typically found in lymphoid organs.Citation43 TLS can be found at sites of inflammation in almost any organ of the body and are thought to facilitate rapid and robust immune responsesCitation44 ().
Figure 1. Proposed B cell functions in the melanoma tumor microenvironment. B cells may arise from the local immune surveillance environment or migrate to the tumor from blood vessels. B cells may accumulate and expand in tumor-associated lymphoid structures (TLS), where they can encounter APCs and T cells, and undergo affinity maturation and clonal amplification. Within the tumor, plasma cells can secrete tumor-specific IgG1 antibodies, effective in inducing ADCC, ADCP and complement-mediated cytotoxicity. On the other hand, in the tumor microenvironment, B cells can be differentially activated to secrete antibody isotypes such as IgA, IgG2 and IgG4, which may induce a weaker immune response through (a) inability to activate the complement cascade, (b) lower affinities for activatory FcRs, (c) higher affinities for inhibitory FcRs, (d) lower potency in triggering ADCC and ADCP compared with IgG1 isotype antibodies and (e) in the case of IgG4, Fab-arm exchange, resulting in antibodies with low antigenic affinity. The tumor microenvironment may also differentially polarize B cells toward a regulatory phenotype (Breg) through the secretion of soluble factors such as IL-10, which, in turn, negatively influences immune cell activation and antibody class switching.
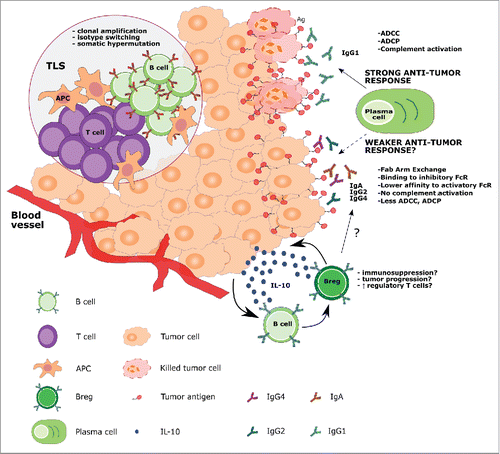
As tumor lesions bear characteristics consistent with chronic inflammation, the presence of tumor-associated TLS, which contain B cell infiltrates, reported in different tumor types including melanomas, may not be surprising.Citation37,Citation45 In a study of 106 primary human melanoma lesions, 26% contained histologically visible aggregates.Citation37 Cipponi et al. recovered and micro-dissected highly ordered TLS, defined as lymphoid follicles which contained clusters of B cells, follicular DCs, T cells and mature DCs from 7 out of 29 human melanoma metastases.Citation45 Sequencing of the immunoglobulin (Ig) repertoire of the lymphoid follicles revealed clonal amplification, isotype switching and somatic hypermutation, suggesting a local antigen-driven responseCitation45 (). These hallmarks of local B cell maturation have also been observed in immunohistochemical analyses of extra-nodal TLS from human germ cell tumorsCitation46 and breast carcinomas.Citation47 In an analysis of TLS in non-small cell lung cancer, characterization of B cell subsets by immunohistochemistry and flow cytometry detected a prevalence of memory B cells and plasma cells producing tumor-specific antibodies, with the density of the follicle correlating with the number of mature plasma cells present.Citation48 In concordance, TLS were found to be prognostic of more favorable patient outcomes.Citation43,Citation48
Collectively, these findings may indicate a role of TLS as local sites of B cell maturation, fostering the generation of in situ adaptive host immune responses.
Evidence for B cells and their functions in experimental models
Murine models of melanoma and other tumors
Initial studies in murine models of melanoma and of other cancers suggest that B cells may exert both pro- and antitumor effects, often depending on the in vivo model system. Reported tumor-permissive properties of B cells include B cell-dependent inhibition of antitumor immunity in lymphoma and melanoma (but not in sarcoma), through a CD40L-dependent mechanism that affects IL-10 secretion in vitro.Citation49 Other studies provide evidence that B cells may support lymphangiogenesis in in vivo lymphoma and melanoma mouse modelsCitation50 and angiogenesis in vitro and also in vivo in melanoma, bladder and lung carcinoma murine tumor models.Citation51 In a murine model of squamous cell carcinoma, antitumor autoantibodies were reported to induce acute inflammation when organized in immune complexes. According to this study, the inflammatory environment regulates recruitment and induces pro-tumoral functions of leukocytes surrounding neoplastic tissue through engagement of Fc gamma receptors (FcγRs) expressed by immune cellsCitation52 (). These pro-tumoral functions engendered by an abnormal secretion of Ig could be reversed by administration of an anti-CD20 treatment in a combined therapy with a chemotherapy agent, which ablated B cells, reprogrammed the chemokine expression profiles of macrophages and increased CD8+ T cell infiltration into mouse tumors.Citation53 In contrast, several other studies suggest that B cells can augment T cell-mediated antitumor responses in in vivo models of melanoma, lymphoma, colorectal and mammary carcinoma.Citation54-58
These in vivo studies not only suggest that B cells can strongly contribute to tumor rejection, but also acquire tolerant or pro-tumorigenic characteristics with disease progression (). It is therefore tempting to envisage a complex orchestration of the immune response mediated by different B cell subsets, perhaps including B cells with immunoregulatory properties, as is the case for different T cell subsets.
The search for regulatory B cells (Bregs): insights from animal models
Mizoguchi et al. first described a subset of gut-associated CD1d-expressing B cells that could suppress inflammatory progression of colitis in mice by secreting the immune regulatory cytokine IL-10, thus coining the term “regulatory B cell” (B10)Citation59 ( and ). In later studies, B10-like IL-10-producing B cells were reported in peripheral human bloodCitation60 and early findings suggest that these cells may also be present in human metastatic melanoma.Citation61 However, possible roles of regulatory B10-like B cells in cancer have to-date only been described in animal models.Citation62,Citation63 A study in a transgenic murine model of prostate cancer identified PD-L1 and IL-10, expressed by a subpopulation of plasma cells, as the factors responsible for CTL inhibition after treatment with the immunogenic chemotherapeutic drug oxaliplatin.Citation64 Bregs have also been shown to regulate immunity to murine breast tumors independently of IL-10 in vivo and in vitro.Citation65,Citation66 Although the exact mechanism of this IL-10-independent immune suppression is yet unknown, some evidence links Breg activity and CD25+ FoxP3+ T regulatory cell accumulation at tumor sites in murine breast tumors.Citation66,Citation67 Moreover, a subset of murine B220+ CD19+ CD25+ Bregs was shown to be enriched in 4T1 breast tumor-bearing mice and to polarize T cells in culture toward a regulatory T cell (Treg)-like phenotype, again in an IL-10-independent manner.Citation65 Tumor-derived Bregs and Tregs were consequently suggested to interact with myeloid-derived suppressor cells, possibly further potentiating Treg responses, thus amplifying immunosuppression and promoting disease progression.Citation65 In the study of Khan et al., Bregs expressing high levels of PD-L1 were observed to inhibit development and expansion of follicular T helper cells in an autoimmune disease in vivo model in mice and ex vivo in human blood, resulting in reduced B cell maturation and T cell-dependent humoral immune responsesCitation68 ().
Figure 2. Potential pro- and antitumor functions of tumor-infiltrating B cells. Tumor-infiltrating B cells may either promote or inhibit growth and metastasis through various immune mechanisms, involving secretion of antibodies, cytokine-mediated activation and recruitment of other immune effector cells and engagement and activation of T cells through antigen presentation via MHC in the presence of co-stimulatory molecules. Regulatory functions may be engendered through secretion of cytokines such as IL-10, T cell inhibition by PD-L1 expression or class switching and production of immunoglobulin isotypes with low immune effector stimulating functions.
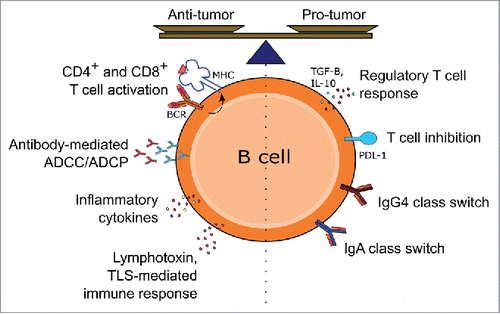
Although pointing to potential roles for Bregs in tumor immune escape, results obtained in animal models are yet to be fully confirmed and elucidated in the human melanoma patient context.
B cells in melanoma immune surveillance
Evidence for reactive mature B cell responses and tumor-specific antibodies
B cells straddle both innate and adaptive immunity, acting as critical effectors of the humoral immune response through the secretion of antibodies.Citation69 In several cancer types, TILs and peripheral B cells have the ability to produce antibodies that could recognize autologous tumor targets, some of which have been investigated as potential diagnostic biomarkers.Citation70-72 The development of the serological identification of recombinant expression cloning (SEREX) approach, a phage display of cDNA libraries derived from tumor samples screened with autologous cancer patient sera, constituted a powerful tool that allowed the identification of more than one hundred melanoma antigens and autoantibodies to these. Findings from SEREX studies supported the notion that tumors such as melanoma are immunogenic and induce temporal tumor-reactive humoral responses.Citation73,Citation74 However, whether tumor-reactive antibodies in vivo have any antitumor protective functions remains under debate. Mature B cells from melanoma patients were able to produce IgG antibodies that recognize melanoma cells, and ex vivo these antibodies could mediate tumor cell cytotoxicityCitation75 (). There is also evidence of a gradual reduction of the human B cell compartment and of tumor-reactive antibodies with melanoma disease progression. This may indicate some functional roles for B cells early on in the disease which may perhaps be modulated as a part of tumor-associated immune escape processes.Citation76 In this regard, Oaks and colleagues described for several human cancers an active anti-inflammatory role of sialylated tumor-specific IgGs that may promote tumor growth.Citation77
Saul et al. report higher mRNA expression of mature B cell markers in human melanoma lesions compared with normal skin and a distinct affinity-matured antibody repertoire. Antibodies from melanoma lesions featured shorter complementarity-determining region 3 (CDR3) sequences, clonal expansion characteristics and differential antigen recognition patterns (demonstrated by homology modeling), suggesting a distinct melanoma-associated B cell responseCitation78 ().
Emerging evidence suggests that the state of maturation of the B cell compartment and subsequent isotype expression of tumor-reactive antibodies can prevent the host from mounting an efficient immune response.Citation79 Depending on the soluble signals released in the tumor microenvironment, B cells can be polarized to undergo class switching and express potentially weak immune-activating antibody isotypes such as IgG480 or IgG2 and IgA,Citation45 as part of chronic inflammation and immune-escape processes associated with human melanomaCitation79 ( and ). It is noteworthy that a subtype of murine B cells (B-1 cells) have been shown to secrete IgM antibodies in a T cell-independent manner after recognition of evolutionally conserved structures of microbial origin through TLR in vitro.Citation81,Citation82 T cell-independent and BAFF-dependent activation has been reported to support production of IgG4 antibodies by human B cells in autoimmune pancreatitis.Citation83 Such innate antibody response triggers may also be envisaged in the context of melanoma tumors, and may give rise to B cell expansion in the absence of specific tumor antigen signal.
IgG4, one of the antibody isotypes produced in melanomas, is known to have poor immune-activating properties, including inability to activate complement,Citation84 a lower capacity to mediate effector functions compared with other isotypesCitation85 and phagocytosis (ADCP) compared with IgG1. These have been associated with specific structural characteristics of IgG4, which determine its poor capacity to bind C1q, its poor affinity for activating receptor FcγRIIa (known to be involved in antibody-dependent macrophage-mediated phagocytosis, ADCP),Citation86 and FcγRIIIa (crucial for NK cell-mediated ADCC functions).Citation87 Structural characteristics of IgG4 are also responsible for relatively high affinity to the inhibitory Fc receptor FcγRIIb, compared with other subclasses, and ability to undergo Fab-arm exchange with other IgG4 molecules.Citation88-90 Collectively, through such functional attributes, IgG4 isotype antibodies are thought to have immunomodulatory properties, and to impair antibody effector functions in cancer.Citation80,Citation91 Elevated IgG4 levels in patient circulation even in the early stages of melanoma has been associated with disease progression and less favorable clinical outcome.Citation80,Citation92 IgG4+ B cells and IgG4s were detected in melanoma tumor microenvironments together with IL-10, IL-4 and vascular endothelial growth factor (VEGF). Ex vivo co-culture experiments with human B cells and melanoma cells suggest that melanoma cells have the ability to promote Th2-biased conditions to support IgG4 production by B cells.Citation80,Citation92 ( and ).
Human melanoma lesion-resident B cells have also been reported to express IgA class antibodies.Citation45 IgA class switching is normally observed in secondary lymphoid organs that drain mucosal tissue and is associated with a tolerance induction to commensal microbiota in the gut.Citation93 IgA class switching is often associated with chronic exposure to antigens and this could facilitate a form of immune subversion mediated by the inflammatory milieu, much like that of the IgG4 class switching. IgA is a poor inducer of complement, ADCC and ADCP.Citation93 A study in a murine model of prostate cancer describes a subset of plasma cells expressing IgA in the tumor region and having immune regulatory functions.Citation64 Yet, more work is needed to investigate the role human IgA response plays in tumor immunity ( and ).
Based on our current knowledge, it is possible that, depending on immune context and microenvironment, B cells will be able to respond to cytokine stimuli in the presence or absence of specific antigenic challenge, to either promote or counteract tumor development. The tumor microenvironment could signal B cells in germinal centers and TLSs to undergo skewed class switching, leading to the secretion of Th2-biased isotypes and thus dampening immune response.
Could B cells act as antigen-presenting cells to enhance T cell responses?
As professional antigen presenting cells (APC), B cells are able to induce antigen-specific T cell priming, which requires both recognition of antigens by their Ig-membrane bound B cell receptor (BCR), as well as engagement of the co-stimulatory molecule CD40 by a CD4+ helper T cell. This is followed by B cell maturation, antigen internalization, processing and presentation on major histocompatibility complexes (MHC)Citation94-98 ().
Depending on their activation status, B cells can also secrete an array of cytokines, notably tumor necrosis factor-α (TNF-α), IL-10, lymphotoxin (LT), IL-2, IL-6, IL-4 and interferon-γ (IFNγ). Through cytokine secretion, B cells can exert dynamic effects on both the local microenvironment and the systemic immune responseCitation99-103 (). In allograft rejection, potent T cell responses are a pivotal component in pathogenesis and tissue destruction. Allograft tissue-reactive B cells can enhance T cell response through antigen presentation and co-stimulation.Citation104,105 Gene-expression profile studies in renal allograft biopsies, corroborated by immunohistochemical analyses, have shown that B cell signatures (comprising of CD20, CD74 and Ig) are associated with acute organ rejection. Similarly, immunohistochemical evaluations revealed dense interstitial CD20+ B cell aggregates in 53% (17/32) of core biopsy samples with graft rejection.Citation105
B cell activation may also be critical for tumor regression in melanoma. Primary human B cells activated in vitro with CD40 ligand and subsequently pulsed with melanoma tumor antigens, efficiently processed and presented MHC class II-restricted peptides to specific CD4+ T cell clones, generating melanoma-specific T cells.Citation106 Ex vivo studies by Von Bergwelt-Baildon et al. on human blood-derived lymphocytes also suggest that in the context of tumor immunology, B cells have the ability to operate as efficient APCs, driving the expansion of both memory and naive tumor-associated antigen-specific CD4+ and CD8+ T cellsCitation96 (). B cells have also been reported to possess direct cytotoxic killing ability against murine 4TI breast cancer cells in a Fas/FasL-dependent manner in the absence of IL-10Citation107 and also in the presence of IL-2.Citation108
B cells may thus potentially contribute a wide variety of functions, including antigen presentation, to promote autoimmunity or tumor rejection.
Therapeutic avenues focused on B cells
Although our understanding of the crosstalk between humoral immunity and melanoma remains incomplete, several therapeutic treatments have been attempted with a view of modulating the B cell compartment to stimulate anticancer immunity. The anti-CD20 monoclonal antibody (mAb) rituximab was administered in 15 patients with renal cell carcinoma and 6 with melanoma before treatment with low doses of IL-2 in a clinical trial, without conferring any beneficial effects.Citation109 In the context of other tumor types, a case study of a primary cutaneous T cell lymphoma showed a temporary remission after a combination therapy with rituximab and chemotherapy, associated with decrease in Tregs and increase in CD8+ T cells.Citation110 In a pilot study on a small cohort of nine stage IV melanoma patients, treatment with rituximab increased the median time without recurrences from 6 to 42 mo (no recurrence in 5 out of 9 patients at 42 mo).Citation111 No correlation was found though between patients with recurrences and immune cell responses. The authors suggested that treatment benefits are due to the antibody's ability to target a subpopulation of CD20-expressing cancer stem cells instead. There are currently several on-going clinical trials testing antibodies targeting CD20 for the indication of metastatic melanoma (e.g., NCT01376713, NCT02142335; www.clinicaltrials.gov). It is the authors' opinion that, apart from anti-CD20 treatments potentially eliminating cancer stem cell populations, generally targeting a widely expressed molecule such as CD20 does not take into account the complex contributions of different B cell subpopulations in cancer. Thus, more fine-tuned approaches to perhaps target specific subpopulations of B cells (e.g., regulatory or IL-10-producing B cells) or inhibitory elements on B cells could constitute possible strategies.
CD40–CD40L interactions have also been the focus of some immunotherapies aimed at driving B cell proliferation and producing sufficient quantities of B cells in vitro for adoptive therapy.Citation112 CD40L-stimulated CD40-expressing B cells can be expanded from small volumes of blood and have been reported to generate antitumor CD8+ T cells.Citation113 In a preclinical setting, adoptive transfer of B cells isolated from draining lymph nodes of 4TI tumor-bearing mice and activated in vitro with LPS and anti-CD40, have been shown to prevent spontaneous metastasis of 4TI breast tumor cells to mouse lungs.Citation114 Given encouraging results from clinical trials and regulatory approval of the adoptive T cell therapy sipeleucel T for the treatment of prostate cancer, it is tempting to consider the possibility that, in future, activated B cells could be used as an adjuvant in adoptive T cell therapies.
Insights from the humoral response in melanoma for the development of future immuno-oncology treatments
B cells play multifaceted roles in melanoma immunity through several signaling and immunological pathways. In this review, we showed numerous evidences supporting this concept, also making parallels with different disease models. Yet, further studies will be needed to dissect the mechanisms by which different components of B cell functions operate in melanoma, and thus offering the potential to identify previously unappreciated translational insights. Initial approaches may involve in-depth dissection of specific B cell subsets and modulatory markers which may affect cell differentiation, migration, functions, antibody expression, maturation, class switching and secretion. Immunologically relevant disease models and ex vivo systems could in future also help delineate specific mechanisms which could be responsible for immunosuppression or for activation of humoral immunity in melanoma.
As melanoma cells may be able to escape host immune responses,Citation115-117 the B cell repertoire and antibodies expressed in the patient context may not be effective enough to confer tumor clearance. Antibody isotypes such as IgG4 and IgA may regulate immune effector functions and support immune evasion. Melanoma-associated IgG4 could perhaps act by restricting Fc-mediated functions of IgG therapeutic antibodies in the circulation and in tumor lesions. These mechanisms should be taken into consideration when designing antibody therapeutic agents. For example, recently approved mAbs for the treatment of melanoma target immune checkpoint molecules on T cells and are designed to act through their Fab-mediated effects, by removing T cell inhibitory signals. However, the potency and mechanisms by which mAbs may also engage immune effector cells through their Fc regions are less well understood. Given the array of Fc receptors expressed by effector cells, including those infiltrating tumors, it may be important to understand how antibodies and effector cells that form part of tumor immune surveillance may influence antitumor immunity, and how they affect the efficacy of therapeutic antibodies.Citation118 Furthermore, it is also tempting to consider new therapeutic opportunities through the design of therapeutic antibodies perhaps less prone to cancer-associated immunosuppressive forces or those better equipped to mount effector functions in the Th2-biased tumor microenvironment. These may include engineered antibodies with enhanced binding to activatory Fc receptors on immune effector cells to redirect them against cancer, or those antibodies with Fc regions of different isotypes such as IgE, perhaps better able to exert immunological surveillance in tissue tumors such as melanoma.Citation119
Underpinning effective therapeutic interventions in melanoma will be the ability to avoid potential tumor blockade mechanisms and enable the host to mount a robust immune response toward a heterogeneous tumor population. In light of encouraging advancements with the use of individual and combination treatments with checkpoint blocking agents, the potential for using B cells and the antibodies they express for therapy or as prognostic or predictive biomarkers of treatment responses remain largely unexplored possibilities. In future, delineating the presence of different subsets of B cells and their impact on immune responses against melanoma could provide opportunities, such as targeting specific populations for elimination (e.g., Bregs or IL-10-producing B cells) or activation (e.g., tumor antigen-specific mature memory B cells), or the use of particular B cell subsets in adoptive therapy regimens. For instance, studies describing checkpoint molecules such as PD-L1 expressed by certain subsets of B cells raise the possibility to exploit specific checkpoint inhibitors to target modulated cell subsets as a strategy that could potentially re-kindle the antitumor functions of these cells. Furthermore, designing molecules that counteract immunosuppressive cytokines such as IL-10, VEGF or TGF-ß from the tumor microenvironment could revert a wider group of immune cells, including B cells, to engage in tumor rejection. Removal of these cytokines may allow B cells to undergo class switching to activatory antibody isotypes (e.g., IgG1), perhaps better able to engage effector cells against tumors. A wider immunotherapy or vaccination approach may also aim to re-establish the antitumor functions of different immune cell populations, to achieve simultaneous stimulation of tumor-neutralizing CTL and humoral immune responses against cancer antigens.
With melanoma continuing to provide the paradigm for clinical translation of cancer treatments based on triggering the activatory potential of T cells, more in-depth focusing on other immune cells including B cells and harnessing their antitumor functions may be of key importance for the development of the next generation of immuno-oncology agents. Dissecting B cells and the patients' humoral immunity and understanding how different compartments may contribute to tumor inflammation, immune responses and clinical course, warrant renewed attention and offer the possibility to widen the scope of immune-based therapies for melanoma and other cancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research was supported by the National Institute for Health Research (NIHR) BRC based at Guy's and St. Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors acknowledge support by the Medical Research Council (MR/L023091/1); The British Skin Foundation (S633); The Dermatrust; Guy's and St. Thomas's Charity Melanoma Special Fund; Cancer Research UK (C30122/A11527; C30122/A15774); the Academy of Medical Sciences; CR UK/EPSRC/MRC/NIHR KCL/UCL Comprehensive Cancer Imaging Centre (C1519/A10331); Breakthrough Breast Cancer/Breast Cancer Now (147); CR UK/NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre (C10355/A15587).
References
- Schadendorf , Hauschild A. Melanoma in 2013: Melanoma-the run of success continues. Nat Rev Clin Oncol 2014; 11:75-6; PMID:24419300; http://dx.doi.org/10.1038/nrclinonc.2013.246
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. The Lancet 2014; 383:816-27; PMID:24054424; http://dx.doi.org/10.1016/S0140-6736(13)60802-8
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C et al. A landscape of driver mutations in melanoma. Cell 2012; 150:251-63; PMID:22817889; http://dx.doi.org/10.1016/j.cell.2012.06.024
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015; 161:1681-96; PMID:26091043; http://dx.doi.org/10.1016/j.cell.2015.05.044
- Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res 2002; 8:3468-74; PMID:12429636
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949-54; PMID:12068308; http://dx.doi.org/10.1038/nature00766
- Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011; 16:5-24; PMID:21212434; http://dx.doi.org/10.1634/theoncologist.2010-0190
- Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Long GV, Lorigan P, McArthur GA et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer 2016; 53:125-34; PMID:26707829; http://dx.doi.org/10.1016/j.ejca.2015.09.013
- Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, Paddock L, Yanik EL, Lynch CF, Kasiske BL et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol 2015; 135:2657-65; PMID:26270022; http://dx.doi.org/10.1038/jid.2015.312
- Shiels MS, Copeland G, Goodman MT, Harrell J, Lynch CF, Pawlish K, Pfeiffer RM, Engels EA. Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer 2015; 121:2063-71; PMID:25739496; http://dx.doi.org/10.1002/cncr.29324
- Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev 2013; Issue 6. Art. No.:CD008955; PMID:23775773; http://dx.doi.org/10.1002/14651858.CD008955.pub2
- Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004; 28:109-23; PMID:15473953; http://dx.doi.org/10.1016/j.cyto.2004.06.010
- Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, Rosenberg SA, Robbins PF. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med 1996; 183:1965-71; PMID:8642306; http://dx.doi.org/10.1084/jem.183.5.1965
- Cole DJ, Weil DP, Shamamian P, Rivoltini L, Kawakami Y, Topalian S, Jennings C, Eliyahu S, Rosenberg SA, Nishimura MI. Identification of MART-1-specific T-cell receptors: T cells utilizing distinct T-cell receptor variable and joining regions recognize the same tumor epitope. Cancer Res 1994; 54:5265-8; PMID:7522957
- Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 1994; 180:347-52; PMID:7516411; http://dx.doi.org/10.1084/jem.180.1.347
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909-15; PMID:15340416; http://dx.doi.org/10.1038/nm1100
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-59; PMID:24667641; http://dx.doi.org/10.1172/JCI73639
- Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med 2005; 201:1257-68; PMID:15824085; http://dx.doi.org/10.1084/jem.20042028
- Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature 2001; 411:1058-64; PMID:11429607; http://dx.doi.org/10.1038/35082583
- Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77:1303-10; PMID:8608507; http://dx.doi.org/10.1002/(SICI)1097-0142(19960401)77:7%3c1303::AID-CNCR12%3e3.0.CO;2-5
- Sim GC, Chacon J, Haymaker C, Ritthipichai K, Singh M, Hwu P, Radvanyi L. Tumor-infiltrating lymphocyte therapy for melanoma: rationale and issues for further clinical development. BioDrugs 2014; 28:421-37; PMID:24890028; http://dx.doi.org/10.1007/s40259-014-0097-y
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348:62-8; PMID:25838374; http://dx.doi.org/10.1126/science.aaa4967
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651-67; PMID:20156973; http://dx.doi.org/10.1084/jem.20091921
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 2012; 18:2039-47; PMID:22271879; http://dx.doi.org/10.1158/1078-0432.CCR-11-1823
- Del Vecchio M. [AACR update on 5-year survival rates, efficacy and long-term safety in previously treated advanced/metastatic melanoma patients receiving mono-immunotherapy with nivolumab]. Recenti Prog Med 2016; 107:414-7; PMID:27571556
- Burns MC, O'Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Expert Opin Orphan Drugs 2016; 4:867-73; PMID:27597930; http://dx.doi.org/10.1080/21678707.2016.1191348
- Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009; 21:1065-77; PMID:19651643; http://dx.doi.org/10.1093/intimm/dxp072
- Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016; 44:955-72;PMID:27192563;http://dx.doi.org/10.1016/j.immuni.2016.05.002
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365-9; PMID:10581077; http://dx.doi.org/10.1038/70932
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/10.1038/nature13954
- Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, Kanomata N, Kawaguchi A, Akiba J, Naito Y et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 2012; 43:1688-94; PMID:22516244; http://dx.doi.org/10.1016/j.humpath.2011.12.013
- Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, Varki N, Shabaik A, Howell S, Kane CJ et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med 2014; 12:30; PMID:24475900; http://dx.doi.org/10.1186/1479-5876-12-30
- Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012; 18:3281-92; PMID:22553348; http://dx.doi.org/10.1158/1078-0432.CCR-12-0234
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678-87; PMID:21846823; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/10.1016/j.immuni.2013.10.003
- Ladányi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, Fejös Z, Gaudi I, Dobos J, Tímár J. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother 2011; 60:1729-38; PMID:21779876; http://dx.doi.org/10.1007/s00262-011-1071-x
- Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012; 72:1070-80; PMID:22266112; http://dx.doi.org/10.1158/0008-5472.CAN-11-3218
- Garg K, Maurer M, Griss J, Brüggen MC, Wolf IH, Wagner C, Willi N, Mertz KD, Wagner SN. Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum Pathol 2016; 54:157-64; PMID:27107457; http://dx.doi.org/10.1016/j.humpath.2016.03.022
- Hussein MR, Elsers DA, Fadel SA, Omar AE. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol 2006; 59:316-24; PMID:16505286; http://dx.doi.org/10.1136/jcp.2005.028860
- Martinez-Rodriguez M, Thompson AK, Monteagudo C. A significant percentage of CD20-positive TILs correlates with poor prognosis in patients with primary cutaneous malignant melanoma. Histopathology 2014; 65:726-8; PMID:24750176; http://dx.doi.org/10.1111/his.12437
- Jones GW, Hill DG, Jones SA. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front Immunol 2016; 7:401; PMID:27752256; http://dx.doi.org/10.3389/fimmu.2016.00401
- Germain C, Gnjatic S, Dieu-Nosjean MC. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front Immunol 2015; 6:67; PMID:25755654; http://dx.doi.org/10.3389/fimmu.2015.00067
- Siliņa K, Rulle U, Kalniņa Z, Linē A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother 2014; 63:643-62; PMID:24695950; http://dx.doi.org/10.1007/s00262-014-1544-9
- Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72:3997-4007; PMID:22850419; http://dx.doi.org/10.1158/0008-5472.CAN-12-1377
- Willis SN, Mallozzi SS, Rodig SJ, Cronk KM, McArdel SL, Caron T, Pinkus GS, Lovato L, Shampain KL, Anderson DE et al. The microenvironment of germ cell tumors harbors a prominent antigen-driven humoral response. J Immunol 2009; 182:3310-7; PMID:19234230; http://dx.doi.org/10.4049/jimmunol.0803424
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 2012; 132:545-53; PMID:21671016; http://dx.doi.org/10.1007/s10549-011-1620-1
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189:832-44; PMID:24484236; http://dx.doi.org/10.1164/rccm.201309-1611OC
- Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res 2006; 66:7741-7; PMID:16885377; http://dx.doi.org/10.1158/0008-5472.CAN-05-3766
- Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia 2011; 13:748-57; PMID:21847366; http://dx.doi.org/10.1593/neo.11756
- Yang C, Lee H, Pal S, Jove V, Deng J, Zhang W, Hoon DS, Wakabayashi M, Forman S, Yu H. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS One 2013; 8:e64159; PMID:23734190; http://dx.doi.org/10.1371/journal.pone.0064159
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 2010; 17:121-34; PMID:20138013; http://dx.doi.org/10.1016/j.ccr.2009.12.019
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 2014; 25:809-21; PMID:24909985; http://dx.doi.org/10.1016/j.ccr.2014.04.026
- DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol 2010; 184:4006-16; PMID:20194720; http://dx.doi.org/10.4049/jimmunol.0903009
- Kobayashi T, Hamaguchi Y, Hasegawa M, Fujimoto M, Takehara K, Matsushita T. B cells promote tumor immunity against B16F10 melanoma. Am J Pathol 2014; 184:3120-9; PMID:25173132; http://dx.doi.org/10.1016/j.ajpath.2014.07.003
- Zhou P, Qiu J, L'Italien L, Gu D, Hodges D, Chao CC, Schebye XM. Mature B cells are critical to T-cell-mediated tumor immunity induced by an agonist anti-GITR monoclonal antibody. J Immunother 2010; 33:789-97; PMID:20842058; http://dx.doi.org/10.1097/CJI.0b013e3181ee6ba9
- Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol 2012; 189:2226-33; PMID:22826317; http://dx.doi.org/10.4049/jimmunol.1200744
- Wennhold K, Weber TM, Thelen M, Garcia-Marquez M, Chakupurakal G, Klein-Gonzalez N, Fiedler A, Schlösser HA, Fischer R, Theurich S et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget 2016 Feb 25; PMID:26934557; http://dx.doi.org/10.18632/oncotarget.7720
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002; 16:219-30; PMID:11869683; http://dx.doi.org/10.1016/S1074-7613(02)00274-1
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117:530-41; PMID:20962324; http://dx.doi.org/10.1182/blood-2010-07-294249
- Egbuniwe I, Mohamad MH, Karagiannis SN, Nestle FO, Lacy KE. Interleukin-10-producing B-cell populations in melanoma. Br J Dermat 2012; 166, No. 4, P-21, 04.2012, p. e35
- Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep 2015; 5:12255; PMID:26193241; http://dx.doi.org/10.1038/srep12255
- Zhang Y, Gallastegui N, Rosenblatt JD. Regulatory B cells in anti-tumor immunity. Int Immunol 2015; 27:521-30; PMID:25999597; http://dx.doi.org/10.1093/intimm/dxv034
- Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015; 521:94-8; PMID:25924065; http://dx.doi.org/10.1038/nature14395
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res 2011; 71:3505-15; PMID:21444674; http://dx.doi.org/10.1158/0008-5472.CAN-10-4316
- Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, Rosenblatt JD. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother 2013; 62:87-99; PMID:22772949; http://dx.doi.org/10.1007/s00262-012-1313-6
- Tadmor T, Zhang Y, Cho HM, Podack ER, Rosenblatt JD. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother 2011; 60:609-19; PMID:21253724; http://dx.doi.org/10.1007/s00262-011-0972-z
- Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun 2015; 6:5997; PMID:25609381; http://dx.doi.org/10.1038/ncomms6997
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood 2008; 112:1570-80; PMID:18725575; http://dx.doi.org/10.1182/blood-2008-02-078071
- Chen H, Werner S, Butt J, Zörnig I, Knebel P, Michel A, Eichmüller SB, Jäger D, Waterboer T, Pawlita M et al. Prospective evaluation of 64 serum autoantibodies as biomarkers for early detection of colorectal cancer in a true screening setting. Oncotarget 2016; 7:16420-32; PMID:26909861; http://dx.doi.org/10.18632/oncotarget.7500
- Dumstrei K, Chen H, Brenner H. A systematic review of serum autoantibodies as biomarkers for pancreatic cancer detection. Oncotarget 2016; 7:11151-64; PMID:26840568
- Katayama H, Boldt C, Ladd JJ, Johnson MM, Chao T, Capello M, Suo J, Mao J, Manson JE, Prentice R et al. An autoimmune response signature associated with the development of triple-negative breast cancer reflects disease pathogenesis. Cancer Res 2015; 75:3246-54; PMID:26088128; http://dx.doi.org/10.1158/0008-5472.CAN-15-0248
- Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A 1995; 92:11810-3; PMID:8524854; http://dx.doi.org/10.1073/pnas.92.25.11810
- Pfreundschuh M. The genealogy of SEREX. Cancer Immun 2012; 12:7; PMID:22896752
- Gilbert AE, Karagiannis P, Dodev T, Koers A, Lacy K, Josephs DH, Takhar P, Geh JL, Healy C, Harries M et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS One 2011; 6:e19330; PMID:21559411; http://dx.doi.org/10.1371/journal.pone.0019330
- Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon TA, Rosenfeld MR, Kaplan DE, Chang KM, Domchek SM, Kanetsky PA et al. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res 2009; 15:4277-87; PMID:19549767; http://dx.doi.org/10.1158/1078-0432.CCR-09-0537
- Oaks M, Taylor S, Shaffer J. Autoantibodies targeting tumor-associated antigens in metastatic cancer: Sialylated IgGs as candidate anti-inflammatory antibodies. Oncoimmunology 2013; 2:e24841; PMID:23894724; http://dx.doi.org/10.4161/onci.24841
- Saul L, Ilieva KM, Bax HJ, Karagiannis P, Correa I, Rodriguez-Hernandez I, Josephs DH, Tosi I, Egbuniwe IU, Lombardi S et al. IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep 2016; 6:29736; PMID:27411958; http://dx.doi.org/10.1038/srep29736
- Karagiannis P, Gilbert AE, Nestle FO, Karagiannis SN. IgG4 antibodies and cancer-associated inflammation: Insights into a novel mechanism of immune escape. Oncoimmunology 2013; 2:e24889; PMID:24073371; http://dx.doi.org/10.4161/onci.24889
- Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 2013; 123:1457-74; PMID:23454746; http://dx.doi.org/10.1172/JCI65579
- Egbuniwe IU, Karagiannis SN, Nestle FO, Lacy KE. Revisiting the role of B cells in skin immune surveillance. Trends Immunol 2015; 36:102-11; PMID:25616715; http://dx.doi.org/10.1016/j.it.2014.12.006
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 2011; 11:34-46; PMID:21151033; http://dx.doi.org/10.1038/nri2901
- Watanabe T, Yamashita K, Fujikawa S, Sakurai T, Kudo M, Shiokawa M, Kodama Y, Uchida K, Okazaki K, Chiba T. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum 2012; 64:914-24; PMID:21971969; http://dx.doi.org/10.1002/art.33386
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods 2005; 306:151-60; PMID:16219319; http://dx.doi.org/10.1016/j.jim.2005.08.009
- Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol 2012; 188:4405-11; PMID:22461693; http://dx.doi.org/10.4049/jimmunol.1200090
- Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008; 7:2517-27; PMID:18723496; http://dx.doi.org/10.1158/1535-7163.MCT-08-0201
- Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 2013; 4:76; PMID:23543707; http://dx.doi.org/10.3389/fimmu.2013.00076
- Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, Killestein J, Polman CH, Aalberse RC, Schuurman J et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009; 27:767-71; PMID:19620983; http://dx.doi.org/10.1038/nbt.1553
- Davies AM, Rispens T, den Bleker TH, McDonnell JM, Gould HJ, Aalberse RC, Sutton BJ. Crystal structure of the human IgG4 C(H)3 dimer reveals the role of Arg409 in the mechanism of Fab-arm exchange. Mol Immunol 2013; 54:1-7; PMID:23164605; http://dx.doi.org/10.1016/j.molimm.2012.10.029
- Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, Sutton BJ. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol 2014; 426:630-44; PMID:24211234; http://dx.doi.org/10.1016/j.jmb.2013.10.039
- Crescioli S, Correa I, Karagiannis P, Davies AM, Sutton BJ, Nestle FO, Karagiannis SN. IgG4 characteristics and functions in cancer immunity. Curr Allergy Asthma Rep 2016; 16:7; PMID:26742760; http://dx.doi.org/10.1007/s11882-015-0580-7
- Karagiannis P, Villanova F, Josephs DH, Correa I, Van Hemelrijck M, Hobbs C, Saul L, Egbuniwe IU, Tosi I, Ilieva KM et al. Elevated IgG4 in patient circulation is associated with the risk of disease progression in melanoma. Oncoimmunology 2015; 4:e1032492; PMID:26451312; http://dx.doi.org/10.1080/2162402X.2015.1032492
- Aleyd E, Heineke MH, van Egmond M. The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol Rev 2015; 268:123-38; PMID:26497517; http://dx.doi.org/10.1111/imr.12337
- Rodríguez-Pinto D. B cells as antigen presenting cells. Cell Immunol 2005; 238:67-75; PMID:16574086; http://dx.doi.org/10.1016/j.cellimm.2006.02.005
- Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol 2001; 13:1583-93; PMID:11717199; http://dx.doi.org/10.1093/intimm/13.12.1583
- Von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, Xia Z, Zeng WY, Wucherpfennig KW, Nadler LM et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 2002; 99:3319-25; PMID:11964299; http://dx.doi.org/10.1182/blood.V99.9.3319
- Becker HJ, Kondo E, Shimabukuro-Vornhagen A, Theurich S, von Bergwelt-Baildon MS. Processing and MHC class II presentation of exogenous soluble antigen involving a proteasome-dependent cytosolic pathway in CD40-activated B cells. Eur J Haematol 2016; 97:166-74; PMID:26561366; http://dx.doi.org/10.1111/ejh.12699
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature 1985; 314:537-9; PMID:3157869; http://dx.doi.org/10.1038/314537a0
- Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis 2013; 72 Suppl 2:ii80-4; PMID:23253921; http://dx.doi.org/10.1136/annrheumdis-2012-202253
- Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015; 74:318-26; PMID:25742773; http://dx.doi.org/10.1016/j.cyto.2015.02.007
- Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol 2004; 172:3422-7; PMID:15004141; http://dx.doi.org/10.4049/jimmunol.172.6.3422
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007; 178:6092-9; PMID:17475834; http://dx.doi.org/10.4049/jimmunol.178.10.6092
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 2000; 1:475-82; PMID:11101868; http://dx.doi.org/10.1038/82717
- Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med 2003; 349:125-38; PMID:12853585; http://dx.doi.org/10.1056/NEJMoa035588
- Zarkhin V, Kambham N, Li L, Kwok S, Hsieh SC, Salvatierra O, Sarwal MM. Characterization of intra-graft B cells during renal allograft rejection. Kidney Int 2008; 74:664-73; PMID:18547992; http://dx.doi.org/10.1038/ki.2008.249
- Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res 2003; 63:2836-43; PMID:12782589
- Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y, Lundy SK, Ito F, Pan Q, Zhang X et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur J Immunol 2015; 45:999-1009; PMID:25545618; http://dx.doi.org/10.1002/eji.201444625
- Xia Y, Tao H, Hu Y, Chen Q, Chen X, Xia L, Zhou L, Wang Y, Bao Y, Huang S et al. IL-2 augments the therapeutic efficacy of adoptively transferred B cells which directly kill tumor cells via the CXCR4/CXCL12 and perforin pathways. Oncotarget 2016; 37:60461-74; PMID:27528023; http://dx.doi.org/10.18632/oncotarget.11124
- Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol 2004; 15:1109-14; PMID:15205206; http://dx.doi.org/10.1093/annonc/mdh280
- Theurich S, Schlaak M, Steguweit H, Heukamp LC, Wennhold K, Kurschat P, Rabenhorst A, Hartmann K, Schlösser H, Shimabukuro-Vornhagen A et al. Targeting tumor-infiltrating B cells in cutaneous T-cell lymphoma. J Clin Oncol 2016; 34:e110-6; PMID:25348001; http://dx.doi.org/10.1200/JCO.2013.50.9471
- Pinc A, Somasundaram R, Wagner C, Hörmann M, Karanikas G, Jalili A, Bauer W, Brunner P, Grabmeier-Pfistershammer K, Gschaider M et al. Targeting CD20 in melanoma patients at high risk of disease recurrence. Mol Ther 2012; 20:1056-62; PMID:22354376; http://dx.doi.org/10.1038/mt.2012.27
- Kondo E, Gryschok L, Klein-Gonzalez N, Rademacher S, Weihrauch MR, Liebig T, Shimabukuro-Vornhagen A, Kochanek M, Draube A, von Bergwelt-Baildon MS. CD40-activated B cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential. Clin Exp Immunol 2009; 155:249-56; PMID:19040609; http://dx.doi.org/10.1111/j.1365-2249.2008.03820.x
- Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 2004; 103:2046-54; PMID:14630810; http://dx.doi.org/10.1182/blood-2003-07-2379
- Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, Li S, Chang AE. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res 2011; 17:4987-95; PMID:21690573; http://dx.doi.org/10.1158/1078-0432.CCR-11-0207
- Ebstein F, Keller M, Paschen A, Walden P, Seeger M, Bürger E, Krüger E, Schadendorf D, Kloetzel PM, Seifert U. Exposure to Melan-A/MART-126-35 tumor epitope specific CD8(+)T cells reveals immune escape by affecting the ubiquitin-proteasome system (UPS). Sci Rep 2016; 6:25208; PMID:27143649; http://dx.doi.org/10.1038/srep25208
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015; 523:231-5; PMID:25970248; http://dx.doi.org/10.1038/nature14404
- Bradley SD, Chen Z, Melendez B, Talukder A, Khalili JS, Rodriguez-Cruz T, Liu S, Whittington M, Deng W, Li F et al. BRAFV600E co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol Res 2015; 3:602-9; PMID:25795007; http://dx.doi.org/10.1158/2326-6066.CIR-15-0030
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695-710; PMID:23897981; http://dx.doi.org/10.1084/jem.20130579
- Josephs DH, Spicer JF, Karagiannis P, Gould HJ, Karagiannis SN. IgE immunotherapy: a novel concept with promise for the treatment of cancer. MAbs 2014; 6:54-72; PMID:24423620; http://dx.doi.org/10.4161/mabs.27029
