ABSTRACT
Natural killer (NK) cells are innate immune effector cells that play a crucial role in immune surveillance and the destruction of cancer cells. NK cells express a low-affinity receptor for the Fc or constant region of immunoglobulin G (FcγRIIIa) and multiple cytokine receptors that respond to antibody-coated targets and cytokines in the tumor microenvironment. In the present work, microarray gene expression analysis revealed that the IL-21 receptor (IL-21R) was strongly upregulated following FcR stimulation. The IL-21R was found to be upregulated on FcR-stimulated NK cells at the transcript level as determined by reverse transcription polymerase chain reaction (RT-PCR). Immunoblot analysis revealed that protein expression of the IL-21R peaked at 8 h post-stimulation of the FcR. Inhibition of the mitogen-activated protein kinase (MAPK) pathway downstream of the FcR blocked the induction of IL-21R expression. Increased expression of the IL-21R sensitized NK cells to IL-21 stimulation, as treatment of FcR-stimulated NK cells led to significantly increased phosphorylation of STAT1 and STAT3, as measured by intracellular flow cytometry and immunoblot analysis. Following FcR-stimulation, IL-21-activated NK cells were better able to mediate the lysis of trastuzumab-coated human epidermal growth factor receptor 2 (HER2+) SK-BR-3 tumor cells as compared to control-treated cells. Likewise, IL-21-induced NK cell secretion of IFNγ following exposure to antibody-coated tumor cells was enhanced following FcR-stimulation. The analysis of NK cells from patients receiving trastuzumab therapy for HER2+ cancer exhibited increased levels of the IL-21R following the administration of antibody suggesting that the presence of monoclonal antibody-coated tumor cells in vivo can stimulate the increased expression of IL-21R on NK cells.
Introduction
Natural killer (NK) cells are bone-marrow-derived, large granular lymphocytes that contain abundant cytolytic granules and express numerous adhesion molecules. NK cells are unique in their constitutive expression of receptors for numerous cytokines and an activating receptor for the Fc region of IgG (FcγRIIIa).Citation1-3 In addition to their ability to mediate antibody-dependent cellular cytotoxicity (ADCC), FcR-activated NK cells can also secrete factors such as IFNγ, TNF-α, and chemokines that inhibit tumor cell proliferation, enhance antigen presentation, and stimulate the chemotaxis of T cells, respectively.Citation4,5 These properties provide NK cells with the ability to directly lyse cellular targets as well as coordinate the developing adaptive immune response.
Interleukin-21 (IL-21) is a pleiotropic cytokine produced primarily by activated CD4+ T cells. IL-21 has been implicated in several disease processes including allergies, autoimmune disorders, viral infections, and cancer.Citation6-11 The IL-21 receptor (IL-21R) is a type I cytokine receptor with four conserved cysteine residues and an extracellular WSXWS motif that shares the common receptor gamma-chain with IL-2, −4, −7, −9, and −15.Citation12,13 The expression of IL-21R on the surface of immune cells helps mediate a variety of effects including its ability to enhance the proliferation, antigen-induced activation, clonal expansion, IFNγ production, and cytotoxicity of CD8+ T cells.Citation13-15 In addition to its effects on CD8+ T cells, IL-21 has context-dependent effects on B cells, amplifies macrophage activation pathways, and inhibits the activation of myeloid dendritic cells.Citation13,16-18 IL-21 also promotes the maturation of murine NK cells and increases human NK cell expression of the NK activation receptors NKp30 and 2B4.Citation19,20 IL-21 is able to mediate the regression of established tumors in a variety of murine models and phase I clinical trials. Its mechanism of action has been variously attributed to NK cell cytolytic activity, perforin-mediated CD8+ T cell cytotoxicity, the differentiation of CD4+ T cell subsets, the growth and activity of NKT cells, and the anti-angiogenic actions of IL-21-induced IFNγ.Citation21-27
Here, we show that the upregulation of IL-21R has positive implications for the activation of NK cells against HER2+ breast cancer cells and that the induction of this receptor is mediated through the MAP kinase pathway. NK cells that had increased IL-21R expression were better able to lyse antibody-coated tumor cells and produced greater amounts of IFNγ than their resting counterparts. In addition, patients receiving the monoclonal antibody (mAb) trastuzumab and exhibiting a clinical response to treatment were found to have greater expression of IL-21R on the surface of their NK cells than those who were not trastuzumab-responders. These findings support further investigation into the role played by IL-21R in mediating NK cell activity during the course of mAb therapy.
Methods
NK cells isolation, cell lines, and reagents
Human natural killer (NK) cells were isolated from fresh peripheral blood leukopacks (American Red Cross, Columbus, OH) by 30-min incubation with RosetteSep cocktail (Stem Cell Technologies) before Ficoll Hypaque (Sigma) density gradient centrifugation and cultured as previously described, with ∼97% purity.Citation28 Peripheral blood mononuclear cells (PBMCs) were also procured from patients with HER2-positive breast cancer who were receiving trastuzumab therapy (OSU Protocol No. OSU-09142 and OSU-08153). Polyclonal human IgG (IgG) was purchased from Sigma-Aldrich. Trastuzumab (Herceptin™), an anti-HER2/neu mAb, was provided by Genentech, Inc. Recombinant human interleukin-21 (IL-21) was supplied by ZymoGenetics, Inc. (Seattle, WA). The YT-CD16 cell line was a gift from Dr. Michael Caligiuri (The Ohio State University, Columbus, OH). The following cell lines were purchased from ATCC: SK-BR-3 (HER2-overexpressing breast cancer), Ramos and HeLa.
FcR-stimulation assays
For FcR-stimulation assays by immobilized IgG, wells of 96-well flat-bottom plates were coated with 100 μg/mL of IgG in cold PBS overnight at 4°C, washed with cold phosphate buffer saline (PBS), and then plated with human NK cells at 2 × 105 cells/well as previously described.Citation29 NK cells classified as “resting” were plated in 96-well flat-bottom plates in a similar fashion to FcR-stimulated NK cells, minus the addition of IgG.
Preparation of labeled RNA and microarray hybridization and gene microarray data analysis
cDNA was prepared from total cellular RNA of NK cells obtained from individual healthy donors and subject to a cleanup protocol as previously described.Citation30 Affymetrix GeneChip expression array U133A was hybridized with each prepared cRNA target in duplicate, according to the manufacturer's instructions. The raw and processed microarray data have been made publicly available in GEO (GSE63038).Citation30 The gene microarray data were analyzed as previously described.Citation30
RT-PCR. The expression values of IL-21R identified via the microarray experiment were validated by real-time PCR. Following RNeasy purification of NK cells from the immobilized IgG assay at various time points, 2 μg of total RNA was reverse transcribed and the resulting cDNA was used as a template to measure IL-21R transcript using pre-designed primer/probe sets according to the manufacturer's recommendations (Applied Biosystems, Foster City, CA). Primers that recognize the human β-Actin sequence were used as the internal control in each reaction well. Real-time PCR data were analyzed using the ABI PRISM® 7900 Sequence Detection system.
Immunoblot analysis
NK cell expression of IL-21R was verified via immunoblot analysis. Lysates were prepared from human NK cells as previously describedCitation31, 32 following FcR-stimulation by immobilized IgG and assayed for the expression of IL-21R (MAB991; R&D Systems Inc., Minneapolis, MN) or β-Actin as a loading control (clone AC-74; Sigma Aldrich, St. Louis, MO). Lysates were separated on a 10% SDS-PAGE gels, transferred to a nitrocellulose membrane, and blocked in 5% nonfat milk prior to incubation with primary and secondary antibodies. For pathway analysis, 25 μM of the ERK inhibitor U0126 was incubated with NK cells for 30 min prior to the preparation of lysates (Sigma Aldrich).
Flow cytometry
NK cells activated through their FcR by immobilized IgG were subject to flow cytometry to determine cell surface expression of IL-21R. Resting or FcR-stimulated NK cells were incubated on ice for 30 min in flow buffer (5% FBS in PBS) with anti-CD56-PE (clone B159) and anti-IL-21R-APC (clone 17A12) or isotype control antibodies (BD Biosciences, San Jose, CA). Cells were then washed and fixed in 1% formalin. Non-specific staining with an isotype control Ab was employed to determine the percent positive population. To detect intracellular levels of phospho (p)-STAT1 and p-STAT3 induced by IL-21 treatment, unstimulated NK cells or FcR-stimulated NK cells were cultured with or without IL-21 (10 ng/mL) for 30 min, washed, and stained with an anti-p-STAT1-FITC mAb or an anti-p-STAT3-FITC mAb in combination with the NK cell marker CD56 as previously described (BD Biosciences).Citation29 Percentages reported are of dual-positive populations (Q2) as determined by the removal of isotype-control background fluorescence.
Cytotoxicity assay
NK cells activated through their FcR by immobilized IgG were plated overnight in RPMI media supplemented with 10% human AB (HAB) serum media with or without IL-21 (10 ng/mL). Following overnight incubation, 51Cr-labeled K562 tumor cells were incubated with NK cells at various effector:target (E:T) ratios. Following a 4 hr incubation, supernatants were harvested and percent lysis was calculated as previously described.Citation33
NK cell cytokine secretion
For in vitro co-culture assays, wells of a 96-well flat-bottom culture plate were seeded with the HER2-overexpressing human breast cancer cell line SK-BR-3 at a density of 5 × 104 cells/well. Tumor cells were grown to confluence overnight and then treated with 100 μg/mL trastuzumab for 1 hr at 37°C. After washing off unbound tumor cells, resting or FcR-stimulated NK cells were added at 2 × 105 cells/well in 200 μL in RPMI media supplemented with 10% human AB (HAB) serum media with or without IL-21 (10 ng/mL). Control conditions consisted of resting or 8 hr FcR-stimulated NK cells incubated with tumor alone or IL-21 alone. Cell-free supernatants were collected following a 48 hr incubation and IFNγ levels were measured using commercially available ELISA kits (R&D Systems Inc.).Citation34
Analysis of apoptosis via Annexin V/propidium iodide (PI) staining
Apoptosis-induced phosphatidyl serine exposure was measured in tumor cells by flow cytometric analysis using propidium iodide, V450-anti-annexin V, and APC-anti-CD56 (BD Biosciences) as previously described.Citation35 Each analysis was performed utilizing at least 10,000 cellular events. The population with values above an isotype control was calculated within each treatment group, gating on APC-anti-CD56-negative cells, for each treatment group.
Statistics. IL-21R expression measured by RT-PCR, IFNγ release as measured by ELISA and tumor cell apoptosis measured by chromium release and annexin V/PtdIns staining was analyzed by the analysis of variance. Expression of IL-21R from patient samples before and after treatment was compared using the Wilcoxon signed rank test.
Results
Human NK cells increase expression of IL-21R transcript levels in response to FcR stimulation
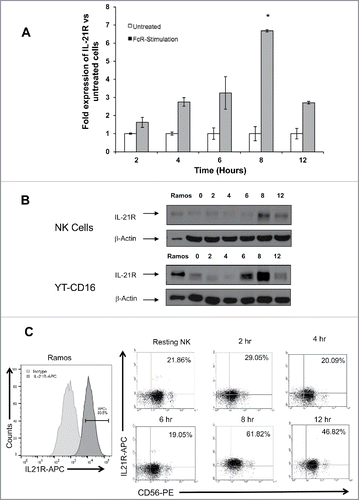
NK cells are known for their ability to upregulate the production of effector cytokines and increase their cytotoxic potential in response to external stimuli.Citation29,31,33,34,36-38 The major goal of this study was to gain insight into the function of NK cells upon encounter with antibody-coated targets as it relates to IL-21 sensitivity. To directly address this question, NK cells were isolated from the peripheral blood of healthy donors and global gene-expression profiling was performed on RNA freshly isolated or isolated after a 12 h stimulation of the NK cell FcR by immobilized IgG. The IL-21R was found to be upregulated in response to FcR stimulation as compared to unstimulated NK cells (). Consistent with the gene expression microarray data, PCR analysis for IL-21R indicated that FcR stimulation upregulates IL-21R expression at the transcript level by approximately 3-fold (p < 0.01; ).
Figure 1. IL-21R gene expression and transcript levels are upregulated on NK cells following FcR stimulation. (A) Heatmap depicting the expression of IL-21R as determined by Affymetrix GeneChip U133A gene chip in untreated NK cells and in NK cells stimulated for 12 hr with immobilized-IgG (100 μg/mL). Expression values were retrieved from the GEO database (GSE63038). Pixel density (highest values are red [+4], lowest are green [−4]) represents average hybridization signal intensity from eight donors pre- and post FcR-stimulation as detected by the probes for IL-21R, 219971_at and 221658_s_at. (B) Validation of IL-21R gene expression data by RT-PCR in untreated NK cells and NK cells exposed to immobilized-IgG (100 μg/mL) for 12 hr to stimulate the FcR. Each group depicts the mean fold increase in IL-21R expression in six donors ± SD. The asterisk (*) denotes p < 0.01 versus untreated NK cells.
![Figure 1. IL-21R gene expression and transcript levels are upregulated on NK cells following FcR stimulation. (A) Heatmap depicting the expression of IL-21R as determined by Affymetrix GeneChip U133A gene chip in untreated NK cells and in NK cells stimulated for 12 hr with immobilized-IgG (100 μg/mL). Expression values were retrieved from the GEO database (GSE63038). Pixel density (highest values are red [+4], lowest are green [−4]) represents average hybridization signal intensity from eight donors pre- and post FcR-stimulation as detected by the probes for IL-21R, 219971_at and 221658_s_at. (B) Validation of IL-21R gene expression data by RT-PCR in untreated NK cells and NK cells exposed to immobilized-IgG (100 μg/mL) for 12 hr to stimulate the FcR. Each group depicts the mean fold increase in IL-21R expression in six donors ± SD. The asterisk (*) denotes p < 0.01 versus untreated NK cells.](/cms/asset/063dcb45-c08b-4a16-9b47-968355f7d33f/koni_a_1312045_f0001_oc.gif)
Upregulation of IL-21R via NK cell FcR stimulation occurs in a time-dependent fashion
RT-PCR, immunoblot analysis, and flow cytometric analysis were used to characterize the upregulation of the IL-21R in NK cells following FcR stimulation. These analyses revealed that the upregulation of the IL-21R occurs in a time-dependent fashion. The expression of IL-21R at the mRNA level peaked at 8 hr post-FcR-stimulation and was upregulated 6.5-fold compared to unstimulated NK cells at this time point (p < 0.01; ). Immunoblot analysis for IL-21R expression was conducted using primary human NK cells and the YT cell line modified to express CD16 (YT-CD16).Citation39 This analysis revealed marked upregulation of IL-21R following FcR stimulation with expression peaking at 8 hr post-stimulation (). NK cells were also analyzed for IL-21R levels by flow cytometry using anti-CD56 Ab and anti-IL-21R fluorescence-conjugated mAbs. This experiment showed that IL-21R was upregulated on the surface of NK cells in a time-dependent fashion, with 62% of NK cells expressing surface IL-21R at 8 hr post-IgG stimulation as compared to 21.9% at baseline ().
Figure 2. The IL-21R is upregulated on NK cells following FcR stimulation in a time-dependent fashion. NK cells stimulated via the FcR by immobilized IgG were analyzed at varying time points for expression of IL-21R transcript by (A) RT-PCR and IL-21R protein by (B) immunoblot analysis, and (C) flow cytometry. (A) RT-PCR for IL-21R transcript in untreated NK cells and NK cells cultured in the presence of immobilized-IgG at the time points indicated. Data represent the mean fold increase in IL-21R expression in three donors ± SD. The asterisk (*) denotes p < 0.01 vs. all time points shown. (B) IL-21R expression at the protein level was confirmed by immunoblot analysis in primary NK cells and YT-CD16 cells at the time points indicated. The Ramos tumor cell line served as a positive control. The membranes were re-probed for β-actin to confirm equal loading. (C) IL-21R expression was measured by flow cytometry in resting NK cells and NK cells stimulated with immobilized-IgG. Cells were stained with anti-CD56-PE and anti-IL-21R-APC Abs at the time points indicated and fluorescence was compared to that obtained with an isotype control antibody. HeLa and Ramos acted as negative and positive controls, respectively. Percentages are reflective of dual positive populations (Q2). Each plot depicts the results from one representative donor. Results are representative of three normal donors tested.
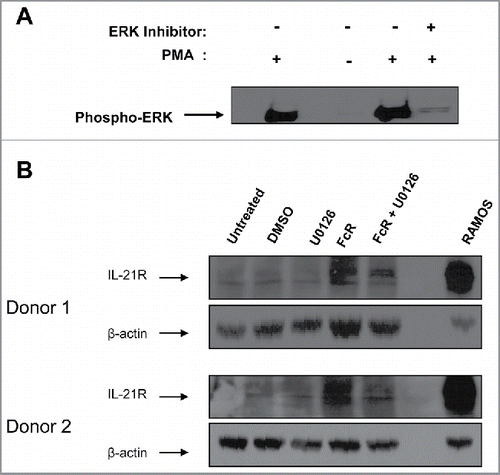
NK cell IL-21R upregulation is dependent on ERK signal transduction
The ERK signal transduction pathway is activated in NK cells following engagement of the FcR and results in the generation of phosphorylated ERK protein, which mediates downstream gene regulation.Citation28 The ERK inhibitor U0126 was used in an effort to determine the contribution of the ERK signaling pathway to the upregulation of IL-21R on the surface of NK cells following FcR stimulation. As expected, the ERK inhibitor was effective in decreasing levels of activated (phosphorylated) ERK in NK cells following PMA stimulation (). When NK cells were activated through the FcR for 8 h, an upregulation of IL-21R protein level was detected, but when these FcR-activated NK cells were treated with an ERK inhibitor, expression of the IL-21R was diminished ().
Figure 3. Upregulation of IL-21R on the NK cell surface is facilitated via the MAP kinase signaling pathway. (A) NK cells were stimulated with PMA or left resting, with the addition or absence of the ERK inhibitor U0126 and subjected to immunoblot analysis for phospho-ERK expression. (B) NK cells were stimulated for 8 hr with immobilized-IgG or left unstimulated in the presence of the ERK inhibitor U0126 (reconstituted in DMSO) and subjected to immunoblot analysis for IL-21R expression. The membranes were re-probed for β-actin to confirm equal loading. NK cells were isolated from two healthy donors.
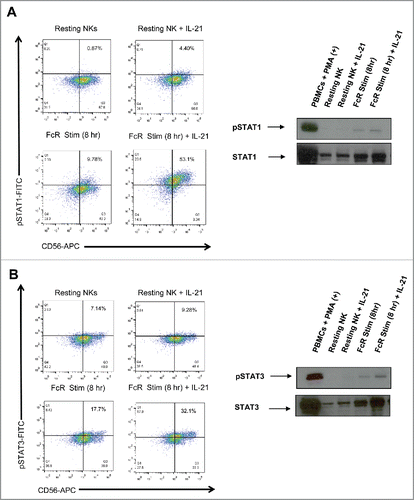
Upregulation of IL-21R on the surface of NK cells leads to enhanced NK cell signal transduction
The IL-21R signals via the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway.Citation40 IL-21 is known to activate JAK1 and JAK3, and this activation leads directly to the phosphorylation of STAT1 and STAT3. In order to determine if FcR-mediated upregulation of IL-21R on the surface of NK cells led to increased sensitivity to exogenous IL-21, intracellular levels of p-STAT1 and p-STAT3 post-IL-21 exposure were measured by flow cytometry and immunoblot analysis (). NK cells stimulated through their FcR for 8 h and then exposed to IL-21 for 45 min showed an increase in the generation of p-STAT1 as compared to unstimulated NK cells via flow cytometry. Immunoblot analysis confirmed that exposure of FcR-stimulated NK cells to IL-21 led to increased levels of p-STAT1 in comparison to unstimulated NK cells (). Similar results were obtained for the generation of p-STAT3 in response to IL-21 (). Interestingly, total STAT1 and STAT3 levels increased as NK cells were stimulated through their FcR. The upregulation of total STAT1 and STAT3 levels may have contributed to the increased p-STAT1 and pSTAT3 signal observed.
Figure 4. IL-21 treatment of FcR-stimulated NK cells enhances IL-21-mediated NK cell signal transduction. Resting NK cells and NK cells stimulated with immobilized-IgG for 8 hr were washed, rested for 1 hr, and then treated with PBS or 10 ng/mL of IL-21. 30 min later, NK cells were analyzed by flow cytometry and immunoblot analysis for (A) p (phospho)-STAT1 and (B) p-STAT3.
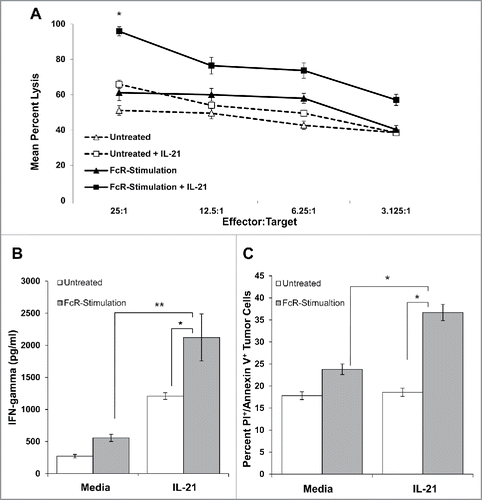
Upregulation of IL-21R on the surface of NK cells leads to enhanced cytotoxicity, IFNγ production, and NK cell-mediated tumor cell apoptosis
In order to characterize the consequences of upregulation of the IL-21R on the surface of NK cells following FcR engagement, NK cell cytotoxicity, production of IFNγ, and NK cell-induced tumor cell apoptosis were measured through chromium release, ELISA and annexin V/PI flow cytometry, respectively (). It was hypothesized that NK cell effector functions in response to IL-21 would be enhanced following FcR stimulation. As predicted, FcR-stimulated NK cells were better able to lyse K562 tumor cells in an NK cell cytotoxicity assay following the addition of IL-21, as compared to unstimulated control NK cells (p < 0.05; ). Furthermore, exposure of FcR-stimulated NK cells to IL-21 resulted in increased production of IFNγ in response to trastuzumab-coated SK-BR-3 tumor cells as compared to unstimulated control NK cells (p < 0.01; ). In addition, FcR-stimulated NK cells mediated greater apoptosis of trastuzumab-coated SK-BR-3 tumor cells in response to IL-21 (p < 0.05; ).
Figure 5. IL-21 treatment of FcR-stimulated NK cells enhances cytotoxicity, NK cell IFNγ production, and tumor cell apoptosis. (A) NK cells were left untreated or stimulated through their FcR for 8 h prior to incubation overnight with or without 10 ng/mL IL-21. The lytic activity of IL-21-activated NK cells was then assessed in a standard 4 h chromium release assay using K562 tumor cells as targets. The percentage of lysis was calculated as previously described. Graph depicts the results from one representative donor ± SD. Three normal donors were tested. The asterisk (*) denotes p < 0.05 vs untreated NK cells. (B) Untreated or 8 h FcR-stimulated NK cells were incubated with or without 10 ng/mL IL-21 and co-cultured with (100 μg/mL) trastuzumab-coated SK-BR-3 tumor cells. Supernatants were harvested at 48 h and analyzed for IFNγ by ELISA. Each graph depicts the mean production of IFNγ from three donors ± SD. The asterisk (**) denotes p < 0.01 and (*) denotes p < 0.05 vs. conditions shown. (C) Untreated or 8 h FcR-stimulated NK cells were incubated with or without 10 ng/mL IL-21 and co-cultured with (100 μg/mL) trastuzumab-coated SK-BR-3 tumor cells. Tumor cells were harvested at 48 h and stained for flow cytometry with anti-CD56-APC, propidium iodide, and anti-annexin V-V450. APC+ values were gated out to account for tumor cell apoptosis only. Percentages reported are of PE+/V450+ populations (Q2). Each graph depicts the results from three donors ± SD. The asterisk (*) denotes p < 0.05 vs. all conditions shown.
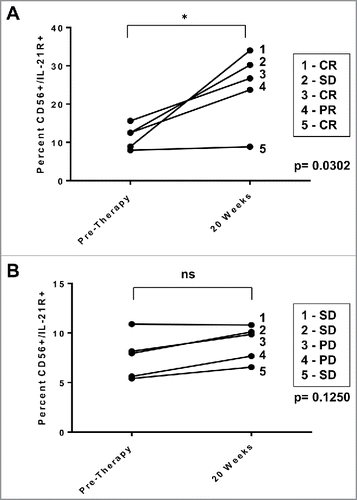
NK cells from patients receiving monoclonal antibody-therapy demonstrate increased IL-21R expression following treatment
PBMCs from HER2-positive breast cancer patients receiving trastuzumab therapy were analyzed for expression of IL-21R on CD56-positive NK cells by flow cytometry. The first analysis was conducted with five patients receiving neoadjuvant chemotherapy, four of whom had either a partial response or a complete response. The pre-surgical treatment regimen of these patients with operable, non-metastatic invasive ductal carcinoma consisted of doxorubicin/cyclophosphamide administered every 3 weeks × 4 cycles followed by weekly trastuzumab/paclitaxel. IL-21R expression on patient NK cells increased significantly at the time of trastuzumab administration compared to pre-therapy levels. The percentage of CD56+ NK cells expressing the IL-21R went from an average of 11.8% pre-therapy to 22.4% at 20 weeks (p = 0.0302; ). In contrast, a second group of patients with metastatic disease receiving trastuzumab in combination with CpG oligodeoxynucleotides weekly for 12 weeks, followed by weekly trastuzumab monotherapy exhibited no clinical responses and there was only a 1.15% increase in IL-21R expression on NK cells at 20 weeks of therapy compared to pre-therapy levels (ns; ).
Figure 6. IL-21R expression is enhanced on NK cells in patients receiving monoclonal antibody therapy. NK cells from patients with HER2-positive breast cancer receiving trastuzumab-based therapy were analyzed for IL-21R expression. PBMC were obtained pre-therapy and at the 20 week time point. Total PBMC were stained with Abs specific for CD56 and IL-21R and analyzed by flow cytometry. (A) IL-21R expression in NK cells of patients with operable breast cancer who successfully received doxorubicin/ plus cyclophosphamide every 3 weeks × 4 cycles followed by weekly trastuzumab plus paclitaxel and exhibited clinical benefit (complete response, partial response, or stable disease = CR, PR, or SD). Time points are pre-therapy and at the 20 week time point at which time patients were receiving trastuzumab. Each data point is reflective of double positive populations determined by a gating strategy utilizing an isotype control antibody. The asterisk (*) denotes p = 0.0302 vs. pre-therapy. (B) IL-21R expression in NK cells of patients with metastatic breast cancer treated with trastuzumab and CpG oligodeoxynucleotides. Each data point is reflective of double positive populations determined by a gating strategy utilizing an isotype control antibody. Time points are pre-therapy and the 20 week mark at which time patients were receiving trastuzumab therapy. All patients eventually experienced progress on of disease. Percentages reported are of dual positive populations (Q2).
Discussion
The major goal of this study was to gain insight into the expression of IL-21R on NK cells upon encountering Ab-coated targets. An oligonucleotide microarray experiment revealed increased expression of the IL-21R on NK cells following FcR activation. Immunoblot analysis and flow cytometry confirmed that levels of IL-21R on the surface of NK cells are increased following FcR-stimulation. Upregulation of IL-21R rendered NK cells more sensitive to IL-21 at the level of signal transduction, lytic activity, and IFNγ production. Finally, it was shown that expression of IL-21R on NK cells was upregulated in patients who successfully received trastuzumab-based neo-adjuvant chemotherapy.
It has been suggested that IL-21 acts in vivo to accelerate the transition between innate and adaptive immunity and that the effects of IL-21 on NK cells may vary depending on the timing and magnitude of the T cell response and subsequent concentrations of IL-21.Citation41 Due to its immune stimulatory properties on both innate and adaptive immunity, IL-21 administration or IL-21 gene transfer has been widely exploited in preclinical models of cancer immunotherapy either alone or in combination with antibodies, other cytokines or immune checkpoint blockers. Early studies in vivo using the systemic expression of IL-21 by plasmid-mediated delivery revealed that IL-21 can inhibit the growth of large melanomas and fibrosarcomas.Citation22 The depletion of select populations of immune cells showed that most of the antitumor activity of IL-21 is mediated by NK cells, with a smaller contribution from CD8+ T cells.Citation21 In syngeneic mouse models of melanoma and renal cell carcinoma, IL-21 administered intratumorally was able to strongly inhibit tumor growth and increase the frequency of tumor-infiltrating CD8+ T cells.Citation42 In addition, delivery of IL-21 showed low toxicity and did not induce vascular-leak syndrome, a dose-limiting toxicity associated with other clinically utilized cytokines, such as IL-2.Citation43 IL-21 antitumor activities have been exploited in association with several other molecules, which showed either additive or synergistic effects.Citation44 The use of an anti-CD25 mAb in combination with an IL-21-secreting mammary carcinoma cell vaccine cured 70% of syngeneic mice from lung micrometastases through the induction of antigen-specific CD8+ T cell responses and production of IFNγ.26 In immunodeficient mice bearing human B cell lymphoma xenografts, the addition of IL-21 to rituximab treatment significantly increased survival relative to either agent alone.Citation45 These findings illustrate the therapeutic potential for IL-21 administration and suggest that FcR-activated NK cells would be sensitive to this cytokine and able to aid in the eradication of tumor. Indeed, the stimulation of the FcR on the surface of NK cells led to an upregulation of IL-21R and increased NK cell ADCC and IFNγ production in response to trastuzumab-coated tumor cells.
Several potential mechanisms of action have been proposed for the antitumor effects of mAbs in vivo, including cross-linking-mediated activation of signaling cascades that lead to tumor cell apoptosis, blockade of ligands required for tumor cell survival, recruitment of the complement cascade, and recruitment of cytotoxic FcR-positive effector cells.Citation46 NK cells are able to directly kill transformed cells due to the interaction of the FcγRIIIa with bound mAb.Citation47, 48 The FcγRIIIa on NK cells initiates cellular activation through an intracellular immunoreceptor tyrosine-based activation motif.Citation49,50 We have also shown that CD56dimCD16+ NK cells co-stimulated via the FcR and IL-12R increase their expression of genes encoding cytotoxicity receptors, apoptotic proteins, intracellular signaling molecules, and cytokines that could mediate enhanced cytotoxicity and interactions with other immune cells within inflammatory tissues.Citation30 Upregulation of the IL-21 cytokine receptor has positive implications for both the proliferation and maturation of NK cell populations from the bone marrow, as IL-21 has been shown to synergistically enhance IL-15- and Flt3L-mediated NK cell generation from CD34+ haematopoietic progenitor cells.Citation13 Based on this data, the use of IL-21 in combination with therapeutic mAbs is thought to exert a positive antitumor effect. The fact that NK cell IL-21R expression was correlated with favorable clinical outcomes in patients receiving trastuzumab alone supports this contention. It should be noted that studies in patients receiving trastuzumab are limited and results are preliminary. The results of correlative studies of NK cells in patients with HER2-positive cancers that received trastuzumab are interesting but are preliminary in nature and must be confirmed in larger prospective studies. Furthermore, it is very possible that the surface expression of other receptors would also increase following mAb therapy, including CD25.Citation51 Functional studies with NK cells isolated from patients receiving trastuzumab therapy could add to this manuscript. However, our group has observed low NK cell activity from patient PBMC that were cryopreserved and then thawed. This limitation reduced our enthusiasm to conduct functional assays using total PBMC in an ex vivo cytotoxicity assay.
In the present study, the IL-21R was shown to be upregulated at the transcriptional and protein levels in NK cells following FcR-stimulation. Upregulation of the IL-21R allowed NK cells to be more sensitive to IL-21 in terms of signal transduction and effector functions. It was also shown that IL-21R expression was upregulated on the surface of NK cells from patients who had a clinical response to antibody-based chemotherapy. These results support further investigation into the use of IL-21 in patients receiving mAb therapy.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
Supplementary_materials.zip
Download Zip (90.8 KB)Funding
This work was supported by NIH Grants P01 CA095426 (M. Caligiuri), P30 CA016058 (M. Caligiuri), CA84402, K24 CA93670 (W.E. Carson, III), T32 GM068412 (ACJ-R), and T32 CA009338.
References
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 1994; 180(4):1395-403; PMID:7523571; https://doi.org/10.1084/jem.180.4.1395
- Carson WE, Lindemann MJ, Baiocchi R, Linett M, Tan JC, Chou CC, Narula S, Caligiuri MA. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood 1995; 85(12):3577-85; PMID:7540068.
- Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol 1999; 162(8):4511-20; PMID:10201989
- Bluman EM, Bartynski KJ, Avalos BR, Caligiuri MA. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J Clin Invest 1996; 97(12):2722-7; PMID:8675682; https://doi.org/10.1172/JCI118726
- Somersalo K, Carpén O, Saksela E. Stimulated natural killer cells secrete factors with chemotactic activity, including NAP-1/IL-8, which supports VLA-4- and VLA-5-mediated migration of T lymphocytes. Eur J Immunol 1994; 24(12):2957-65; PMID:7805722; https://doi.org/10.1002/eji.1830241206
- Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173(9):5361-71; PMID:15494482; https://doi.org/10.4049/jimmunol.173.9.5361
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 2005; 175(12):7867-79; PMID:16339522; https://doi.org/10.4049/jimmunol.175.12.7867
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298(5598):1630-4; PMID:12446913; https://doi.org/10.1126/science.1077002
- Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cϵ transcription of IL-4-stimulated B cells. Blood 2002; 100(13):4565-73; PMID:12393685; https://doi.org/10.1182/blood-2002-04-1115
- Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, Skak K, Lundsgaard D, Frederiksen KS, Thygesen P et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res 2007; 13(12):3630-6; PMID:17575227; https://doi.org/10.1158/1078-0432.CCR-07-0410
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science 2009; 324(5934):1569-72; PMID:19423777; https://doi.org/10.1126/science.1174182
- Habib T, Senadheera S, Weinberg K, Kaushansky K. The common γ chain (γc) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry 2002; 41(27):8725-31; PMID:12093291; https://doi.org/10.1021/bi0202023
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408(6808):57-63; PMID:11081504; https://doi.org/10.1038/35040504
- Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood 2007; 109(10):4135-42; PMID:17234735; https://doi.org/10.1182/blood-2006-10-054973
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008; 111(11):5326-33; PMID:18276844; https://doi.org/10.1182/blood-2007-09-113050
- di Carlo E, de Totero D, Piazza T, Fabbi M, Ferrini S. Role of IL-21 in immune-regulation and tumor immunotherapy. Cancer Immunol Immunother 2007; 56(9):1323-34; PMID:17447063; https://doi.org/10.1007/s00262-007-0326-z
- Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res 2007; 13(23):6926-32; PMID:18056166; https://doi.org/10.1158/1078-0432.CCR-07-1238
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 2008; 26:57-79; PMID:17953510; https://doi.org/10.1146/annurev.immunol.26.021607.090316
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol 2004; 172(4):2048-58; PMID:14764669; https://doi.org/10.4049/jimmunol.172.4.2048
- Burgess SJ, Marusina AI, Pathmanathan I, Borrego F, Coligan JE. IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J Immunol 2006; 176(3):1490-7; PMID:16424177; https://doi.org/10.4049/jimmunol.176.3.1490
- Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res 2003; 63(24):9016-22; PMID:14695220
- Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol 2003; 171(2):608-15; PMID:12847225; https://doi.org/10.4049/jimmunol.171.2.608
- Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol 2004; 172(3):1540-7; PMID:14734732; https://doi.org/10.4049/jimmunol.172.3.1540
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med 2005; 201(12):1973-85; PMID:15967825; https://doi.org/10.1084/jem.20042280
- Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, Lanier LL. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 2005; 175(4):2167-73; PMID:16081783; https://doi.org/10.4049/jimmunol.175.4.2167
- Comes A, Rosso O, Orengo AM, Di Carlo E, Sorrentino C, Meazza R, Piazza T, Valzasina B, Nanni P, Colombo MP et al. CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J Immunol 2006; 176(3):1750-8; PMID:16424205; https://doi.org/10.4049/jimmunol.176.3.1750
- McMichael EL, Jaime-Ramirez AC, Guenterberg KD, Luedke E, Atwal LS, Campbell AR, Hu Z, Tatum AS, Kondadasula SV, Mo X et al. IL-21 enhances natural killer cell response to cetuximab-coated pancreatic tumor cells. Clin Cancer Res 2017; 23(2):489-502; PMID:27435400; https://doi.org/10.1158/1078-0432.CCR-16-0004
- Kondadasula SV, Roda JM, Parihar R, Yu J, Lehman A, Caligiuri MA, Tridandapani S, Burry RW, Carson WE 3rd. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood 2008; 111(8):4173-83; PMID:18174382; https://doi.org/10.1182/blood-2007-01-068908
- Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest 2002; 110(7):983-92; PMID:12370276; https://doi.org/10.1172/JCI0215950
- Campbell AR, Regan K, Bhave N, Pattanayak A, Parihar R, Stiff AR, Trikha P, Scoville SD, Liyanarachchi S, Kondadasula SV et al. Gene expression profiling of the human natural killer cell response to Fc receptor activation: unique enhancement in the presence of interleukin-12. BMC Med Genom 2015; 8:66; PMID:26470881; https://doi.org/10.1186/s12920-015-0142-9
- Lesinski GB, Badgwell B, Zimmerer J, Crespin T, Hu Y, Abood G, Carson WE 3rd. IL-12 pretreatments enhance IFN-alpha-induced Janus kinase-STAT signaling and potentiate the antitumor effects of IFN-alpha in a murine model of malignant melanoma. J Immunol 2004; 172(12):7368-76; PMID:15187113; https://doi.org/10.4049/jimmunol.172.12.7368
- Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker M, Carson WE 3rd. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J Natl Cancer Inst 2004; 96(17):1331-42; PMID:15339971; https://doi.org/10.1093/jnci/djh252
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol 2001; 31(10):3016-25; PMID:11592078; https://doi.org/10.1002/1521-4141(2001010)31:10%3c3016::AID-IMMU3016%3e3.0.CO;2-J
- Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE 3rd. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol 2006; 177(1):120-9; PMID:16785506; https://doi.org/10.4049/jimmunol.177.1.120
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184(1):39-51; PMID:7622868; https://doi.org/10.1016/0022-1759(95)00072-I
- Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol 2004; 5(10):996-1002; PMID:15454923; https://doi.org/10.1038/ni1114
- Carson WE, Dierksheide JE, Jabbour S, Anghelina M, Bouchard P, Ku G, Yu H, Baumann H, Shah MH, Cooper MA et al. Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-gamma production and STAT-mediated signal transduction. Blood 2000; 96(4):1465-73; PMID:10942393
- Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, Parihar R, Karpa V, Papenfuss TL, LaPerle KM et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-γ production. J Immunol 2011; 186(6):3401-9; PMID:21321106; https://doi.org/10.4049/jimmunol.1000328
- Deaglio S, Zubiaur M, Gregorini A, Bottarel F, Ausiello CM, Dianzani U, Sancho J, Malavasi F. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood 2002; 99(7):2490-8; PMID:11895784; https://doi.org/10.1182/blood.V99.7.2490
- Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 2014; 13(5):379-95; PMID:24751819; https://doi.org/10.1038/nrd4296
- Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002; 16(4):559-69; PMID:11970879; https://doi.org/10.1016/S1074-7613(02)00295-9
- Søndergaard H, Galsgaard ED, Bartholomaeussen M, Straten PT, Odum N, Skak K. Intratumoral interleukin-21 increases antitumor immunity, tumor-infiltrating CD8+ T-cell density and activity, and enlarges draining lymph nodes. J Immunother 2010; 33(3):236-49; PMID:20445344; https://doi.org/10.1097/CJI.0b013e3181c0c1cb
- Sivakumar PV, Garcia R, Waggie KS, Anderson-Haley M, Nelson A, Hughes SD. Comparison of vascular leak syndrome in mice treated with IL21 or IL2. Comp Med 2013; 63(1):13-21; PMID:23561933
- Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008; 123(4):575-83; PMID:18005035; https://doi.org/10.1111/j.1365-2567.2007.02730.x
- Krejsa CM, Holly RD, Heipel M, Bannink KM, Johnson R, Roque R, Heffernan J, Hill J, Chin L, Wagener F et al. Interleukin-21 enhances rituximab activity in a cynomolgus monkey model of B cell depletion and in mouse B cell lymphoma models. PLoS One 2013; 8(6):e67256; PMID:23825648; https://doi.org/10.1371/journal.pone.0067256
- Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol 2008; 20(4):444-9; PMID:18585457; https://doi.org/10.1016/j.coi.2008.05.011
- Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 2013; 4:76; PMID:23543707; https://doi.org/10.3389/fimmu.2013.00076
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331(6013):44-9; PMID:21212348; https://doi.org/10.1126/science.1198687
- Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retrovir 2002; 18(16):1197-205; PMID:12487826; https://doi.org/10.1089/08892220260387940
- Nimmerjahn F, Ravetch JV. FcγRs in health and disease. Curr Top Microbiol Immunol 2011; 350:105-25; PMID:20680807
- Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transpl 2014; 20(4):463-73; PMID:24434782; https://doi.org/10.1016/j.bbmt.2014.01.006
