ABSTRACT
NKp46 is a major determinant of natural killer (NK) cell function and it is implicated in tumor immune surveillance in acute myeloid leukemia (AML). The purpose of this study was to investigate the prognostic significance of NKp46 expression in an independent cohort of patients with AML, and to investigate the impact of NKp46 on clinical outcome after allogeneic stem cell transplantation (allo-SCT).
NKp46 expression was assessed at diagnosis on NK cells by flow cytometry (N = 180 patients). Clinical outcome was evaluated with regard to NKp46 expression. Patients with NKp46high phenotype at diagnosis had better progression-free survival (PFS) and overall survival (OS) than patients with NKp46low phenotype (74.3% vs. 46.6%, p = 0.014; 82.6% vs. 57.1%, p = 0.010, respectively). In multivariate analysis, high NKp46 was an independent factor for improved OS (HR = 0.409, p = 0.010) and PFS (HR = 0.335, p = 0.011). Subgroup analysis revealed that allo-SCT had a favorable impact on PFS in patients with NKp46high phenotype (p = 0.025). By contrast, allo-SCT did not impact PFS in patients with low NKp46 expression (p = 0.303).
In conclusion, we validate the prognostic value of NKp46 expression at diagnosis in AML. However, the prognostic value of NKp46 expression is limited to patients treated with allo-SCT, thus suggesting that NKp46 status may be predictive for allo-SCT responsiveness.
Introduction
Acute myeloid leukemia (AML) is a hematologic disorder characterized by variable responsiveness to treatment. Induction chemotherapy based on cytarabine and anthracyclines induces complete remission (CR) in most patients; however, relapse concerns most patients.Citation1 In this context, precise and accurate patient stratification criteria are mandatory to enable identification of patients likely to benefit from allogeneic stem cell transplantation (allo-SCT). Therefore, discovery and validation of novel prognostic biomarkers is crucial for outcome prediction. However, most biomarkers lack formal validation on independent multicenter cohorts of patients, which is a challenging but mandatory step before clinical applications.Citation2,3
Actual prognostic groups are based on cytogenetics,Citation4,5 and European LeukemiaNet (ELN) genetic classification.Citation6 These classifications define three groups of patients, i.e., favorable, adverse and intermediate prognosis; the benefits of post-remission therapy (PRT) with allo-SCT in patients with intermediate prognosis remain controversial,Citation7-9 and additional prognostic parameters are necessary to refine this classification. New molecular markers have been shown to impact prognosis and may be included in future revisions of ELN classification.Citation6,10,11 However, molecular markers do not account for the entire prognostic heterogeneity of AML and new markers are warranted.
Beside genetic alterations, accumulating evidence highlights the microenvironment and, in particular, deficient immunity as factors strongly implicated in tumor progression and resistance to chemotherapy.Citation12 Thus, immune parameters are currently being extensively developed as prediction tools in solid tumors.Citation13-16 Natural killer (NK) cells are key components of the innate immunity and substantially contribute to antitumor immune responses.Citation17-19 In AML patients, NK cells play a major role in maintaining prolonged remission, especially in the context of allo-SCT.Citation20 Among crucial parameters linked to NK antitumor activity, NK-activating receptors expression such as natural cytotoxic receptors (NCR), notably NKp46 play a crucial role.Citation21-23 In line with this, our group previously reported that low NKp46 expression on NK cells was significantly associated with reduced overall survival (OS) in AML.Citation21 However, formal validation of this finding is an absolute prerequisite before considering any clinical application. Therefore, the aim of this study was to validate the prognostic value of NKp46 expression on clinical outcome in an independent multicenter cohort of patients with AML. A subgroup analysis in patients treated with allo-SCT in first complete remission (CR1) revealed that NKp46 expression impacts clinical response to allo-SCT.
Results
NKp46 expression at diagnosis
Baseline NKp46 expression on NK cells was assessed by flow cytometry. Patients were classified according to NKp46 rMFI. The threshold used to discriminate patients with NKp46low and NKp46high phenotype was based on dispersion criteria (Fig. S1), and defined as the intersection between the two Gaussian distributions among patients (rMFI = 43.5; see Patients and Methods for further details). Among 180 patients, 35 (19.4%) had high NKp46 expression on NK cells (NKp46high phenotype), and 145 (80.6%) had low NKp46 expression on NK cells (NKp46low phenotype) (). Frequency of patients with NKp46 high and low phenotype did not differ between age groups, cytogenetics or number of inductions (). Median follow-up after documentation of CR was 55.3 mo.
Table 1. Baseline patient characteristics.
Prognostic value of NKp46 expression at diagnosis
In univariate analysis, 4-y progression-free survival (PFS) after CR was better in the NKp46high group, with 74.3% (95%CI, 61.1% to 90.3%) versus 46.6% (95%CI, 38.8% to 55.9%) in the NKp46low group (p = 0.014; ). Four-year OS after CR was better in the NKp46high group, with 82.6% (95%CI, 70.8% to 96.3%) versus 57.1% (95%CI, 49.3% to 66.1%) in the NKp46low group (p = 0.010; ).
Figure 1. Kaplan–Meier estimates of progression-free survival (A) and overall survival (B) by NKp46 expression at diagnosis. Cumulative incidence of relapse (C) and non-relapse mortality (D) by NKp46 expression at diagnosis. The numbers at the bottom of each plot represent the number at risk at the beginning of each 12-mo period for each group of patients. CR: complete remission. Statistical analyses were performed using a log Rank tests. p < 0.05 was considered significant.
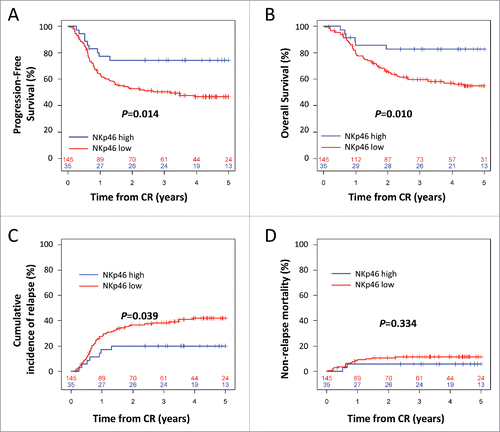
High NKp46 expression at diagnosis was inversely correlated with cumulative incidence of relapse (CIR), with 20.0% (95%CI, 5.6% to 32.2%) versus 42.1% (95%CI, 33.1% to 49.9%) in the NKp46low group (p = 0.039; ). There was no significant difference in non-relapse mortality between patients with high and low NKp46 expression (p = 0.334; ).
Multivariate Cox regression analysis was performed to assess the predictive value of NKp46 expression while adjusting for the prognostic factors in the population (age at transplantation, cytogenetics, white blood cells and number of inductions). In multivariate analysis, high NKp46 expression was significantly associated with improved PFS (HR = 0.409; 95%CI = [0.20–0.81]; p = 0.010) and OS (HR = 0.335; 95%CI = [0.14–0.78]; p = 0.011) (). Notably, in multivariate analysis, NKp46 status was more significantly associated with clinical outcome than cytogenetic risk group for both OS and PFS.
Table 2. Multivariate analysis of PFS and OS.
Prognostic value of NKp46 expression is restricted to patients treated with allogeneic haematopoietic stem cell transplantation (allo-SCT)
We then assessed the impact of NKp46 expression at diagnosis on clinical outcome after allo-SCT. Of 180 patients, 66 (36.7%) received allo-SCT in CR1. Four-year clinical outcome was assessed using Cox regression models after controlling for allo-SCT as a time-dependent covariate. Patients were stratified by NKp46 expression and post-remission treatment.
In the group of patients with high NKp46 expression, allo-SCT significantly improved PFS, with PFS rates of 100% (95%CI, 100% to 100%) versus 60.4% (95%CI, 42.9% to 85.1%) in the conventional PRT group (p = 0.025; ). Consistently, allo-SCT was associated with improved OS with 100% (95%CI, 100% to 100%) versus 71.2% (95%CI, 54.0% to 93.8%) in the conventional PRT group (). The significance for OS could not be tested due to the absence of death in the group of patients with high NKp46 expression, which precludes multivariate analyses ().
Figure 2. Kaplan–Meier estimates of progression-free survival (A, C) and overall survival (B,D) according to post-remission therapy in patients with low (A, B) or high (C, D) NKp46 expression at diagnosis. The numbers at the bottom of each plot represent the number at risk at the beginning of each 12-mo period for each group of patients. CR: complete remission. Statistical analyses were performed using a log Rank tests. p < 0.05 was considered significant.
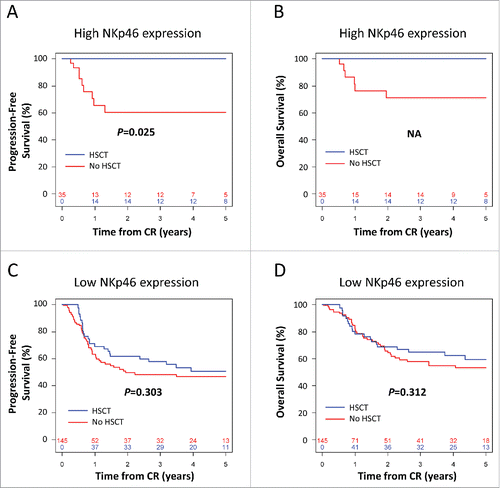
By contrast, in the group of patients with low NKp46 expression, the impact of allo-SCT on PFS was not significant, with PFS rates of 50.8% (95%CI, 38.4% to 67.1%) versus 46.6% (95%CI, 37.0% to 58.6%) in the conventional PRT group (p = 0.303; ). Consistently, allo-SCT did not impact OS in the group of patients with low NKp46 expression, with OS rates of 62.3% (95%CI, 50.3% to 77.3%) versus 55.0% (95%CI, 45.2% to 66.8%) in the conventional PRT group (p = 0.312; ).
These results clearly suggest that the prognostic effect of NKp46 expression on survival observed in the total population is limited to the subgroup of patients with high NKp46 expression treated with allo-SCT. In addition, since allo-SCT selectively impacts survival in patients with high NKp46 expression, our data strongly suggest that NKp46 expression at diagnosis can be considered as a predictive biomarker of response to allo-SCT.
Discussion
NK cells are potent immune effectors that mediate graft-versus-leukemia effects.Citation24,25 Among NK activating receptors, NCR such as NKp46 are among the most important, acting by triggering cytolytic responses to tumor target cells.Citation21,25-28 The prognostic value of NKp46 expression at diagnosis in AML patients described in the present multicenter study is a formal validation of previous results published by our team.Citation21 Patients' distribution histograms confirmed the existence of two distinct populations of patients based on NKp46 intensity of expression (Fig. S1). In the present study, the threshold validation for NKp46 expression was based on objective dispersion criteria. The first group of patients characterized by a low expression of NKp46 was statistically associated with poor prognosis compared with the second group, which had a higher NKp46 expression. In addition, our study confirms that NKp46 is an independent prognostic biomarker at diagnosis. In contrast to our previous study with an exploratory cohort, we have included in our multivariate analyses all the currently admitted confounding variables (leukocytosis, age, cytogenetics, number of inductions), and NKp46 appeared as the most important risk factor compared with other variables in multivariate models.
We then assessed the impact of NKp46 expression at diagnosis on clinical outcome after allo-SCT. Our data suggest that the clinical outcome after allo-SCT is strongly dependent on NKp46 status at the time of diagnosis. Indeed, the clinical benefit of allo-SCT is exclusively observed in the subgroup of patients with high NKp46 expression, whereas in the subgroup of patients with low NKp46 expression, there was no significant effect of allo-SCT on survival and relapse. These results support the idea that NKp46 can be considered as a predictive biomarker for clinical outcome after allogeneic transplantation, since the observed benefit only occurs in case of treatment by allo-SCT. Interestingly, this biomarker is assessable at diagnosis, which is a great advantage compared with most surrogate biomarkers in allo-SCT assessed after transplantation.Citation29-32
The impact of NKp46 expression on patients' NK cells at diagnosis on clinical outcome after transplantation is puzzling. Indeed, it is difficult to understand how the phenotype of NK cells at the diagnosis of AML can impact of clinical events taking place several months later and in an allogeneic context, where NK cells come from the donor.
NKp46 is a triggering receptor implicated in malignant cell recognition and destruction. Low NKp46 expression on NK cells is responsible for poor blast recognition,Citation21 and has been correlated with high minimal residual disease (MRD) after induction therapy in acute lymphoid leukemia.Citation33 In our study, the impact of NKp46 expression on NK cells at diagnosis on clinical outcome after transplantation may be explained, at least, by a higher MRD in patients with low NKp46 expression, favoring emergence of clones leading to relapse.
Beside relapse, failure of allo-SCT is mainly due to severe graft-versus-host disease (GvHD). Importantly, we noticed the absence of death by GvHD in the group of NKp46high patients. In a recent study in a NKp46 knockout mouse model, it was evidenced an exacerbated GvHD in an experimental transplantation setting.Citation34 Although not directly transferrable to human setting, questions raise from this study concerning the potential of NKp46high expression to prepare the immune cells to control GvH reaction. The number of patients with NKp46high phenotype in our cohort did not enable us to analyze the impact of NKp46 expression on GvHD but should prompt further study in a larger cohort. If confirmed, this observation could provide additional arguments to stimulate NKp46 expression to enhance NK-mediated graft-versus-leukemia effect without inducing GvHD.
Mechanistically, the mechanisms of subversion of NK cells by leukemic cells remain challenging. This immune subversion has been observed in other cancer settings.Citation23,35
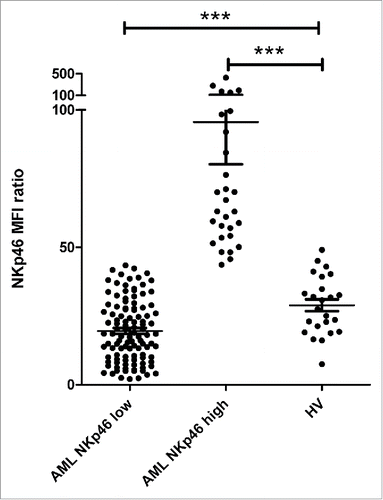
Interestingly, in the group of NKp46high patients, NKp46 expression was higher than that of healthy volunteers (HV) (median ± SD = 60.2 ± 16.0 vs. 29.2 ± 10.6, p < 0.0001, ). This abnormal high expression may reflect some degree of activation, since activated NK cells display increase in activating receptor expression. This appeals for further investigation to test whether activation markers are also expressed in this group of patients. Additionally, a recent study have shown that NK cell gene expression, which was altered at diagnosis, was completely restored after in vitro activation and expansion.Citation36 Therefore, it would be interesting to analyze whether gene expression of these NKp46high AML-NK corresponds to activated NK cells. Alternatively, a gene expression comparison between NKp46low and NKp46high patients may provide cues for membrane-bound or soluble factors provided by leukemic cells and responsible for NK cell defects. This strategy has been used by Khaznadar et al. in a recent study, and reveals that patients with a “normal” NK phenotype, gene data sets included pathways related to immune reaction, which is in line with our findings regarding NKp46high patients.Citation37
Figure 3. Comparison of NKp46 expression in AML patients and HV. NKp46 expression on NK cells was assessed by flow cytometry at diagnosis. Patients were stratified by NKp46 expression. NKp46 expression in each subgroup of patients (NKp46high and NKp46low) was compared with HV. Abbreviations: HV: healthy volunteers. Differences were assessed with a Student's t test. p < 0.05 was considered significant. ***p < 0.0001.
On the other hand, further investigations should focus on the different isoforms of NKp46. Indeed, four isoforms of NKp46 have been described, with isoform d having the highest activity,Citation38 and whose relative expression might be modulated by the microenvironment.Citation39 Moreover, recent studies demonstrated that NCR isoforms relative expression impact clinical outcome, as demonstrated for splice variants of NKp30 in gastrointestinal stromal tumorsCitation35 as well as splice variants of NKp44 in AML.Citation40
Beside applications for AML patients, a perspective of the present work is to investigate the generalization of our finding to other pathologies, such as refractory Hodgkin lymphoma or high-grade lymphoma. In the case of these pathologies, allo-SCT is a therapeutic option likely to be balanced with alternative treatment with anti-PD1 therapy. The possibility of the prediction of success of allo-SCT in these contexts would be highly relevant for clinical decision making.
In conclusion, our results formally validate the prognostic value of NKp46 expression in AML described in our previous work.Citation21 However, the prognostic value of NKp46 expression is limited to patients treated with allo-SCT, thus suggesting that NKp46 status may actually be predictive for allo-SCT responsiveness, as opposed to strictly prognostic.Citation41 Although this conclusion requires further validation on an independent cohort of patients, our study provides a strong rationale to develop interventional therapy to induce NKp46 expression after allo-SCT.
Patients and methods
Patients
All participants gave written informed consent in accordance with the Declaration of Helsinki. The entire research procedure was approved by the ethical review boards from the IPC and the GOELAMS. The patient characteristics, stratified by NKp46 expression groups, are summarized in . Baseline NKp46 expression on NK cells at diagnosis was assessed by flow cytometry in 180 patients with newly diagnosed AML. Two cohorts of patients were merged for this study. The Paoli Calmettes Institute (IPC) prospective cohort included 114 patients with newly diagnosed non-acute promyelocytic leukemia (APL) AML admitted between November 2007 and November 2012, aged 18 to 65 y and treated with conventional 3+7 induction chemotherapy as described previously.Citation42 The Groupe Ouest Est d'Etude des Leucémies Aiguës et autres Maladies du Sang (GOELAMS) cohort included 66 patients. The median age at induction was 48 y (range: 19–59). All patients were included in the LAM2006IR prospective multicenter randomized trial between November 2007 and April 2012 (NCT00860639). All patients had previously untreated AML with intermediate cytogenetics, as defined by Slovak et al.Citation43 Patients received conventional 3+7 induction chemotherapy with or without the addition of Gemtuzumab Ozogamicin.Citation44 Patients with APL AML, patients above 66 y and patients without CR after one or two courses of induction chemotherapy were not eligible for this study. The median age at induction was 47 y (range: 18–65). Out of 180 patients, 22 had favorable cytogenetics (12.2%), 139 had intermediate cytogenetics (77.2%) and 19 had unfavorable cytogenetics (10.6%). Sixty-six (36.7%) patients received allo-SCT in their CR1.
Clinical samples
Fresh total peripheral blood samples (IPC cohort) or peripheral blood mononuclear cells (PBMC) cryopreserved in 90% fetal calf serum / 10% Dimethyl Sulfoxide (DMSO) (GOELAMS cohort) were obtained from randomly selected patients at diagnosis before induction chemotherapy and analyzed by flow cytometry. Fresh total peripheral blood from age-matched (range: 18–65) HV was obtained from the Etablissement Français du Sang (EFS). Samples were stained and analyzed according to the procedure used for the IPC cohort (N = 24). For the GOELAMS validation cohort, handling, conditioning and storing of patients samples were performed by the FILOtheque AML (N° BB-0033–00073), tumor bank of the FILO group, Cochin hospital, Paris.
Flow cytometry
For the IPC cohort, analyses were performed in the Biopathology department of the Paoli Calmettes Institute. A FACS Canto II (BD Biosciences), and FACS Diva Software (BD Biosciences) were used for flow cytometry. For the GOELAMS cohort, analyses were performed in the Immunomonitoring platform of the Paoli Calmettes Institute. A LSR Fortessa (BD Biosciences) was used for flow cytometry. Cells were immunostained with Krome Orange (KOTM)- or allophycocyanin (APC)-conjugated anti-CD45, fluorescein isothiocyanate (FITC)–conjugated or Phycoerythrin-Cyanine 7 (PC7)- or Phycoerythrin-Texas Red-xTM (ECD)-conjugated anti-CD3, and PC7-, APC AF700- or APC–conjugated anti-CD56. Triggering receptor expression NKp46 was measured with Phycoerythrin-Cyanine 5 (PC5)-conjugated monoclonal antibodies. Isotype controls were mouse immunoglobulin G conjugated to PC5. All antibodies used in this study were kindly provided by Beckman-Coulter. For total blood, red blood cells were lysed with BD FACS Lysing solution (BD Biosciences) before data acquisition. The NKp46 mean fluorescence intensity (MFI) ratio (NKp46 MFI / isotype control MFI, referred to as rMFI) was calculated for each patient. Assays were performed blinded to the study end point.
Threshold determination
Thresholds were calculated based on the results of the IPC cohort. Patients were classified into two groups, NKp46low and NKp46high, according to NKp46 rMFI. The dichotomy between NKp46low and NKp46high patients was based on dispersion criteria of the population. Inter-individual variability of NKp46 expression in AML patients (Fig. S1A) was represented on a distribution histogram. The distribution of NKp46 expression was a juxtaposition of two Gaussian distributions (d'Agostino-Pearson normality test and Kernel density estimation). The threshold between these two peaks was NKp46 rMFI = 43.5 (Fig. S1A). Of note, this threshold was above the 90th percentile of HV ().
All the possible thresholds were tested in the range of NKp46 expression for OS and PFS (Fig. S1B and S1C, respectively). The threshold based on dispersion criteria was also the most discriminant threshold for survival analyses. For the rest of the study, patients were classified into two distinct subgroups (NKp46high and NKp46low phenotype) for survival analyses according to this threshold. For samples from the GOELAMS cohort, analyses were performed on a different cytometer. A correction factor was applied for NKp46 rMFI so the cohorts could be merged. The correction factor was the equation of the regression of paired samples (fresh samples analyzed on a CantoII and paired frozen samples analyzed on a LSRII Fortessa).
Statistical analysis
Statistical analyses were performed using Graph Pad Prism (Graph Pad Software, San Diego, CA) and R software (http://www.r-project.org). The limit of significance was set at p < 0.05. OS from CR was defined by the time between CR achievement after induction therapy until death from any cause, and PFS as time between CR achievement and relapse or death, whatever occurred first. Patients without an event were censored at the time of their last follow-up. Survival times were estimated by the Kaplan–Meier method and compared using the log-rank test. For OS and PFS stratified by post-remission therapy, hazard ratios for OS and PFS were determined by Cox regression analysis, while treating allo-SCT as a time-dependent covariate. The cumulative incidences of leukemia relapse and death in CR1 were calculated using the Prentice estimator, considering relapse and death in CR1 as mutually competing events. The impact of NKp46 on these cumulative incidences was evaluated with the Gray test.Citation45 Cox regression analysis was performed to adjust the impact of NKp46 using age (continuous variable), white blood cell count at diagnosis (continuous variable), cytogenetics (low vs. intermediate vs. high) and number of induction course to obtained CR1 (1 vs. 2). Analyses for the main end points were performed on an intention-to-treat basis. The X2 or Fisher's exact test was used to assess association between variables. The subgroup analysis of patients treated with allo-SCT was defined post hoc, and those results therefore have to be considered hypothesis generating.
Authors' disclosures of potential conflicts of interest
Anne-Sophie Chretien: Patents, Royalties, Other Intellectual Property : Inserm Transfert. Raynier Devilliers: No relationship to disclose. Cyril Fauriat: Patents, Royalties, Other Intellectual Property : Inserm Transfert. Florence Orlanducci: No relationship to disclose. Samia Harbi: No relationship to disclose. Aude Le Roy: No relationship to disclose. Jérôme Rey: Consulting or Advisory Role: Novartis. Travel, Accommodations, Expenses : Novartis. Gaelle Bouvier Borg: Employment: Immunotech Beckman Coulter. Emmanuel Gautherot: Employment: Immunotech Beckman Coulter. Jean-François Hamel: No relationship to disclose. Norbert Ifrah: No relationship to disclose. Catherine Lacombe: No relationship to disclose. Pascale Cornillet-Lefebvre: No relationship to disclose. Jacques Delaunay: No relationship to disclose. Antoine Toubert: No relationship to disclose. Christine Arnoulet: Patents, Royalties, Other Intellectual Property : Inserm Transfert. Norbert Vey: Consulting or Advisory Role : Novartis, Roche. Travel, Accommodations, Expenses : Amgen, Novartis Patents, Royalties, Other Intellectual Property : Inserm Transfert. Didier Blaise: Patents, Royalties, Other Intellectual Property : Inserm Transfert. Daniel Olive: Stock or Other Ownership : Imcheck Therapeutics. Research Funding : GSK. Patents, Royalties, Other Intellectual Property : Inserm Transfert, GSK.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_materials.zip
Download Zip (3.7 MB)Acknowledgments
The authors thank Beckman Coulter for their technical advices and for providing the antibodies used in this study. The authors thank the Paoli Calmettes Institute immunomonitoring platform for their valued contributions to this work. The authors thank the Groupe Ouest Est d'Etude des Leucémies Aiguës et autres Maladies du Sang (GOELAMS), the FILOtheque AML (N° BB-0033–00073), and Lamya Haddaoui, for their implication in this study.
Funding
This work has been financially supported by the INCa, the SIRIC (grant INCa-DGOS-INSERM 6038), the GS IBiSA. D.O. team was labeled “Equipe FRM DEQ 201 40329534.” D.O. is Senior Scholar of the Institut Universitaire de France. All antibodies used were kindly provided by Beckman-Coulter, Marseille, France. Beckman Coulter and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc. in the United States and other countries. All other trademarks are the property of their respective owners.
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Eng J Med 2015; 373:1136-52; PMID:26376137; https://doi.org/10.1056/NEJMra1406184
- Poste G. Bring on the biomarkers. Nature 2011; 469:156-7; PMID:21228852; https://doi.org/10.1038/469156a
- Buyse M, Sargent DJ, Grothey A, Matheson A, de Gramont A. Biomarkers and surrogate end points–the challenge of statistical validation. Nat Rev Clin Oncol 2010; 7:309-17; PMID:20368727; https://doi.org/10.1038/nrclinonc.2010.43
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood 1998; 92:2322-33; PMID:9746770
- Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH, Medical Research Council Adult Leukemia Working Party. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood 2001; 98:1312-20; PMID:11520776; https://doi.org/10.1182/blood.V98.5.1312
- Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115:453-74; PMID:19880497; https://doi.org/10.1182/blood-2009-07-235358
- Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A, Ligges S, Sauer T, Tschanter P, Thoennissen GB et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol 2013; 50:5768; PMID:24366930; doi:10.1200/JCO.2013.50.5768
- Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009; 301:2349-61; PMID:19509382; https://doi.org/10.1001/jama.2009.813
- Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood 2016; 127:62-70; PMID:26660427; https://doi.org/10.1182/blood-2015-07-604546
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK, National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010; 116:354-65; PMID:20385793; https://doi.org/10.1182/blood-2009-11-254441
- Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Eng J Med 2012; 366:1079-89; PMID:22417203; https://doi.org/10.1056/NEJMoa1112304
- Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia 2012; 26:2019-26; PMID:22446501; https://doi.org/10.1038/leu.2012.87
- Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol 2006; 24:1997-2005; PMID:16648500; https://doi.org/10.1200/JCO.2005.03.9594
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; https://doi.org/10.1126/science.1129139
- Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016; 44:698-711; PMID:26982367; https://doi.org/10.1016/j.immuni.2016.02.025
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610-8; PMID:21245428; https://doi.org/10.1200/JCO.2010.30.5425
- Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Human natural killer cells: Molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett 2005; 100:7-13; PMID:16109445; https://doi.org/10.1016/j.imlet.2005.07.004
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; https://doi.org/10.1126/science.1198687
- Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012; 12:239-52; PMID:22437937; https://doi.org/10.1038/nri3174
- Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SG, Geraghty D, Spellman S, Haagenson MD et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010; 116:2411-9; PMID:20581313; https://doi.org/10.1182/blood-2010-05-283051
- Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 2007; 109:323-30; PMID:16940427; https://doi.org/10.1182/blood-2005-08-027979
- Garcia-Iglesias T, del Toro-Arreola A, Albarran-Somoza B, del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Dueà ± as MG, Balderas-Peña LM, Bravo-Cuellar A, Ortiz-Lazareno PC, Daneri-Navarro A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer 2009; 9:186; PMID:19531227; https://doi.org/10.1186/1471-2407-9-186
- Pasero C, Gravis G, Granjeaud S, Guerin M, Thomassin-Piana J, Rocchi P, Salem N, Walz J, Moretta A, Olive D. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget 2015; 6:14360-73; PMID:25961317; https://doi.org/10.18632/oncotarget.3965
- Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nat Rev Immunol 2003; 3:108-22; PMID:12563295; https://doi.org/10.1038/nri999
- Triplett BM, Horwitz EM, Iyengar R, Turner V, Holladay MS, Gan K, Behm FG, Leung W. Effects of activating NK cell receptor expression and NK cell reconstitution on the outcomes of unrelated donor hematopoietic cell transplantation for hematologic malignancies. Leukemia 2009; 23:1278-87; PMID:19212329; https://doi.org/10.1038/leu.2009.21
- Fauriat C, Marcenaro E, Sivori S, Rey J, Gastaut JA, Moretta A, Olive D, Costello RT. Natural killer cell-triggering receptors in patients with acute leukaemia. Leuk Lymphoma 2003; 44:1683-9; PMID:14692519; https://doi.org/10.1080/1042819031000104006
- Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Euro J Immunol 1999; 29:1656-66; https://doi.org/10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1
- Martner A, Rydström A, Riise RE, Aurelius J, Anderson H, Brune M, Foà R, Hellstrand K, Thorén FB. Role of natural killer cell subsets and natural cytotoxicity receptors for the outcome of immunotherapy in acute myeloid leukemia. Oncoimmunology 2016; 5:e1041701; PMID:26942055; https://doi.org/10.1080/2162402X.2015.1041701
- Clave E, Lisini D, Douay C, Giorgiani G, Busson M, Zecca M, Charron D, Bernardo ME, Toubert A, Locatelli F. A low thymic function is associated with leukemia relapse in children given T-cell-depleted HLA-haploidentical stem cell transplantation. Leukemia 2012; 26:1886-8; PMID:22430571; https://doi.org/10.1038/leu.2012.59
- Forcina A, Noviello M, Carbone M, Bonini C, Bondanza A. Predicting the clinical outcome of allogeneic hematopoietic stem cell transplantation: the long and winding road toward validated immune biomarkers. Front Immunol 2013; 4:71; PMID:23531639; https://doi.org/10.3389/fimmu.2013.00071
- Le Blanc K, Barrett AJ, Schaffer M, Hagglund H, Ljungman P, Ringden O, Remberger M. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant 2009; 15:1108-15; PMID:19660724; https://doi.org/10.1016/j.bbmt.2009.05.015
- Savani B, Mielke S, Adams S, Uribe M, Rezvani K, Yong A, Zeilah J, Kurlander R, Srinivasan R, Childs R et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia 2007; 21:2145-52; PMID:17673900; https://doi.org/10.1038/sj.leu.2404892
- Sullivan EM, Jeha S, Kang G, Cheng C, Rooney B, Holladay M, Bari R, Schell S, Tuggle M, Pui CH et al. NK cell genotype and phenotype at diagnosis of acute lymphoblastic leukemia correlate with postinduction residual disease. Clin Cancer Res 2014; 20:5986-94; PMID:25281696; https://doi.org/10.1158/1078-0432.CCR-14-0479
- Ghadially H, Ohana M, Elboim M, Gazit R, Gur C, Nagler A, Mandelboim O. NK cell receptor NKp46 regulates graft-versus-host disease. Cell Rep 2014; 7:1809-14; PMID:24882008; https://doi.org/10.1016/j.celrep.2014.05.011
- Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011; 17:700-7; PMID:21552268; https://doi.org/10.1038/nm.2366
- Venton G, Labiad Y, Colle J, Fino A, Afridi S, Torres M, Monteuil S, Loriod B, Fernandez-Nunez N, Farnault L et al. Natural killer cells in acute myeloid leukemia patients: from phenotype to transcriptomic analysis. Immunol Res 2016; 64:1225-36; PMID:27481509; https://doi.org/10.1007/s12026-016-8848-0
- Khaznadar Z, Boissel N, Agaugue S, Henry G, Cheok M, Vignon M, Geromin D, Cayuela JM, Castaigne S, Pautas C et al. Defective NK cells in acute myeloid leukemia patients at diagnosis are associated with blast transcriptional signatures of immune evasion. J Immunol 2015; 195(6):2580-90; PMID:26246143; doi:10.4049/jimmunol.1500262
- Shemer Avni Y, Kundu K, Shemesh A, Brusilovsky M, Yossef R, Meshesha M, Solomon-Alemayehu S, Levin S, Gershoni-Yahalom O, Campbell KS et al. Expression of NKp46 splice variants in nasal lavage following respiratory viral infection: domain 1-negative isoforms predominate and manifest higher activity. Front Immunol 2017; 8:161; PMID:28261217; https://doi.org/10.3389/fimmu.2017.00161
- Siewiera J, Gouilly J, Hocine H-R, Cartron G, Levy C, Al-Daccak R, Jabrane-Ferrat N. Natural cytotoxicity receptor splice variants orchestrate the distinct functions of human natural killer cell subtypes. Nat Commun 2014; 6:10183; PMID:26666685; https://doi.org/10.1038/ncomms10183
- Shemesh A, Brusilovsky M, Hadad U, Teltsh O, Edri A, Rubin E, Campbell KS, Rosental B, Porgador A. Survival in acute myeloid leukemia is associated with NKp44 splice variants. Oncotarget 2016; 7:32933; PMID:27102296; doi:10.18632/oncotarget.8782
- Ballman KV. Biomarker: predictive or prognostic? J Clin Oncol 2015; 63:3651; PMID:26392104; doi:10.1200/JCO.2015.63.3651
- Devillier R, Gelsi-Boyer V, Murati A, Prebet T, Rey J, Etienne A, D'Incan E, Charbonnier A, Blaise D, Mozziconacci MJ et al. Prognostic significance of myelodysplasia-related changes according to the WHO classification among ELN-intermediate-risk AML patients. American journal of hematology 2015; 90:E22-E4; PMID:25219760; https://doi.org/10.1002/ajh.23850
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96:4075-83; PMID:11110676
- Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, Estey EH, Dombret H, Chevret S, Ifrah N et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 2014; 15:986-96; PMID:25008258; https://doi.org/10.1016/S1470-2045(14)70281-5
- Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de Lavallade H, Sarvaria A et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014; 99:836-47; PMID:24488563; https://doi.org/10.3324/haematol.2013.087536
