ABSTRACT
Up to now, the efficacy of programmed death protein 1/programmed death ligand 1 (PD-1/PD-L1) blockade in pancreatic cancer (PC) remains uncertain. Serum levels of soluble PD-1 and PD-L1 (sPD-1/sPD-L1) have been reported to be independent prognostic factors in solid tumors susceptible to checkpoint blockade. Provenience, regulation and immunologic function of sPD-1 and sPD-L1 in cancer are poorly understood. To the best of our knowledge, sPD-1 and sPD-L1 have not been measured conjointly in any cancer type yet.
In contrast to other tumor entities, sPD-1/sPD-L1 levels did not indicate an adverse outcome in a cohort of 41 patients with advanced PC. We observed a close positive correlation of sPD-L1 levels with sPD-1 in patients with advanced PC, suggesting a common provenience and regulation of sPD-1 and sPD-L1 in cancer patients. Higher sPD-L1 levels were present in patients with elevated C-reactive protein or strong tumoral T cell infiltration, while no correlation of sPD-L1 levels with tumoral PD-L1 expression was found. Our findings indicate that sPD-1 and sPD-L1 are markers of systemic inflammation in (pancreatic) cancer. In a subset of PC patients, elevation in sPD-L1 levels might be caused by an inflammatory tumor type – independent of tumoral PD-L1 expression.
| Abbreviations | ||
| CA 19–9 | = | carbohydrate antigen 19–9 |
| CEA | = | carcinoembryonic antigen |
| CRP | = | C-reactive protein |
| ECOG | = | Eastern Cooperative Oncology Group |
| HCC | = | hepatocellular carcinoma |
| HR | = | hazard ratio |
| IHC | = | immunohistochemistry |
| OS | = | overall survival |
| PC | = | pancreatic cancer |
| PD-1 | = | programmed death protein 1 |
| PD-L1 | = | programmed death receptor ligand 1 |
| sPD-1 | = | soluble programmed death protein 1 |
| sPD-L1 | = | soluble programmed death receptor ligand 1 |
Introduction
Pancreatic cancer (PC) is predicted to become the second leading cause of cancer-related death in the United States and Germany by 2030.Citation1,2 The vast majority of patients are diagnosed with advanced disease and do not qualify for potentially curative resection. Despite minor advances using intensified chemotherapeutic regimens like FOLFIRINOX and gemcitabine plus nab-paclitaxel, prognosis in advanced PC remains dire with a median overall survival (OS) of less than 1 y.Citation3,4 Accordingly, novel and innovative treatment strategies are urgently needed.
Inflammation is an important step in PC initiation and progression.Citation5,6 Almost a decade ago, checkpoint blockade targeting the programmed death protein 1/programmed death ligand 1 (PD-1/PD-L1) pathway was described as a promising therapeutic target in PC and has been proven to be successful in a variety of other solid tumor entities.Citation7-9 While the importance of PD-L1 in PC immune evasion was confirmed very recently, only preliminary – rather disappointing – data on the potential efficacy of PD-1/PD-L1 blockade is available in PC yet.Citation10-12 High levels of soluble plasma PD-L1 (sPD-L1) were described as an adverse prognostic factor in several malignancies susceptible to PD-1/PD-L1 blockade, including renal cell cancer and hepatocellular carcinoma (HCC).Citation13,14 To date, provenience, regulation and immunologic function of sPD-1 and sPD-L1 in cancer remain uncertain. Plasma levels of sPD-1 and sPD-L1 have not yet been determined conjointly in any cancer type. To our knowledge, neither sPD-1 nor sPD-L1 has previously been analyzed in patients with advanced PC.
Materials and methods
Patient characteristics
Serum samples of 41 patients with advanced PC (locally advanced: n = 6, metastatic: n = 35) diagnosed and/or treated at our high-volume comprehensive cancer center between 2011 and 2015 were prospectively collected before initiation of palliative chemotherapy. Clinical characteristics were obtained from a prospectively maintained database. All patients were followed up for survival status until September 2016. This translational research study was approved by the local ethics committee of Ludwig-Maximilians-University of Munich (approval number 284–10) and all patients gave written informed consent for the collection of blood and data analysis.
Blood sampling
Blood samples were obtained from each subject during routine venipuncture on day one of the first cycle of chemotherapy. To remove blood cells, serum tubes were centrifuged at 3,000 rounds per minute for 10 min at room temperature. Serum samples were aliquoted and stored at −80°C subsequently. Complete blood count, levels of C-reactive protein (CRP), carbohydrate antigen 19–9 (CA 19–9) and carcinoembryonic antigen (CEA) were measured at the central laboratory of LMU Munich University Hospital.
sPD-1 and sPD-L1 ELISAs
Soluble PD-1 (sPD-1) and PD-L1 (sPD-L1) were quantified by enzyme-linked immunosorbent assays (ELISA) using the human PD-1 antibody duosets for ELISA development (for PD-1 catalog number DY1086, for PD-L1 / B7-H1 DY156) from R&D Systems (Minneapolis, MN) that were applied on the MSD Mesoscale Quickplex SQ120 platform (MSD Mesoscale Diagnostics, Rockville, ML) enabling highly sensitive chemoluminescent detection. Antibody duosets contained streptavidin- and biotin-labeled capture and detection antibodies as well as appropriate standard material for PD-1 and PD-L1.
In brief, high bind microtiter plates from MSD were incubated with 25 µL/well capture antibodies (concentrations 2 µg/mL for PD-1, and PD-L1, respectively), sealed and incubated overnight. On the next day, plates were washed (3 × 200 µL/well PBS with 0.05% Tween). Then, 150 µL/well BSA (5% in PBS) was added as blocking agent, the plate was sealed and shaken at 700 rpm for 1 h. After a washing step (3 × 200 µL/well PBS-T), 25 µL of calibrators or patient samples were added, sealed and incubated for 2 h under shaking conditions. Calibration curve consisted of 1:4 dilutions of the standard material ranging from 30 ng/mL to 7 pg/mL. After a further washing step (3 × 200 µL/well PBS-T), 25 µL/well unlabeled detection antibodies were added (concentrations 200 ng/mL for PD-1, and 100 ng/mL for PD-L1, respectively), sealed and incubated for 2 h under shaking conditions. Once again plates were washed (3 × 200 µL/well PBS-T) and 25 µL/well streptavidin-sulfo-tag antibodies were added and incubated for 2 h. After a further washing step (3 × 200 µL/well PBS-T), 150 µL/well reading buffer was added and chemiluminescent measurement was performed on the SQ120 QuickPlex reader. Absolute concentrations of sPD-1 and sPD-L1 (in ng/mL) in the patient samples were calculated by use of a four-point-fit calibration curve of the standard dilutions.
Prior to clinical testing, the assays were optimized regarding plate selection (high-bind vs. standard plate), buffers antibody concentrations (by titrating different combinations) and streptavidin-labeling. Best antibody combinations were identified by highest signal-to-noise ratio over several standard concentrations. In addition, intra- and inter-assay imprecision as well as dilution linearity were tested.
Defining a cohort with high sPD-1 and sPD-L1 serum levels
Up to now, there is no defined cut-off value for sPD-1 and sPD-L1 in patients with cancer. In other cancer entities, different approaches to determine prognostic cut-off values have been used: median sPD-L1 serum level in the analyzed cancer patient cohort, sPD-L1 serum levels in a corresponding cohort of healthy individuals or receiver operating characteristic (ROC) curve models to define an ideal prognostic cut-off value.Citation14-18 In all aforementioned studies, the prognostic cut-off value was in the range of the 50th (i.e., median) and 75th percentile of the observed sPD-L1 levels in the whole analyzed patient cohort. When designing our study we therefore decided to use the median and the 75th percentile of sPD-1 and sPD-L1 levels observed in our study to define a cohort of patients with high versus low levels of the two molecules.
Tumor samples and immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue containing histologically confirmed exocrine pancreatic cancer was retrieved from the archives of the Institute of Pathology of the Ludwig-Maximilians-University or from external pathologists. Tumor tissue of eight patients was included in a tissue microarray (TMA) consisting of three 1 mm diameter tissue cores of different tumor regions from resection or biopsy specimens of each patient. To examine tumoral CD3+ lymphocyte infiltration and PD-L1 expression using immunohistochemistry (IHC) on 4-µm thick whole mount tissue sections or TMA sections, a Ventana Benchmark Ultra autostainer was used (Ventana, Tucson, AZ, USA). Briefly, the slides were dewaxed and antigenicity was retrieved using the Ventana antigen retrieval solution CC1 (pH 8.4, Ventana) for 64 min. The slides were then incubated with the rabbit monoclonal anti-PD-L1 antibody (1:100 dilution, clone E1L3N, Cell Signaling Technology, Danvers, MA, USA) for 32 min and after secondary antibody incubation (UltraView DAB Kit; Ventana) the staining was visualized using a diaminobenzidine system (Ventana). As a validation experiment, exemplary slides were also stained using a commercial Ventana PD-L1 assay kit. Briefly, slides were dewaxed and antigenicity was retrieved as described before. Slides were then incubated with the pre-diluted rabbit monoclonal anti PD-L1 antibody (clone SP263, concentration 1.61 µg/mL, Ventana) and staining was visualized using a diaminobenzidine kit after secondary antibody incubation (OptiView DAB Kit; Ventana). Appropriate positive (human tonsil) and negative controls (human liver) if not already present on the slides were included in each staining run. Only membranous PD-L1 staining in tumor cells was scored using the percentage of positive cells in the whole tissue sample. Rarely occurring pure cytoplasmic staining was considered as artifact and not counted. The amount of CD3+ lymphocyte infiltration was scored semiquantitatively using a three-tier score. Briefly, score 0 was considered very few (0–5) CD3+ per high power field (HPF) in 200× magnification. Score 1 was given when there were up to 50 CD3+ cells per HPF. Cases showing more than 50 CD3+ lymphocytes per HPF in 200-fold magnification were considered as score 2. All three tissue cores on TMA tissue as well as all the tumor tissue present on biopsy samples were read and CD3+ infiltrate was counted. Only true tumor infiltrating or tumor adjacent CD3+ cells were counted, excluding CD3+ cells in occasionally present lymph follicles or lymphatic aggregates.
Statistical analyses
OS was estimated using Cox-regression analysis and the Kaplan–Meier method. Median follow-up was calculated using the reversed Kaplan–Meier method. Differences in mean tumor marker levels were tested using an unpaired Student's t-test. Differences in variance were tested for statistical significance using an F-test. Correlation analyses were performed using the Pearson coefficient analysis. SPSS PASW 23.0 (SPSS Inc., Chicago, IL, USA) software was used for survival analyses. Graphpad Prism 7.01 (GraphPad Software Inc., La Jolla, CA USA) was used for comparison and correlation analyses.
Results
Patient characteristics
Between 2011 and 2015, serum samples of 41 consecutive patients with non-resectable PC (ductal adenocarcinoma: n = 40, acinar cell carcinoma n = 1) were prospectively collected before initiation of first-line chemotherapy. At the time of database lock in September 2016, 35 patients were deceased. Median OS for the whole study population was 10.8 mo (95% CI: 6.1 to 15.5 mo) with a median follow-up of 24.7 mo (95% CI: 19.6 to 30.0 mo). First-line palliative chemotherapy consisted of gemcitabine monotherapy (n = 11), different gemcitabine-based combination regimens (n = 11), 5-FU-based combination chemotherapy (n = 18; FOLFIRINOX = 10) or palliative chemoradiotherapy (n = 1).
Correlation of sPD-1 and sPD-L1 levels in patients with advanced pancreatic cancer
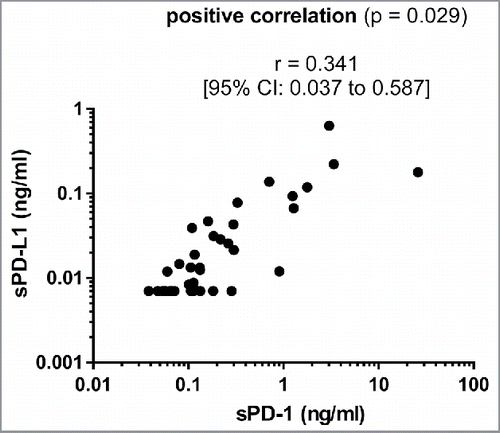
While sPD-1 was detectable in the serum of all patients analyzed, sPD-L1 was below the lower limit of detection in 15 cases. Median values for sPD-1 and sPD-L1 were 0.117 (range 0.038–25.93 ng/mL) and 0.012 ng/mL (range 0.007–0.632 ng/mL), respectively. We observed a close correlation between levels of sPD-1 and sPD-L1 suggesting a common provenience and a simultaneous release of these two soluble checkpoint molecules ().
Correlation of sPD-1 and sPD-L1 serum levels with overall survival using cox regression
To correlate levels of sPD-1 and sPD-L1 with OS, we applied a cox regression model including established prognostic factors in advanced PC (). As expected, CRP levels and a high pre-treatment CA 19–9 value (> 1.000 U/mL) independently predicted an unfavorable prognosis in our patient cohort. Neither sPD-1 nor sPD-L1 was correlated with a shorter OS in advanced PC (HR for sPD-1: 0.74 [0.44–1.24]; HR for sPD-L1: 0.23 [0.01–13.52]).
Table 1. Prognostic relevance of sPD-1 and sPD-L1.
Clinical characteristics of patients with high vs. low sPD-1 and sPD-L1 serum levels
Patients were grouped into sPD-1 / sPD-L1 high vs. low using the median sPD-1 or sPD-L1 concentrations, respectively. Clinical characteristics such as age, Eastern Cooperative Oncology Group (ECOG) score and stage of disease were comparable in patients with sPD-1 and sPD-L1 high vs. low subgroups ( and ).
Table 2A. Clinical characteristics of patients with high vs. low levels of sPD-1.
Table 2B. Clinical characteristics of patients with high vs. low levels of sPD-L1.
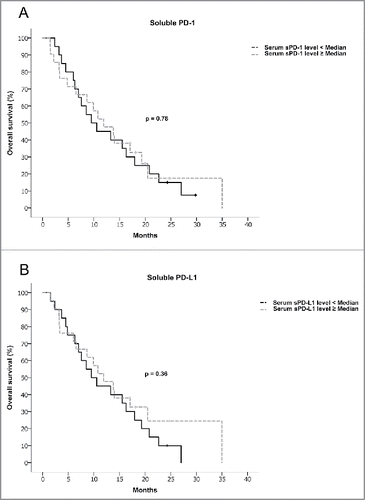
To compare OS in patients with high vs. low sPD-1 and sPD-L1 serum levels, we used the Kaplan–Meier method. We observed no adverse outcome in the high sPD-1 or sPD-L1 group, respectively (11.93 vs. 9.53 mo for high sPD-1 vs. low sPD-1, p = 0.78; 11.92 vs. 9.53 mo for high sPD-L1 vs. low sPD-L1, p = 0.36) (see and ). Similar results were obtained when using the 75th percentile or very high levels of sPD-1 /sPD-L1 (as defined by sPD-1 > 1 ng/mL and sPD-L1 > 0.1 ng/mL) instead of the median sPD-1 and sPD-L1 levels (Fig. S1).
CA 19–9 and CEA in patients with high sPD-1 and sPD-L1 levels
CA 19–9 and CEA are established serum tumor markers in patients with advanced PC and indicative of prognosis and burden of disease.Citation19-22 As expected, we found a significant positive correlation of pretreatment CA 19–9 and CEA levels in our patient cohort (Fig. S2A). Mean levels of CA 19–9 and CEA in patients with high vs. low sPD-L1 levels did not differ significantly (Fig. S2B). Further, we correlated levels of CA 19–9 and CEA to sPD-L1 levels in individual patients (Fig. S2C): No correlation between tumor marker levels and sPD-L1 serum levels was observed. Similar results were observed for sPD-1 (data not shown).
Correlation of sPD-1 and sPD-L1 levels with levels of C-reactive protein, tumoral T cell infiltration and tumoral PD-L1 expression
We hypothesized that sPD-1 and sPD-L1 might be upregulated in patients with elevated markers of systemic inflammation (e.g., CRP). Very high sPD-1 and sPD-L1 levels (as defined by sPD-1 > 1 ng/mL and sPD-L1 > 0.1 ng/mL) were almost exclusively present in patients with CRP elevation (p < 0.01 [sPD-1] and p < 0.0001 [sPD-L1] for variances between patients with normal vs. elevated CRP). Mean sPD-1 and sPD-L1 levels tended to be higher in patients with an elevated CRP (mean sPD-1: 490 pg/mL vs. 230 pg/mL, p = 0.36 and mean sPD-L1 60 pg/mL vs. 18 pg/mL, p = 0.28 for patients with elevated vs. normal CRP values, respectively) (). Archival tumor specimens to analyze tumoral infiltration by CD3+ T cells and tumoral PD-L1 expression were available from 30 of 41 patients. Reasons for missing tumor samples were diagnosis of PC at an external institution in eight cases and no remaining tumor tissue after diagnostic workup in three cases (). Tumor tissue was from metastatic sites in 17 cases and from the primary tumor in 13 cases. Two patients received neoadjuvant treatment before acquisition of the analyzed tumor sample. Median time from acquisition of tumor sample to blood draw was 12 d (range: −317 d [neoadjuvant treatment] to 1,205 d [relapse after surgery in curative intent]) (). A high infiltration by CD3+ T cells was found in 10/30 cases (33%) (Fig. S3 and ). Interestingly – a subset of patients with a strong tumoral T cell infiltration had very high sPD-L1 levels (). Of note in one patient with low sPD-L1 levels despite a strong T cell infiltration – neoadjuvant treatment was administered after acquisition of blood for sPD-L1 analysis (patient number 23, ). Using adequate positive and negative controls, PD-L1 expression was found to be present in 9/30 cases (30%) ( and ). When correlating levels of sPD-L1 with tumoral PD-L1 expression, no correlation was found ().
Figure 3. (A) Distribution of sPD-1 (left figure) and sPD-L1 (right figure) in patients with normal vs. elevated CRP. Differences in mean were tested using a Student's t-test, differences in distribution pattern were tested using an F-test. (B) Immunohistochemical staining of PD-L1 in controls (A, B) and exemplary pancreatic cancer tissue (C, D). Magnification = × 200. Scale bars indicate 50 µm. (C) Correlation analysis of sPD-L1 levels with tumoral CD3+ T cell infiltration (left figure) and tumoral PD-L1 expression (right figure) in advanced pancreatic cancer patients; Left figure: Correlation of sPD-L1 with tumoral CD3+ T cell infiltration (tCD3 count), differences between groups were tested using an F-test. Right figure: Correlation of sPD-L1 levels with tumoral PD-L1 expression, Pearson coefficient analysis was used to test for a potential correlation.
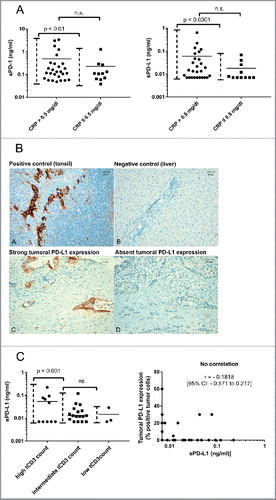
Table 3. Correlation of sPD-L1 levels with tumoral PD-L1 expression and tumoral CD3+ T cell infiltration in advanced pancreatic cancer.
Discussion
Clinical results of phase II/III trials investigating the efficacy of PD-1/PD-L1 blockade as monotherapy or in combination with other drugs in PC are still pending.Citation11,23 Serum levels of sPD-1 and sPD-L1 have been reported to be independent prognostic factors in different solid tumors susceptible to immunotherapy targeting the PD-1 axis.Citation13,14,24 Regulation, provenience and function of the soluble forms of PD-1 and PD-L1 in cancer are still under discussion.Citation24 This is the first study to examine serum levels of sPD-1 and sPD-L1 in advanced PC.
The soluble forms of PD-1 and PD-L1 were initially described in autoimmune disorders where both sPD-1 and sPD-L1 are thought to be produced by immune cells upon stimulation with proinflammatory cytokines.Citation25,26 To the best of our knowledge, sPD-1 and sPD-L1 have not yet been measured conjointly in any cancer type. As seen in autoimmune disorders, we observed a close positive correlation of sPD-1 and sPD-L1 levels in patients with advanced PC, suggesting a common provenience and regulation of sPD-1 and sPD-L1 in cancer patients.Citation25
While sPD-L1 has not been determined in PC before, conflicting findings on the prognostic relevance of membrane bound PD-L1 on PC tumor cells exist.Citation7,35 Using a cox regression model including established prognostic factors for patients with advanced PC, we did not find an adverse influence of sPD-1 or sPD-L1 on OS. Likewise, we did not find a significant survival difference between patients with high vs. low sPD-1 or sPD-L1, respectively. This suggests, that neither sPD-1 nor sPD-L1 serum levels serve as adverse prognostic marker in advanced PC. This is in line with the recent report on the absent prognostic effect of PD-L1 expression on PC tumor cells and the rather disappointing early trial results of checkpoint blockade as monotherapy in advanced PC and could suggest that PD-L1 blockade alone might possibly not be sufficient to improve prognosis in advanced PC patients.Citation35-37 Serum CA 19–9 and CEA are established tumor markers in PC.Citation19-22 Higher CA 19–9 and CEA levels indicate higher tumor burden and poor outcome.Citation21,22 In accordance with the observation that high sPD-1 or sPD-L1 values are not correlated with an adverse prognosis, no correlation between mean sPD-L1 levels and tumor marker levels was found in our cohort.
Levels of sPD-L1 have been reported to be elevated in patients with cancer and systemic inflammation (as defined by an elevated CRP or elevated sCD163) in HCC with a similar trend for CRP in gastric cancer.Citation14,16 Accordingly, very high serum levels of sPD-1 and sPD-L1 were only observed in patients with elevated CRP levels in our study population (with a similar trend for higher mean sPD-1 and sPD-L1 levels in patients with elevated CRP). Importantly, serum levels of soluble PD-L1 were markedly elevated in (a subset of) patients with strong tumoral CD3+ T cell infiltration. This could indicate that systemic inflammation and subsequently elevated sPD-L1 levels are provoked by an inflammatory tumor type in a subset of advanced PC patients. Given the small number of patients included in our study, further studies are clearly necessary to confirm this observation. Importantly, such studies should aim to elucidate the reason for the missing upregulation of sPD-L1 in a subset of patients despite a strong tumoral T cell infiltrate.
Besides tumoral T cell infiltration, we analyzed tumoral PD-L1 expression in archival tissue samples: Using two different PD-L1 IHC antibodies, we were not able to confirm the high expression of PD-L1 reported very recently.Citation27 In line with previous reports only a minority (30%) of all analyzed tumor specimens were classified as PD-L1 positive.Citation7,28-30 However, PD-L1 expression might differ between primary tumor and metastases as described for renal cell carcinoma and lung adenocarcinoma.Citation31-33 Therefore, it must be pointed out that a majority (57%) of tumor samples in our study was from metastatic sites, while previous studies in PC determined PD-L1 expression in the pancreatic primary.Citation7,27-30 In lymphoma patients, levels of sPD-L1 have been reported to be independent of tumoral PD-L1 expression.Citation15 Likewise, tumoral expression of PD-L1 did not correlate to serum levels of its soluble counterpart in our patient population. As reported very recently, PD-L1 expression in PC is not associated to an inflammatory tumor type as defined by an immunogenic gene signature.Citation39 This could explain the correlation of high sPD-L1 levels with a strong CD3+ T cell infiltrate in the absence of a correlation between tumoral PD-L1 expression with sPD-L1 levels.
In conclusion, our study indicates that sPD-1 and sPD-L1 are markers of systemic inflammation in (pancreatic) cancer. In a subset of PC patients, elevated sPD-L1 levels might be caused by an inflammatory tumor type – independent of tumoral PD-L1 expression. Future studies should attempt to define the immune cell population(s) responsible for release of sPD-1 and sPD-L1 in cancer and possible functional consequences. A special focus should be on T cells and innate immune cells given the positive correlation of sPD-L1 with sCD163 (the soluble form of CD163, exclusively expressed on macrophages).Citation34
Disclosure of potential conflicts of interest
Stefan Boeck has received honoraria for scientific presentations from Celgene and Roche, research funding from Celgene, Clovis Oncology and Roche, travel grants from Celgene and Roche and acted as consultant for Celgene and Baxalta.
Michael Haas has received honoraria for scientific presentations from Celgene, research funding (for his institution) from Boehringer Ingelheim and Roche, travel grants from Boehringer Ingelheim and Celgene.
Dominik Modest has received Honoraria and research funding from Roche.
Volker Heinemann has received honoraria for scientific presentations from Baxalta, Celgene and Roche, research funding from Celgene and Roche and acted as consultant for Baxalta, Celgene and Roche.
All other authors declare no conflict of interest.
Author contributions
Stephan Kruger and Stefan Boeck: analysis and interpretation of data; drafting of the manuscript. Stephan Kruger, Stefan Holdenrieder and Stefan Boeck: study concept and design. Marie-Louise Legenstein and Verena Roesgen: acquisition of data, collection of serum and tumor samples. Steffen Ormanns and Thomas Kirchner: pathological review of tumor samples and analysis of tumoral PD-L1 expression and tumoral CD3C T cell infiltration. Stephan Kruger, Michael Haas, Dominik Paul Modest, Christoph Benedikt Westphalen, Volker Heinemann and Stefan Boeck: provision of patient data. Stephan Kruger, Marie-Louise Legenstein, Verena R€osgen, Michael Haas, Dominik Paul Modest, Christoph Benedikt Westphalen, Steffen Ormanns, Thomas Kirchner, Volker Heinemann, Stefan Holdenrieder and Stefan Boeck: critical revision of the final manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local ethics committee (Approval number 284–10, ethics committee Ludwig-Maximilians-University Munich).
Supplementary_materials.zip
Download Zip (5.9 MB)Acknowledgements
This work is part of the doctoral thesis of Marie-Louise Legenstein and Verena Rösgen.
Funding
Stephan Kruger is supported by a grant from the Friedrich-Baur-Stiftung, Munich.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5-29; PMID: 25559415; https://doi.org/10.3322/caac.21254
- Quante AS, Ming C, Rottmann M, Engel J, Boeck S, Heinemann V, Westphalen CB, Strauch K. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med 2016; 5:2649-56; PMID: 27356493; https://doi.org/10.1002/cam4.767
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817-25; PMID: 21561347; https://doi.org/10.1056/NEJMoa1011923
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369:1691-703; PMID: 24131140; https://doi.org/10.1056/NEJMoa1304369
- Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 2012; 18:4266-76; PMID: 22896693; https://doi.org/10.1158/1078-0432.CCR-11-3114
- Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, Bruno M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev 2014; 40:513-22; PMID: 24315741; https://doi.org/10.1016/j.ctrv.2013.11.005
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007; 13:2151-7; PMID: 17404099; https://doi.org/10.1158/1078-0432.CCR-06-2746
- Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, W Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett 2008; 268:98-109; PMID: 18486325; https://doi.org/10.1016/j.canlet.2008.03.056
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: The beginning of the end of cancer? BMC Med 2016; 14:73; PMID: 27151159; https://doi.org/10.1186/s12916-016-0623-5
- Wang J, Reiss KA, Khatri R, Jaffee E, Laheru D. Immune therapy in GI malignancies: A review. J Clin Oncol 2015; 33:1745-53; PMID: 25918295; https://doi.org/10.1200/JCO.2015.60.7879
- Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer 2016; 4:14; PMID: 26981244; https://doi.org/10.1186/s40425-016-0119-z
- Zheng L. PD-L1 expression in pancreatic cancer. J Natl Cancer Inst. 2017 Jan 28; 109(6); PMID: 28131993; https://doi/org/10.1093/jnci/djw304
- Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res 2011; 17:1915-23; PMID: 21355078; https://doi.org/10.1158/1078-0432.CCR-10-0250
- Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 2016; 59:152-9; PMID: 27039170; https://doi.org/10.1016/j.ejca.2016.03.002
- Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014; 28:2367-75; PMID: 24732592; https://doi.org/10.1038/leu.2014.137
- Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res 2014; 26:104-11; PMID: 24653632; https://doi.org/10.3978/j.issn.1000-9604.2014.02.08
- Wang L, Wang H, Chen H, Wang WD, Chen XQ, Geng QR, Xia ZJ, Lu Y. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 2015; 6:41228-36; PMID: 26515600; https://doi.org/10.18632/oncotarget.5682
- Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T, Yamada Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 2016; 142:1727-38; PMID: 27256004; https://doi.org/10.1007/s00432-016-2184-6
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016 Jul 2; 388(10039):73-85; PMID: 26830752; https://doi.org/10.1016/S0140-6736(16)00141-0
- Kanemasa Y, Kamisawa T, Tabata T, Kuruma S, Iwasaki S, Chiba K, Kuwata G, Fujiwara T, Egashira H, Koizumi K et al. Mixed acinar-endocrine carcinoma of the pancreas treated with S-1. Clin J Gastroenterol 2013; 6:459-64; PMID: 26182137; https://doi.org/10.1007/s12328-013-0416-8
- Haas M, Laubender RP, Stieber P, Holdenrieder S, Bruns CJ, Wilkowski R, Mansmann U, Heinemann V, Boeck S. Prognostic relevance of CA 19–9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol 2010; 31:351-7; PMID: 20480409; https://doi.org/10.1007/s13277-010-0044-6
- Haas M, Heinemann V, Kullmann F, Laubender RP, Klose C, Bruns CJ, Holdenrieder S, Modest DP, Schulz C, Boeck S. Prognostic value of CA 19–9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: Results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol 2013; 139:681-9; PMID: 23315099; https://doi.org/10.1007/s00432-012-1371-3
- Johansson H, Andersson R, Bauden M, Hammes S, Holdenrieder S, Ansari D. Immune checkpoint therapy for pancreatic cancer. World J Gastroenterol 2016; 22:9457-76; PMID: 27920468; https://doi.org/10.3748/wjg.v22.i43.9457
- Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer 2016; 100:77-84; PMID: 27597284; https://doi.org/10.1016/j.lungcan.2016.08.001
- Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 2006; 177:8844-50; PMID: 17142787; https://doi.org/10.4049/jimmunol.177.12.8844
- Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol 2005; 235:109-16; PMID: 16171790; https://doi.org/10.1016/j.cellimm.2005.07.007
- Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, Oberlies NH, Pearce C, Liu K. The MLL1-H3K4me3 Axis-Mediated PD-L1 expression and pancreatic cancer immune evasion. J Natl Cancer Inst. 2017 Jan 28; 109(6); PMID: 28131992; https://doi.org/10.1093/jnci/djw283
- Wang Y, Lin J, Cui J, Han T, Jiao F, Meng Z, Wang L. Prognostic value and clinicopathological features of PD-1/PD-L1 expression with mismatch repair status and desmoplastic stroma in Chinese patients with pancreatic cancer. Oncotarget. 2017 Feb 7; 8(6):9354-9365; PMID: 28030840; https://doi.org/10.18632/oncotarget.14069
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID: 25428504; https://doi.org/10.1038/nature14011
- Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg 2010; 34:1059-65; PMID: 20145927; https://doi.org/10.1007/s00268-010-0448-x
- Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res 2015; 3:1158-64; PMID: 26014095; https://doi.org/10.1158/2326-6066.CIR-15-0043
- Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y, Chen L, Kluger HM. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer 2014; 5:166-72; PMID: 24563671; https://doi.org/10.7150/jca.8167
- Uruga H, Bozkurtlar E, Huynh TG, Muzikansky A, Goto Y, Gomez-Caraballo M, Hata AN, Gainor JF, Mark EJ, Engelman JA et al. Programmed Cell Death Ligand (PD-L1) expression in stage II and III lung Adenocarcinomas and nodal metastases. J Thorac Oncol. 2017 Mar; 12(3):458-466; PMID: 27815126; https://doi.org/10.1016/j.jtho.2016.10.015
- Akahori H, Karmali V, Polavarapu R, Lyle AN, Weiss D, Shin E, Husain A, Naqvi N, Van Dam R, Habib A et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat Commun 2015; 6:7792; PMID: 26242746; https://doi.org/10.1038/ncomms8792
- Diana A, Wang LM, D'Costa Z, Allen P, Azad A, Silva MA, Soonawalla Z, Liu S, McKenna WG, Muschel RJ et al. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016 Jul 5; 7(27):40992-41004; PMID: 27329602; https://doi.org/10.18632/oncotarget.10038
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33:828-33; PMID: 20842054; https://doi.org/10.1097/CJI.0b013e3181eec14c
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID: 22658128; https://doi.org/10.1056/NEJMoa1200694
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007; 67:9518-27; PMID: 17909062; https://doi.org/10.1158/0008-5472.CAN-07-0175
- Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin Cancer Res. 2016 Dec 22; PMID: 28007776; https://doi.org/10.1158/1078-0432.CCR-16-2128