ABSTRACT
Among reported advantages of umbilical cord blood (CB) in transplantation is lower leukemia relapse probability. Underlying cellular mechanisms of graft-vs.-leukemia (GVL) are thought to include a prominent role for T cells. Cells of the CB's mother, maternal microchimerism (MMc), were recently strongly, but indirectly, implicated in this GVL benefit. We assayed MMc directly and hypothesized benefit accrues from CB maternal T cells. MMc was quantified in 51 CBs and, within memory T, naïve T, B, NK cells, and monocytes in 27 CBs. Polymorphism-specific quantitative-PCR assays targeted maternal genotypes non-shared with CBs. Overall MMc was common and often at substantial levels. It was present in 52.9% of CB and in 33.3–55.6% of tested subsets. Remarkably, MMc quantities were greater in memory T cells than other subsets (p < 0.001). Expressed as genome equivalents (gEq) per 105 total gEq tested (gEq/105), memory T cell MMc averaged 850.2 gEq/105, while other subset mean quantities were 13.8–30.1 gEq/105. After adjustment for proportionality in CB, MMc remained 6–17 times greater in memory T, and 3–9 times greater in naïve T, vs. non-T-cell subsets. Further, CB-origin MMc was detected in vivo in a patient up to 6 mo post-transplantation, including among T cells. Overall, results revealed levels and phenotypes of CB MMc with potential relevance to CB transplantation and, more broadly, to offspring health.
Introduction
Microchimerism (Mc) refers to the presence of a small number of cells (or DNA) from a genetically disparate individual,Citation1 and is a natural phenomenon deriving from bi-directional cell exchange during pregnancy.Citation2 Both maternal and fetal Mc are detected during pregnancy and are known to persist decades later in respective individuals.Citation3-7 Maternal cells in the fetus are detected as early as the secondCitation5 and third gestational trimesters,Citation6 and persist in her progeny into adult life.Citation7 Studies were first conducted on umbilical cord blood (CB) samples from immunocompetent subjects in the 1990s, using polymerase chain reaction (PCR)-based methodsCitation8-10 or fluorescence in situ hybridization (FISH) probes specific to the X and Y chromosomes to identify female cells when the CB was from a male fetus.Citation11 These studies were initially motivated by concerns about maternal cells in CB potentially contributing to graft-vs.-host disease (GVHD) when used as a source of hematopoietic stem cells for allogeneic transplantation.Citation12 Yet, despite drawbacks, GVHD is also associated with a graft-vs.-leukemia (GVL) effect, beneficial against recurrent malignancy in the patient.Citation13 Such GVL reactivity was recently strongly suggested to be mediated, at least in part, by maternal Mc (MMc) originating in the transplanted CB.Citation14 In that study, a significant reduced relapse rate of acute leukemia was observed after CB transplantation when the Human Leukocyte Antigens (HLA) of the fetus (CB), that were not present in the CB mother (i.e. paternally inherited HLA), were shared with the CB recipient. MMc, however, was not directly assessed.
Among the advantages of CB for use in transplantation are ready availability as a source of hematopoietic cells for approximately 70% of recipients who do not have an HLA-matched alternative source.Citation15 CB can be used with greater allowance for HLA mismatch, thus increasing its availability to nearly all patients.Citation16 A recent study demonstrated that leukemia patients with minimal residual disease risk had significantly reduced risk of relapse in recipients of CB compared with both unrelated HLA-matched and HLA-mismatched donors.Citation17 While maternal cells in CB are an attractive, if not compelling candidate for some of the benefits of CB in transplantation, surprisingly little is known about the prevalence, quantity, and immuno-phenotypes of maternal cells in CB. Just one CB was studied in one report (for CD3+, CD19+, CD16+, and CD14+),Citation18 and another was limited to testing for CD34+ and CD8+ cells of CB samples.Citation11
To address this gap in knowledge, we conducted studies to determine the prevalence, quantities and immuno-phenotypes of maternal cells in CB. Because of the acknowledged central role that mature allo-reactive T cells play in the mediation of GVHD/GVL effects,Citation19 we hypothesized greater MMc quantities among T cells and focused on two subsets: naïve and memory T lymphocytes. The former would constitute most of neonatal T cells, but the latter are known to live longer, to be antigen-educated, and to have a lower threshold of activation compared with naïve T lymphocytes. Additional studied subsets included B and NK cells, and monocytes. Mother–CB (child) pairs were first genotyped for HLA and other polymorphisms, to identify a maternal-specific polymorphism to target for MMc. Fluorescence-activated cell sorting (FACS) was then conducted and MMc was interrogated in the cellular subsets using a panel of polymorphism-specific sensitive quantitative PCR (qPCR) assays we have developed. Additionally, studies were extended to include testing for cells from the mother of the CB donor in a leukemia patient post-transplantation, up to 6 mo after undergoing double CB transplantation.
Results
Sixty-one CB samples were obtained from uncomplicated term pregnancies that resulted in a single live birth for which both maternal and neonatal health were confirmed as normal. Genotyping of HLA class-II loci, as well as polymorphisms in GSTT1, TG, ATIII, and TNN loci, was conducted for mother–CB (child) pairs. Of the 61 mother–CB pairs, 52 had a maternal polymorphism not transmitted to the fetus and unique to the mother that could be targeted to assay MMc. CB mononuclear cells (CBMC) were isolated and, of the 52 samples, 27 were selected in a blind fashion and sorted into five cellular subsets, based on previously established flow cytometry panels:Citation20,21 CD3+ CD45RA− and/or CD27− memory T cells; CD3+ CD45RA+ CD27+ naïve T cells; HLA-DR+ CD19+ B cells; CD3− CD56+ and/or CD16+ NK cells; and CD14+ monocytes (Fig. S1). Of the 27 sorted samples, one did not have any DNA left to test on unsorted CBMC (51 samples). To measure MMc the appropriate maternal-specific qPCR assay was selected from a panel of polymorphism-specific qPCR assays we previously developed for this purpose.Citation22-24 In the current study, a new assay was developed and used, targeting a multi-nucleotide insertion in an intronic region of the TNN gene (Fig. S2).
Clinical characteristics of study subjects are provided in and Table S1. Maternal age at delivery ranged from 19 to 40 y (mean: 32). Twenty-eight births were the mother's first, 20 a second, and 3 a third birth. Gestational age ranged from 36 weeks to 41 weeks and 6 days (mean: 39). Children's birthweights ranged from 2,752 to 4,555 g (mean: 3,543).
Table 1. Characteristics of the subjects for whom cord blood was tested for maternal microchimerism.
MMc was present in 52.9% (27/51) of CBs, and in 65.4% (17/26) of CBMCs investigated for cellular subsets. In the sorted T cells for memory and naïve phenotypes, B cells, NK cells, and monocytes, MMc was identified in at least one cellular subset in 85.2% of the CBs (23/27). Among memory T cells MMc was present in 51.9% (14/27) of CBs and among naïve T cells 40.7% (11/27). Among B cells MMc was present in 33.3% (9/27) of CBs, and in NK cells 44.4% (12/27) had MMc. Among monocytes 55.6% (15/27) were positive for MMc. There was no significant difference in MMc prevalence between the groups (overall chi-square test: p = 0.49; one-on-one comparisons using logistic regression adjusted for “total DNA tested:” p values ranged from 0.11 to 0.81).
The concentration of MMc varied across a wide range from none detected to substantial levels in some CB cellular subsets. The median concentration of MMc (characterized by a skewed distribution) in all groups ranged from 0 to 5.1 genome equivalents (gEq) per 105 total gEq tested (gEq/105). The mean MMc concentration in memory T cells was 850.2 gEq/105 and in naïve T cells 30.1 gEq/105. In B cells and NK cells mean MMc concentrations were 14.3, and 13.8 gEq/105, respectively. In monocytes mean MMc was 26.4 gEq/105. The mean MMc in unsorted CBMC was 28.9 gEq/105. shows MMc quantities in the CBMC and the five subsets.
Figure 1. Maternal microchimerism (MMc) data in cord blood mononuclear cells (CBMC) samples and by cellular subsets from healthy women. (A) Data from N = 51 CBMC samples are represented. The filled triangles represent 26 CBMC also selected for fluorescence-activated cell sorting into five cellular subsets in panel B. The open triangles are the remaining CBMC. (B) Data from N = 27 CBMC sorted into five cellular subsets. To help illustrate that measurements are not independent, each participating CB is represented by a slightly different shade of gray across the five subsets. Detection rate ratios (DRR), derived from the negative binomial regression model, are shown. DRR are interpreted as the fold-change in expected MMc quantities from one subset compared with a subset on its right. Numbers between brackets are 95% confidence intervals. Concentrations are measured in genome equivalent (gEq) of maternal DNA per 105 gEq of total cellular DNA and plotted in log scale. n/d = not detected.
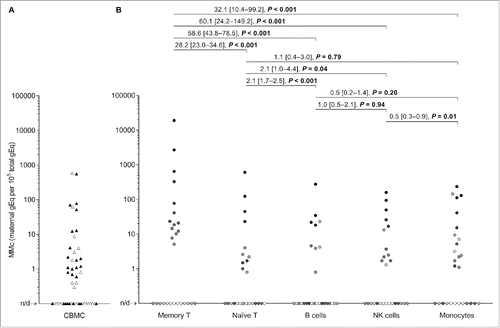
Detection rate ratios (DRR) derive from negative binomial regressions, recently described to best model Mc data.Citation25 MMc DRR, interpreted as the fold-change in expected MMc quantities from one vs. another subset, were highest for memory T cells compared with all other subsets with values [and 95% confidence intervals] ranging from 28.2 [23.0–34.6] to 60.1 [24.2–149.2], p < 0.001 for each comparison (). MMc concentrations were also significantly higher in naïve T cells compared with B and NK cells (DRR: 2.1 [1.7–2.5] and 2.1 [1.0–4.4]), but not compared with monocytes (). For MMc rates in the non-T-cell groups, the difference was significant only for NK cells vs. monocytes. With Bonferroni correction (10 comparisons), MMc DRR of memory T cells vs. other subsets, and that of naïve T vs. B cells remained significantly increased.
MMc was assayed in all DNA obtained for a cellular subset or in 105 gEq i.e., 660 ng of total DNA if more than this amount was obtained after sorting. The total amount assayed was lowest for memory T cells, for which median [and interquartile range, or IQR] of total DNA tested was 5.4 × 103 [3.2 × 103–2.4 × 104] (Fig. S3A). Overall, the cumulative total DNA tested for the five subsets for each CB sample was (median and [IQR]) 3.0 × 105 [1.9 × 105–3.6 × 105] (Fig. S3B).
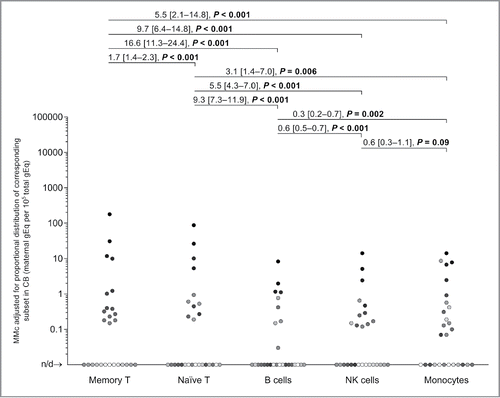
In the current study, T cells, B cells, and NK cells were found to respectively constitute a median [and IQR] of 65% [46–69%], 11% [9–14%], and 15% [13–18%], of lymphocytes in CB, comparable to reported percentages.Citation26 Naïve and memory phenotypes formed 93% [88–94%] and 7% [6–12%] of total CB T cells, respectively. The estimated proportions of lymphocytes and monocytes in whole CB are around 38% and 6%, respectively.Citation26 Evaluating the MMc data for each subject while also taking into account the proportional distribution of all cells across cellular subsets, maternal cells with memory T phenotype remained the most abundant. Maternal memory T cells were 16.6 [11.3–24.4] more abundant than B cells, 9.7 [6.4–14.8] more than NK cells and 5.5 [2.1–14.8] times more than monocytes. Maternal naïve T cells were 9.3 [7.3–11.9] times more abundant than B cells, 5.5 [4.3–7.0] times more than NK cells, and 3.1 [1.4–7.0] times more than monocytes (p ≤ 0.006; values are DRR and [95% confidence intervals], ). A Bonferroni correction (10 comparisons) did not change the outcome of the analysis, except in the case of naïve T cells vs. monocytes where a statistically significant result became a tendency (Bonferroni-corrected p = 0.06).
Figure 2. Maternal microchimerism (MMc) data by cellular subset, adjusted by the fraction of the corresponding subset in cord blood (CB) samples. To help illustrate the non-independence of measurements, each participating CB is represented by a slightly different shade of gray across the five subsets. Detection rate ratios (DRR), derived from the negative binomial regression model, are shown. DRR are interpreted as the fold-change in expected MMc quantities from one subset compared with a subset on its right. Numbers between brackets are 95% confidence intervals. Concentrations are measured in genome equivalent (gEq) of maternal DNA per 105 gEq of total cellular DNA and plotted in log scale. n/d = not detected.
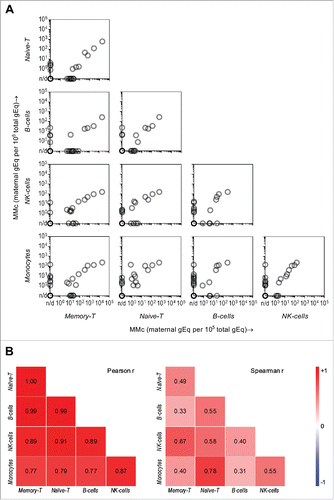
Because MMc was detected in subsets with diverse immuno-phenotypes and functions, and quantities were higher among T cells even after distribution-proportion adjustments, we asked further whether the data supported preferential transfer (or not) of some types of maternal cells over others to the child. A pairwise correlation analysis of MMc distribution within CB subsets for each CB sample, therefore, was conducted. In , three categories of data-points can be distinguished: (a) subsets on the X and Y axes are both MMc-positive for a particular CB sample and a positive correlation can be perceived across the population for almost all pairs of subsets; (b) one of the subsets (either the X or the Y axis) is MMc-positive, while the MMc in the other subset was not detected; and (c) both subsets have no detectable MMc for a particular CB sample (darker circles on bottom left of squares). Overall, a high degree of positive correlation was observed both by parametric and non-parametric tests; Pearson's and Spearman's r values ranged from 0.77 to 1.00 and 0.31 to 0.78, respectively. The correlation was significant in all Pearson's tests, as well as in Spearman's tests (), except for memory T cells vs. B cells and monocytes vs. B cells. This analysis indicated higher MMc in a given subset often predicts higher MMc in other subsets of the same subject, favoring the view of no preferential maternal cellular subset passage to the fetus.
Figure 3. Pairwise correlation of maternal microchimerism (MMc) distribution in cellular subsets of cord blood (CB) and respective CB subjects. (A) The “matrix-like” figure represents the correlation between MMc levels in a particular subset vs. another subset for the same participating CB (each semi-transparent gray circle). Data is represented in genome equivalent (gEq) of maternal DNA per 105 gEq of total cellular DNA. n/d = not detected. (B) The heat maps show Pearson and Spearman correlation coefficients where blue, red, and white are negative (r tends to −1), positive (r tends to 1), and no (r = 0) correlation, respectively. p < 0.05 in all Pearson and Spearman's tests except in the two cases, where r = 0.31 and 0.33.
Overall the data showed substantial presence of MMc in different cellular subsets of CB. Whether these maternal cells persist in CB recipients after transplantation is not known. To address this, we identified a patient with leukemia who underwent myeloablative mismatched double CB transplantation. HLA-genotyping for the patient, her mother, both CBs, and both CB mothers was obtained and reviewed to seek polymorphisms that could be targeted for each of the CB mothers. provides a synopsis of HLA-genotyping results and indicates MMc for the CB unit that achieved dominance (as is expected in double CB transplantation) could be assayed by targeting DRB1*15. MMc from the non-dominant CB unit was also targetable by assaying for DRB1*04. The patient was a Caucasian female with precursor-B acute lymphoblastic leukemia. She was 40-y-old at the time of transplant, had never given birth or been pregnant, and was the eldest among her siblings. The double CB transplant was administered 11 weeks post CAR-T therapy, and had been preceded by multiple cycles of chemotherapy over four years. Engraftment occurred at day 17 post-transplantation and dominance of a “winning” CB unit as the sole source of hematopoiesis was established in the recipient at day 21 by routine clinical testing. Three weeks post-transplantation, the patient did not show signs of acute GVHD in any organsystems. At the second month, there was biopsy proven grade-II gut GVHD with no other organsystem involvement. This was treated by steroids and the GVHD resolved at the third month. No formal GVHD evaluation was obtained at day 180, but the comment was made that a skin rash and elevated liver function test values may suggest GVHD if other causes were excluded. Clinical evaluation at one year after transplantation noted a scaly neck rash, suggesting mild chronic GVHD, but skin biopsy pathology was interpreted as dermatitis rather than GVHD. The patient was relapse-free at one year post-transplantation.
Figure 4. Maternal microchimerism (MMc) from the cord blood (CB) in cellular subsets of a leukemia patient who received double-CB transplantation. (A) HLA-DRB1 genotype of patient “P,” her mother “M,” the winning and losing received CB units: “CBWin” and “CBLose,” and the mothers of these CB: “M-CBWin” and “M-CBLose.” (B) From left to right, gating on T, B, NK cells, and monocytes sorted from patient's peripheral blood mononuclear cells (PBMC) at days 30, 57, 100, and 180 post-transplantation. Numbers in gates represent percentages. (C) Heat map revealing quantities of CB-origin MMc from winning (upper panel) and losing (lower panel) units at various time-points for various subsets, measured in genome equivalent (gEq) of maternal DNA per 105 gEq of total cellular DNA (gEq/105). Graft-vs.-host disease (GVHD) evaluation is also reported.
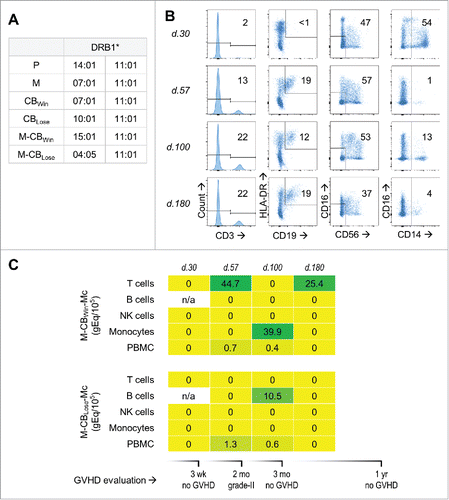
A whole blood sample was collected one week before transplantation. DNA extracted from the pre-transplantation sample was assayed, and was negative for MMc of both the winning and losing CB units. Peripheral blood mononuclear cells (PBMC) were isolated from days 30, 57, 100, and 180 post-transplantation. Initial screening of DNA from PBMC revealed MMc at two time-points. PBMC from days 30, 57, 100, and 180 were sorted by FACS for T cells, B cells, NK cells, and monocytes. The plenitude of collected cells per subset varied significantly among time-points, but the subset of NK cells consistently remained the most abundant (). MMc from the winning unit was present at two time-points only in T cells, including 180 d after transplant (). At day 100, MMc from both the winning and the losing CB unit were detectable among the patient's monocytes and B cells, respectively. DNA from the cellular subsets ranged from 7.1 × 102 to 1.9 × 105, except for B cells from day 30, where no usable DNA was recovered.
Discussion
In the current study, we determined the prevalence and quantities of MMc in CBMC and in memory and naïve T cells, B cells, NK cells, and monocytes isolated from CB from healthy women. MMc was found across all the cellular subsets tested and was present in at least one subset in 85.2% of CB samples. MMc quantities were highest among memory T cells compared with all other subsets. Phenotypes of neonatal blood cells have been described previouslyCitation26,27 and proportions of the various cellular subsets are unequal. After adjusting MMc data with the proportional distribution of subsets in respective CB samples, MMc concentration remained the highest among memory T cells, and MMc concentration emerged significantly higher in naïve T cells compared with non-T subsets.
The prevalence of MMc was somewhat lower in unsorted CBMC than across the five subsets (52.9% vs. 85.2%). This difference may be the result of a greater total cumulative gEq from the five subsets compared with total gEq tested from bulk CBMC (Fig. S3B), suggesting MMc may be widely prevalent in CB, with increasing likelihood of detection with larger quantities tested. Alternatively, MMc may be detectable in a subset if quantitatively enriched in that subset, whereas the overall quantity of MMc in unsorted CBMC could fall below detection limits.
MMc in CB was recently strongly implicated in reduced relapse of leukemia after CB transplantation.Citation14 With total nucleated cells from a single unit of CB reaching the acceptable 1.75 × 109 for CB transplantation in adults,Citation28 maternal memory and naïve T cells transferred to the recipient from one CB unit can be estimated to be around 1.5 × 105 and 8.6 × 104, respectively. The numbers of maternal B, NK, and monocytes infused would be in the range of 8.8 × 103, 1.6 × 104, and 2.8 × 104, respectively. Maternal T-cell numbers are likely greater than any cell-type, not including granulocytes that generally do not survive CB processing. In all probability, maternal T cells, including long-lived and antigen-educated memory cells, would bear potential immunological effectsCitation29-31 at such numbers.
In addition, MMc originating from the CB mother was identified post-transplantation in a patient who underwent double CB transplantation for acute lymphoblastic leukemia. Moreover, MMc from the winning CB unit was detected in T cells at 2 mo and 6 mo post-transplantation. Although standard clinical chimerism analysis showed as expected the dominance of one CB unit as the source of sustained hematopoiesis, the non-engrafting “losing” unit might not be entirely lost, as recently reported in another study.Citation32 MMc from both the winning and losing CB were detected at 2+ mo transplantation. Other studies are needed but this also raises the question whether MMc in CB plays any role in the dominance of one CB unit or in GVHD. Overall our findings indicate MMc of the CB can persist in a patient post-transplant. The presence of MMc in immune cell subsets points to MMc functional potential, and further investigation is needed to address potential functional contribution to GVL and GVHD after CB transplantation.
The presence of MMc at various quantities in different subsets raises the question whether there is preferential transfer of maternal cells to the fetus. The mechanisms by which maternal cells reach the fetus are not known but may involve passive transfer through fetomaternal transfusions or active transfer involving signaling pathways triggered by fetal chemokines or growth factors.Citation33 In our study, the pairwise correlation analysis suggested presence of maternal cells without preference for a specific subset, lending support to the view of passive transfer, and MMc levels in CB may reflect the magnitude of transfusions from the mother. This appears to be true at least at the time of delivery, the last point of contact between mother and newborn. Greater MMc quantities in T cells, even after adjustment for CB subset proportional distribution, may reflect greater T-cell proportions in the maternal blood, reported to be increased in the last stages of pregnancyCitation34 as well as relative to T-cell proportions of neonates.Citation35,36 The correlation remains nevertheless imperfect, and the question merits further investigation in a larger cohort that would also explore whether mechanisms of fetomaternal cell transfer could be active rather than passive at earlier gestational time points. Additionally, MMc could be influenced by maternal-fetal HLA compatibility with MMc increased when the mother is HLA-homozygous or HLA-identical with her child. An association between HLA-class II compatibility and Mc has been implied in other studies.Citation37,38 In our study, the mother was compatible at the DRB1–DQA1–DQB1 haplotype from the perspective of the fetus in six of the 52 mother–child pairs (HLA-identical in two, HLA-homozygous mother in four) and the child was compatible from the maternal perspective for five (HLA-identical in two, HLA-homozygous child in three; Table S1). The numbers in each of these relationship categories was too small to permit definitive conclusions.
A limitation of the current study was the number of cells available for sorting from a CB was insufficient to test additional subsets of potential interest, such as other T-cell subsets, CD34+, or CD34+CD38+ progenitor cells, that can contain Mc.Citation4,39 Non-CBMC, such as short-lived granulocytes, were also not evaluated and are known to harbor MMc.Citation40 Also, no tissue biopsies could be obtained for the assessment of potential CB-origin MMc in the transplant recipient. A strength of the study is the approach and methodology allowing specific MMc identification and quantification based on family genotyping and qPCR targeting non-shared polymorphisms with validated assays that are sensitive and specific.
In summary, in the current study we determined the MMc prevalence, concentrations, and distribution among cellular subsets in CBs from women known to be healthy with uncomplicated pregnancies. MMc was detected in the majority of samples often at substantial concentrations, suggesting it may be universal in CB. While suspected to transfer passively into CB at least at delivery, MMc was present among multiple cell populations, and was particularly notable among T cells. We also identified MMc of the CB donor in a recipient up to 6 mo post-transplantation. The frequent and substantive presence of MMc in CB with greatest concentrations in memory T cells underscores the importance of further investigations to evaluate potential benefit of MMc in the context of CB transplantation for leukemia, as well as to elucidate potential effects on offspring health.
Materials and methods
Subjects
CBs were collected from healthy women with uncomplicated pregnancies derived from the Seattle, Washington area. CB was obtained by double clamping the umbilical cord segment, carefully cleaning, and drawing CB by venipuncture into acid citrate dextrose solution A-vacutainer tubes. Criteria for inclusion included that the woman was healthy, had no history of malignancy, autoimmune, immunologic, or other disease. Subjects with a twin or a history of transfusion were excluded as these are alternative sources of Mc.Citation41,42 Medical records and self-reported questionnaires were obtained and reviewed for clinical and demographic information. A patient who was undergoing double CB transplantation for treatment of leukemia at the Seattle Cancer Care Alliance, Seattle, WA, was also recruited for inclusion in the study. Samples from the patient's mother, both CBs and both CB mothers were obtained and HLA genotyping revealed targetable markers for the mothers of both CB units for this patient. Participants gave written informed consent and the study was approved by the institution's Internal Review Board.
Identifying a non-inherited maternal polymorphism to target for MMc testing
Whole blood aliquots and/or buccal swabs were obtained from mothers and CB. HLA genotyping was conducted on extracted DNA using Luminex-based PCR sequence-specific oligonucleotide probe techniques (One Lambda, Canoga Park, CA, USA), and determined alleles for the class II loci HLA-DRB1, HLA-DQA1, and HLA-DQB1. When the non-transmitted maternal HLA could not be distinguished from both HLA alleles of the fetus, genotyping was performed for four other loci: GSTT1, TG, ATIII, and TNN (respectively: glutathione S-transferase theta-1, thyroglobulin, antithrombin III, and tenascin N). Development of non-HLA genotyping and detection assays was based on an approach described previously,Citation43 and resulted in additional qPCR assays that identified and targeted multiple-nucleotides insertion/deletion/substitution polymorphisms.Citation24
Fluorescence-Activated Cell Sorting (FACS)
CBMC were isolated by gradient centrifugation, and cryopreserved in dimethylsulfoxide 7%. For sorting, 5–20 × 106 CBMC were resuspended, first stained with LIVE/DEAD®-Aqua-Fluorescent fixable dead cell stain (Life Technologies, Carlsbad, CA, USA), followed by staining with an eight-color cocktail (CD14-BV711, HLADR-AlexaFluor700, CD19-BUV737, CD16-APC-Cy7, CD45RA-PE-Cy7, CD27-BV786 [BD Biosciences, Franklin Lakes, NJ, USA], CD56-BV605 [BioLegend, San Diego, CA, USA], and CD3-PE-TexasRed [Beckman Coulter, Brea, CA, USA]). Reagents were individually titrated to achieve optimal staining concentrations. Cells were sorted on Becton Dickinson FACSAria™ II 4-way sorter (BD Biosciences, Franklin Lakes, NJ, USA). Gating included “alive” cells and avoided doublets. Purity was assessed for the sorted cells and the overall median [IQR] purity was 93% [90–96%]. Subpopulations were stored as dry pellets at −80 °C for DNA extraction.
DNA extraction for qPCR
DNA was extracted using a QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany) and resuspended in Tris-HCl (pH 8.5). In situations with low cell numbers, DNA was extracted by direct lysis at 56°C, in the presence of proteinase K (1 mg/mL), and using a homemade lysis buffer composed of Tris-HCl (10 mM), NaCl (50 mM), MgCl2 (6.25 mM), NP40 (0.045%), and Tween-20 (0.45%), as recommended.Citation44 The DNA recovery rate was similar in all subpopulations, and had a median [and IQR] of 30% [17–47%] overall.
Polymorphism-specific qPCR for Mc detection and quantification
Each CBMC sample, cellular subset, or peripheral blood sample was assayed for MMc by selecting a qPCR assay specific to the mother of the CB from a panel of HLA-specific qPCRCitation22,23 or non-HLA polymorphism-specific qPCRCitation24 assays we have developed for this purpose. The assays were all previously validated for specificity for the intended polymorphism, and to be able to amplify up to a single DNA copy in a background of four orders of magnitude of non-specific copies per test aliquot.Citation22-24 A new assay that targeted an insertion polymorphism in the TNN gene was also used. The newly developed assay followed the same design, with rigorous specificity and sensitivity validation steps as described previously (Fig. S2).Citation22 Real-time qPCR reactions were performed on ABI Prism® 7700 (Applied Biosystems, Waltham, MA, USA), as described previously.Citation22
Statistical analysis
Categorical variables were reported as counts and percentages, and comparisons were performed using chi-square, or logistic regression analysis (with adjustment for the total gEq tested per subset for each CB sample), which provided presence-vs.-absence interpretation of MMc data.
A continuous approach was used to analyze MMc quantities by cellular subset. Mc occurs by definition at low concentrations approximating a Poisson distribution. The data distribution is skewed to the right, often with excess of zeros and occasional large outlying values. Such challenges are addressed using negative binomial regression models, as they have recently been reported to better account for the higher level of variability in Mc data than expected in a Poisson model,Citation25 with the same interpretation of the “mean” as in a Poisson model. In brief, MMc concentrations were modeled as a rate of detection defined as a count of gEq maternal cells detected per total count of gEq CB cells tested. A negative binomial regression model was then applied to estimate a rate ratio comparing the rates between groups. The model accounts for the number of MMc gEq detected (whether zeros or positives) as well as the number of total gEq assessed in each sample. The output of this model is a DRR, derived from exponentiating the coefficients in the model and interpreted as the fold-change of MMc quantities from one vs. another group. An important aspect considered was the fact that measurements in the five subsets were not independent, but related per participating CB. To account for this, a command ensuring clustering of data-points from the cellular subsets in connection with their respective CB sample was included in the model.
Adjustments for multiple comparisons, when applied, were Bonferroni. Pairwise correlation tests of MMc in the subsets with respect to subjects were assessed with both Pearson's (parametric) and Spearman's rank (non-parametric) correlation tests. The gEq was defined as the amount of DNA per human cell and corresponded to 6.6 pg of DNA.Citation45 Means and medians were used to estimate the central tendency of data sets and all values, including zeros were incorporated. Analyses were performed using GraphPad Prism 7 (La Jolla, CA, USA) and STATA 14 (College Station, TX, USA).
Disclosure of potential conflicts of interest
JLN conceived the study, SBK, SCD, and JLN designed the experiments. SBK, AMF and EC performed the experiments. SBK, HSG, WH, PAS, SCD, JA, KB, CSD, and JLN analyzed the data and critically contributed to paper drafting. SBK wrote the paper. The authors declare no competing financial interests.
Supplementary_materials.zip
Download Zip (1.1 MB)Acknowledgments
The authors thank all of the study subjects and families for their participation in this research.
Funding
This research was supported by the Thrasher Research Fund, and the National Institutes of Health by grants R01 HL117737, K08HD067221, and T32 HD007233.
References
- Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol 2012; 33:421-7; PMID:22609148; https://doi.org/10.1016/j.it.2012.03.002
- Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem 2000; 46:1301-9; PMID:10973858
- Adams Waldorf KM, Gammill HS, Lucas J, Aydelotte TM, Leisenring WM, Lambert NC, Nelson JL. Dynamic changes in fetal microchimerism in maternal peripheral blood mononuclear cells, CD4+ and CD8+ cells in normal pregnancy. Placenta 2010; 31:589-94; PMID:20569981; https://doi.org/10.1016/j.placenta.2010.04.013
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA 1996; 93:705-8; PMID:8570620; https://doi.org/10.1073/pnas.93.2.705
- Lo ES, Lo YM, Hjelm NM, Thilaganathan B. Transfer of nucleated maternal cells into fetal circulation during the second trimester of pregnancy. Br J Haematol 1998; 100:605-6; PMID:9504651; https://doi.org/10.1046/j.1365-2141.1998.0636a.x
- Petit T, Dommergues M, Socié G, Dumez Y, Gluckman E, Brison O. Detection of maternal cells in human fetal blood during the third trimester of pregnancy using allele-specific PCR amplification. Br J Haematol 1997; 98:767-71; PMID:9332337; https://doi.org/10.1046/j.1365-2141.1997.2603076.x
- Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest 1999; 104:41-7; PMID:10393697; https://doi.org/10.1172/JCI6611
- Socié G, Gluckman E, Carosella E, Brossard Y, Lafon C, Brison O. Search for maternal cells in human umbilical cord blood by polymerase chain reaction amplification of two minisatellite sequences. Blood 1994; 83:340-4; PMID:8286734
- Petit T, Gluckman E, Carosella E, Brossard Y, Brison O, Socié G. A highly sensitive polymerase chain reaction method reveals the ubiquitous presence of maternal cells in human umbilical cord blood. Exp Hematol 1995; 23:1601-5; PMID:8542953
- Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, Wainscoat JS. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood 1996; 88:4390-5; PMID:8943877
- Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood 1995; 86:2829-32; PMID:7545474
- Gluckman E. History of cord blood transplantation. Bone Marrow Transplant 2009; 44:621-6; PMID:19802032; https://doi.org/10.1038/bmt.2009.280
- Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, Storb R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979; 300:1068-73; PMID:34792; https://doi.org/10.1056/NEJM197905103001902
- van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci USA 2012; 109:2509-14; PMID:22232664; https://doi.org/10.1073/pnas.1119541109
- Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 2014; 371:339-48; PMID:25054717; https://doi.org/10.1056/NEJMsa1311707
- Ruggeri A, Paviglianiti A, Gluckman E, Rocha V. Impact of HLA in cord blood transplantation outcomes. HLA 2016; 87:413-21; PMID:27060588; https://doi.org/10.1111/tan.12792
- Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, Witherspoon R, Mielcarek M, Deeg JH, Sorror M et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med 2016; 375:944-53; PMID:27602666; https://doi.org/10.1056/NEJMoa1602074
- Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008; 322:1562-5; PMID:19056990; https://doi.org/10.1126/science.1164511
- Miller JS, Warren EH, van den Brink MRM, Ritz J, Shlomchik WD, Murphy WJ, Barrett AJ, Kolb HJ, Giralt S, Bishop MR et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biology underlying recurrence of malignant disease following allogeneic HSCT: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 2010; 16:565-86; PMID:20152921; https://doi.org/10.1016/j.bbmt.2010.02.005
- Moncunill G, Dobaño C, McElrath MJ, De Rosa SC. OMIP-025: evaluation of human T- and NK-cell responses including memory and follicular helper phenotype by intracellular cytokine staining. Cytom Part J Int Soc Anal Cytol 2015; 87:289-92; PMID:25407958; https://doi.org/10.1002/cyto.a.22590
- McGowan I, Anton PA, Elliott J, Cranston RD, Duffill K, Althouse AD, Hawkins KL, De Rosa SC. Exploring the feasibility of multi-site flow cytometric processing of gut associated lymphoid tissue with centralized data analysis for multi-site clinical trials. PloS One 2015; 10:e0126454; PMID:26010577; https://doi.org/10.1371/journal.pone.0126454
- Lambert NC, Erickson TD, Yan Z, Pang JM, Guthrie KA, Furst DE, Nelson JL. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum 2004; 50:906-14; PMID:15022334; https://doi.org/10.1002/art.20200
- Loubiere LS, Lambert NC, Flinn LJ, Erickson TD, Yan Z, Guthrie KA, Vickers KT, Nelson JL. Maternal microchimerism in healthy adults in lymphocytes, monocyte//macrophages and NK cells. Lab Invest 2006; 86:1185-92; PMID:16969370; https://doi.org/10.1038/labinvest.3700471
- Gammill HS, Stephenson MD, Aydelotte TM, Nelson JL. Microchimerism in recurrent miscarriage. Cell Mol Immunol 2014; 11:589-94; PMID:25242272; https://doi.org/10.1038/cmi.2014.82
- Guthrie KA, Gammill HS, Kamper-Jørgensen M, Tjønneland A, Gadi VK, Nelson JL, Leisenring W. Statistical methods for unusual count data: examples from studies of microchimerism. Am J Epidemiol 2016; 184:779-86; PMID:27769989; https://doi.org/10.1093/aje/kww093
- D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica 1998; 83:197-203; PMID:9573672
- Holm BC, Svensson J, Akesson C, Arvastsson J, Ljungberg J, Lynch K, Ivarsson S-A, Lernmark A, Cilio CM. Diabetes prediction study in Skåne (DiPiS). Evidence for immunological priming and increased frequency of CD4+ CD25+ cord blood T cells in children born to mothers with type 1 diabetes. Clin Exp Immunol 2006; 146:493-502; PMID:17100770; https://doi.org/10.1111/j.1365-2249.2006.03243.x
- Allan D, Petraszko T, Elmoazzen H, Smith S. A review of factors influencing the banking of collected umbilical cord blood units. Stem Cells Int 2013; 2013:e463031; PMID:23533442; https://doi.org/10.1155/2013/463031
- Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, Shlomchik WD. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest 2003; 112:101-8; PMID:12840064; https://doi.org/10.1172/JCI17601
- Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, Jain D, McNiff J, Shlomchik WD. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood 2008; 111:2476-84; PMID:18045967; https://doi.org/10.1182/blood-2007-08-109678
- Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, Gooley TA, Sommermeyer F, Riddell SR, Shlomchik WD. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest 2015; 125:2677-89; PMID:26053664; https://doi.org/10.1172/JCI81229
- Oliver DC, Milano F, Gammill HS, Gentil C, Kanaan SB, Allen J, Nelson JL, Delaney C. 3109 Persistence of the Losing Unit Following Double Umbilical Cord Blood Transplant: Finding the Unseen [Internet]. Orlando, FL: American Society of Hematology; 2015. Available from: https://ash.confex.com/ash/2015/webprogramscheduler/Paper86600.html
- Chen C-P, Lee M-Y, Huang J-P, Aplin JD, Wu Y-H, Hu C-S, Chen P-C, Li H, Hwang S-M, Liu SH et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells Dayt Ohio 2008; 26:550-61; PMID:17975225; https://doi.org/10.1634/stemcells.2007-0406
- Kühnert M, Strohmeier R, Stegmüller M, Halberstadt E. Changes in lymphocyte subsets during normal pregnancy. Eur J Obstet Gynecol Reprod Biol 1998; 76:147-51; PMID:9481564; https://doi.org/10.1016/S0301-2115(97)00180-2
- Zhao Y, Dai ZP, Lv P, Gao XM. Phenotypic and functional analysis of human T lymphocytes in early second- and third-trimester fetuses. Clin Exp Immunol 2002; 129:302-8; PMID:12165087; https://doi.org/10.1046/j.1365-2249.2002.01920.x
- Köhler C, Adegnika AA, van der Linden R, Luty AJ, Kremsner PG. Phenotypic characterization of mononuclear blood cells from pregnant Gabonese and their newborns. Trop Med Int Health 2011; 16:1061-9; PMID:21702869; https://doi.org/10.1111/j.1365-3156.2011.02812.x
- Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, Bean MA, Ober C, Bianchi DW. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet 1998; 351:559-62; PMID:9492775; https://doi.org/10.1016/S0140-6736(97)08357-8
- Berry SM, Hassan SS, Russell E, Kukuruga D, Land S, Kaplan J. Association of maternal histocompatibility at Class II loci with maternal microchimerism in the fetus. Pediatr Res 2004; 56:73-8; PMID:15128924; https://doi.org/10.1203/01.PDR.0000129656.10005.A6
- Adams K, Lambert N, Heimfeld S, Tylee T, Pang J, Erickson T, Nelson J. Male DNA in female donor apheresis and CD34-enriched products. Blood 2003; 102:3845-7; PMID:12869496; https://doi.org/10.1182/blood-2003-05-1570
- Sunku Cuddapah C, Gadi V, deLavaldeLacoste B, Guthrie K, Nelson J. Maternal and fetal microchimerism in granulocytes. Chimerism 2010; 1:11-4; PMID:21327147; https://doi.org/10.4161/chim.1.1.13098
- Lee TH, Paglieroni T, Ohto H, Holland PV, Busch MP. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood 1999; 93:3127-39; PMID:10216112
- de Bellefon LM, Heiman P, Kanaan SB, Azzouz DF, Rak JM, Martin M, Roudier J, Roufosse F, Lambert NC. Cells from a vanished twin as a source of microchimerism 40 years later. Chimerism 2010; 1:56-60; PMID:21327048; https://doi.org/10.4161/chim.1.2.14294
- Yan Z, Lambert NC, Ostensen M, Adams KM, Guthrie KA, Nelson JL. Prospective study of fetal DNA in serum and disease activity during pregnancy in women with inflammatory arthritis. Arthritis Rheum 2006; 54:2069-73; PMID:16804866; https://doi.org/10.1002/art.21966
- van der Burg M, Kreyenberg H, Willasch A, Barendregt BH, Preuner S, Watzinger F, Lion T, Roosnek E, Harvey J, Alcoceba M et al. Standardization of DNA isolation from low cell numbers for chimerism analysis by PCR of short tandem repeats. Leukemia 2011; 25:1467-70; PMID:21681189; https://doi.org/10.1038/leu.2011.118
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239:487-91; PMID:2448875; https://doi.org/10.1126/science.2448875
