ABSTRACT
Treatment of Erdheim–Chester disease (ECD), a rare non-Langerhans histiocytosis, relies on interferon-α, chemotherapeutic agents such as purine analogs, cytokine-blocking agents and BRAF inhibitors. Since interleukin (IL)-6 levels are elevated in serum and lesions of ECD patients, we evaluated the therapeutic efficacy and safety of IL-6 blockade with tocilizumab. We conducted an open-label, single-arm, phase II, prospective study of tocilizumab in three patients with multisystem ECD and poor tolerance/contraindications to IFN-α. Modifications of symptoms attributed to ECD represented the criteria for evaluation of clinical response. Changes at positron emission tomography scan, computed tomography scan, and magnetic resonance imaging at month 6 represented the main criteria for the evaluation of radiological response.
Sustained complete clinical response and partial radiological improvement were observed in two patients, paralleled by modulation of systemic pro-inflammatory mediators. In spite of disease stabilization or improvement at extra-neurological sites, a third patient experienced a radiologic and clinical progression of central nervous system involvement, mirrored by a dramatic increase of circulating IL-6 and related cytokines. These findings indicate that IL-6 inhibition can be effective in ECD, but caution is advisable in patients with neurologic involvement. IL-6 emerges as a central mediator in ECD pathogenesis.
Introduction
Erdheim–Chester disease (ECD) is a rare, multisystem histiocytic neoplasm characterized by tissue infiltration or organ encasement by CD68+, CD1a−, S100-low/negative foamy histiocytes.Citation1,2 Heterogeneous clinical manifestations depend on site and degree of involvement. Skeletal involvement with sclerotic lesions and radiotracer uptake is common, often asymptomatic and incidentally detected by radiological studies.Citation3 Other disease manifestations include neurologic symptoms due to central nervous system (CNS) involvement, diabetes insipidus, constitutional symptoms, and heart and lung involvement.Citation3 When affected, CNS, lungs, heart, and retroperitoneal space account for a severe prognosis.Citation1,4,5
Interferon (IFN)-α is the first-line treatment for ECD, but efficacy is limited against severe manifestations, specifically CNS and cardiovascular involvement.Citation6,7 Recent evidence indicates that deregulated activation of the mitogen-activated protein kinase (MAPK) pathway due to oncogenic mutations in the BRAF, NRAS, PIK3CA, and MAP2K1 genes is central to the pathogenesis of ECD.Citation8-10 Following these discoveries, treatment with small-molecule inhibitors of BRAFV600E (vemurafenib) or MEK (cobimetinib, trametinib) came to fruition (reviewed inCitation11). However, there are several concerns regarding the safety and tolerability of long-term treatment, as these agents are often associated with severe or life-threatening adverse effects,Citation12 while unequivocal therapy end-points and maintenance regimens are yet to be identified. In addition, some cases of ECD have no targetable kinase mutations, and thereby remain devoid of treatment options. New strategies are thereby needed to address the unmet clinical needs of ECD patients.
A complex milieu of cytokines and chemokines orchestrates macrophage activation and recruitment into ECD lesions.Citation13,14 Interleukin (IL)-6, a pleiotropic cytokine involved in the regulation of immune responses and bone metabolism, may be central to this inflammatory network. In fact, IL-6 is abundantly produced by foamy histiocytes in ECD lesions, and serum levels correlate with disease severity.Citation13-15 Tocilizumab, a monoclonal antibody blocking the IL-6 receptor, is an effective treatment option for rheumatoid arthritis. In this study, we evaluated IL-6 blockade with tocilizumab in ECD. We treated three patients with contraindications or unresponsive to IFN-α therapy, and evaluated the clinical and radiologic changes, as well as the modulation of pro-inflammatory mediators.
Patients and methods
Patients and trail design
We conducted an open-label, single-arm, phase II, prospective, pilot study of tocilizumab in ECD (ClinicalTrials.gov NCT01727206; Eudra-CT 2012-003151-11). Studies were approved by our Institutional Review Board and conducted according to the Declaration of Helsinki. All patients had histologically confirmed ECD (lipid-laden CD68+, CD163+, CD1a−, CD207−, S100− histiocytes with admixed or surrounding fibrosis, as evaluated by an experienced hemopathologist), with contraindications or unresponsive to IFN-α therapy. Of note, at the time this study was conceived and started, small-molecule inhibitors were not yet available for ECD patients; enrollment was interrupted upon availability.
Treatment regimen
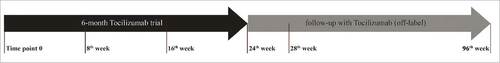
Tocilizumab was administered intravenously at a standard dose of 8 mg/kg at time 0, and at weeks 4, 8, 12, 16, 20, and 24 (). Responders continued to receive tocilizumab every 4 weeks for 72 additional weeks. Enrolled patients were allowed on a stable dose of steroids (maximum 10 mg/day of prednisone) and/or methotrexate, provided these medications had been administered for at least 4 mo.
Figure 1. Timeline of the 96-week-TCZ course of the patients treated highlighting key weeks. We monthly collected clinical and laboratory data (complete blood count and chemistry panel). Disease burden was assessed by means of total-body computed tomography (CT) scan, technetium-99m methylene diphosphonate (99mTc-MDP) bone-scan, fluorine-18-2-fluoro-d-glucose positron emission tomography (FDG-PET), brain and cardiac magnetic resonance imaging (MRI) at day 0 and at week 28. Specific imaging examinations at other different timepoints were performed if considered necessary by clinical judgment. Blood samples were obtained for each patient at day 0, than monthly in order to evaluate the levels of IL-6, CXCL-8, IL-12, CXCL10, CCL-2, CCL-4, soluble-TNF receptors (TNF-Rs) before, during and after 6-mo course of therapy.
Clinical and radiological criteria of response to therapy
Clinical and laboratory data were collected every 4 weeks. For clinical response, we used the following criteria: complete response (complete resolution of symptoms attributed to ECD), partial response (partial resolution of symptoms attributed to ECD), stable disease (no change in symptoms attributed to ECD), and progressive disease (worsening of symptoms attributed to ECD). These criteria are reasonable and accepted by the physician community that take care and treat the patients with ECD.
For radiological response, we used the following criteria: complete response (complete resolution of lesion due to ECD), partial response (partial resolution of lesion due to ECD), stable disease (no significant changes in lesion due to ECD for at least N months), and progressive disease (progression or worsening of lesion due to ECD). Changes in disease burden were evaluated at day 0 and at week 28 with total-body computed tomography (CT) scan, technetium-99m methylene diphosphonate bone scan, positron emission tomography (PET), and brain and cardiac magnetic resonance imaging (MRI). Target lesions were identified as the most metabolically active lesions on FDG-PET/CT study before treatment. Changes in target lesions were evaluated by comparing baseline and post-treatment PET/CT images, by means of a side-by-side image analysis to evaluate dimensional as well as FDG uptake changes.Citation7,16 Similarly, follow-up cardiac and cerebral MRIs were compared to baseline images, and lesion size was measured before treatment and during follow-up evaluations. Coated aorta, pericardial effusion, and pseudotumoral atrial infiltration were measured at the first and last visits. For CNS involvement, location (supra- and infra-tentorial) and changes in size of parenchymal lesions were evaluated using T2-weighted images at MRI.Citation16,17 Repeated biopsies to evaluate histologic or cellular changes induced by treatment were not performed. Changes in quality of life were assessed with the 36-Item Short Form Health Survey (SF-36) questionnaire, administered by healthcare workers during monthly re-evaluations.
Cytokine studies
Plasma levels of IL-6, IL-1β, CXCL8, CXCL10, CCL-2, CCL-4, and CCL5 were determined at time 0 and every 4 weeks with the Bio-Plex Multiple-Cytokine Assay (Bio-Rad, Hercules, CA).
Statistics
Differences were evaluated with the Mann–Whitney U unpaired test. Significance level was set at 0.05 (two-tailed p distribution). Analyses were performed with SPSS 16.0.2 for Windows (SPSS, Chicago, IL).
Results
Patient features
Three patients were enrolled and completed the protocol. The BRAFV600E mutation was detected in all cases. summarizes the clinical findings at enrollment and after 28 and 96 weeks of tocilizumab treatment.
Table 1. Clinical, radiological and laboratory features of ECD patients before tocilizumab and after 24 and 96 mo of therapy. Skeletal involvement was assessed by means of FDG-PET imaging and 99mTc-MDP bone scans, thoracic and/or retroperitoneal involvement by means of high resolution CT-scan, cardiac involvement by means of cardiac cine MRI, central nervous system (CNS) and/or retro-orbital involvement by means of brain MRI, quality of life was assessed by means of health assessment questionnaire (HAQ). All the data presented in the table, with the exception of countable data, namely erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were expressed in a semi-quantitative manner (from the worst to the best; –, +, ++, +++)
Efficacy on skeletal manifestations and systemic inflammation
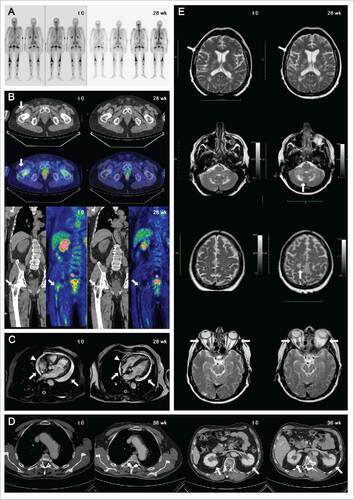
Patients 1 and 2 had a rapid and sustained improvement of skeletal manifestations, with resolution of bone pain within 1 mo of treatment. This improvement was also evident at bone scan and was consistent with a partial response on FDG-PET, first documented at week 28 and maintained until week 96 in both patients. In Patient 1, tracer uptake was reduced in all affected sites. In Patient 2, humeral lesions were no longer detectable as tracer uptake or radiologic abnormalities at CT-PET, while femoral lesions improved markedly ( and ). Treatment also curbed constitutional symptoms and systemic inflammation (). In all patients, fever, fatigue and asthenia resolved, and erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) normalized. Patients 1 and 2 experienced a considerable improvement in quality of life within 8 weeks of treatment; in both patients, SF-36 scores remarkably improved with regards to bodily pain and physical function, particularly for indexes of moderate physical activity (e.g. walking for more than a mile, climbing stairs, or lifting weight such as grocery bags, etc.). Conversely, patient 3 did not report substantial changes.
Figure 2. Skeletal involvement of patient 2 at time point 0 and at 28th week assessed by 99mTc-MDP bone scans (A) and by FDG-PET scan (B): upon treatment, humeral lesions were no longer detectable as tracer uptake or radiologic abnormalities at CT-PET, while femoral lesions improved markedly (arrows). Marked reduction of pericardial effusion (arrows) and right atrium infiltration (from 12 to 7 mm in diameter, arrowheads) in patient 1 before and after 28 week of treatment, as assessed by cardiac MRI (C). Improvement in thoracic aorta involvement and bilateral perinephric fat infiltration (arrows) in patient 1 at time point 0 and 96th week assessed by contrast enhanced CT-scan of the abdomen (D). Retro-orbital, cerebral and cerebellar disease progression in patient 3, as assessed by brain-MRI at baseline (t = 0; E, left) and after 28 weeks of tocilizumab therapy (t = 28; E, right).
Efficacy on cardiovascular and retroperitoneal involvement
Patients 1 and 3 had cardiovascular involvement. Patient 1 had symptomatic pericardial effusion and heart failure (New York Heart Association (NYHA) functional class III; left ventricular ejection fraction (LVEF) evaluated with cardiac magnetic resonance: 37%; pro-BNP 7200 pg/mL). Upon treatment pericardial effusion reduced, cardiac contractility increased, and symptoms improved to NYHA class I. Specifically, cardiac MRI performed at 28 weeks showed improved diastolic and systolic function (LFEV 45%), a 50% reduction of pericardial effusion, and a marked reduction of pseudotumoral infiltration of the right atrium, from 12 to 7 mm in diameter ( and ). Patient 3 also had a mass infiltrating the right atrium, which was again reduced by treatment.
In Patients 1 and 2, treatment led to regression of retroperitoneal fibrosis. Given this clear efficacy, treatment with tocilizumab was continued in Patients 1 and 2 for a total of 24 mo ().
Efficacy of tocilizumab on CNS involvement
One patient (Patient 3) had CNS involvement (), and developed ataxia and dysarthria between weeks 24 and 28. MRI at week 28 demonstrated a clear progression of numerous retro-orbital, cerebral and cerebellar localizations, for which the patient ultimately received supportive care. Baseline MRI studies documented involvement of peritrigonal regions bilaterally, of the white matter of semioval centers, and of cerebellar hemispheres and middle cerebellar peduncles bilaterally (). At week 28 (), lesions had increased in number and size. Conversely, cardiac involvement and other disease manifestations outside the CNS (skeletal, retroperitoneal) improved also in this patient.
Tolerability of tocilizumab
Adverse effects of treatment were neither reported by patients, nor detected at clinical or biochemical monitoring. In particular, neither infection (airway, GI or urinary tract), nor metabolic (increase in serum liver enzymes) and hematological (neutropenia) abnormalities were observed.
Cytokine measurements
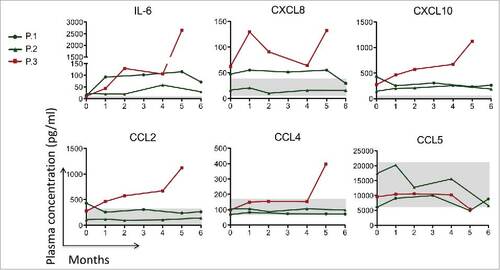
In Patients 1 and 2, treatment was associated with a stabilization or reduction of the circulating levels of CXCL8, CXCL10, CCL2, CCL4, and CCL5, all pro-inflammatory mediators typically increased in ECD patientsCitation13,14 (). In Patient 3, these mediators raised dramatically between months 5 and 6, mirroring the neurological disease progression at these time points (). IL-1β levels were within normal range in all patients throughout the study (not shown). IL-6 receptor blockade resulted in an increase in serum IL-6, as described.Citation18
Figure 3. Circulating levels of IL-6, CXCL-8, IL-12, CXCL10, CCL-2, CCL-4 and CCL-5 before, during and after 6-month course of TCZ therapy. The gray area in the figure corresponds to the physiological concentration of every cytokine of 10 healthy controls. In patients 1 and 2 (green lines) we observed a slight reduction or stabilization of these cytokine/chemokines level. In patient 3 (red line) a worsening of all the circulating mediators except CCL5 was seen at week 20 (month 5).
Discussion
The present study prospectively evaluated the therapeutic potential of tocilizumab in ECD. IL-6 blockade with tocilizumab was effective in the treatment of various ECD manifestations, including severe involvement such as cardiovascular, but was not effective against CNS localizations in one patient.
A crucial finding was the efficacy of tocilizumab on cardiac involvement (Patients 1 and 3; ), which was relatively unexpected, since cardiovascular involvement is poorly responsive to most currently available treatments.Citation6,19 Additional confirmation of treatment efficacy came from improvement of retroperitoneal and aortic involvement, and from rapid amelioration of skeletal manifestations, paralleled by improvements in biochemical and radiologic findings ( and ), as already reported.Citation15,18
In Patient 3, treatment with tocilizumab was again associated with improvement of cardiovascular and stabilization of skeletal and retroperitoneal involvement (). However, in contrast to these favorable responses, he also had CNS involvement, and experienced neurological progression despite treatment. Given the concomitant improvement of other disease manifestations outside the CNS, this lack of efficacy against CNS involvement may be due to reduced penetration through the blood brain barrier, a phenomenon already observed for other biologic drugs.Citation20
BRAF-mutated histiocytes in ECD activate oncogene-induced senescence, a protective mechanism against oncogenic events characterized by robust production of pro-inflammatory cytokines, including IL-6.Citation10,21,22 The pro-inflammatory milieu induced by senescent cells favors the recruitment and activation of histiocytes into ECD lesions.Citation21 The clinical response observed in Patients 1 and 2 was indeed associated with a decrease or stabilization of these mediators (). Therapeutic inhibition of IL-6 likely dampened runaway inflammation and disrupted the vicious cycle leading to histiocyte recruitment, thus reducing lesion size. Consistently, disease progression in Patient 3 was paralleled by a rampant increase in the levels of pro-inflammatory mediators ().
Previous studies reported the use of agents blocking TNFα and IL-1 in the treatment of ECD.Citation17,23 Intriguingly, our findings highlight the role of deregulated activation of IL-6 and related cytokines in the pathogenesis of ECD, and suggest that curbing this aberrant inflammatory response can be beneficial to ECD patients.
In parallel to this and other ongoing clinical trials evaluating the safety and efficacy of immediately available therapies for ECD, translational studies may also identify new key mechanisms responsible for this orphan disease. For example, molecular studies may allow the identification of mutations amenable to target therapy with small molecule inhibitors.Citation8 As part of this effort in dissecting and contrasting the molecular and genetic bases of ECD, combination therapy with small molecules and anti-cytokine agents is particularly appealing, as it may be effective against both the oncogenic transformation and inflammatory activation.
The present study indicates that tocilizumab can be effective and is well tolerated in the treatment of ECD. However, while the efficacy of tocilizumab was remarkable even on severe and difficult-to-treat disease manifestations such as cardiac involvement, patients with CNS disease may respond poorly to IL-6 inhibition and should receive alternative treatments. Further studies are needed to confirm this preliminary observation and validate the clinical utility of tocilizumab in ECD.
Disclosure of potential conflicts of interest
All the authors have neither funding sources nor conflicts of interest relevant to the present work.
Author contributions
All the authors had access to the data, had an active role in the study, approved the present version of the manuscript, and agreed with its submission.
Funding
This work was supported by a research grant from the Italian Ministry of Health to Lorenzo Dagna (GR-2009-1594586).
References
- Campochiaro C, Tomelleri A, Cavalli G, Berti A, Dagna L. Erdheim–Chester disease. Eur J Intern Med 2015; 26(4):223-9; PMID:25865950; https://doi.org/https://doi.org/10.1016/j.ejim.2015.03.004
- Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, Requena-Caballero L, Jordan MB, Abdel-Wahab O, Allen CE et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016; 127(22):2672-81; PMID:26966089; https://doi.org/https://doi.org/10.1182/blood-2016-01-690636
- Cavalli G, Guglielmi B, Berti A, Campochiaro C, Sabbadini MG, Dagna L. The multifaceted clinical presentations and manifestations of Erdheim–Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis 2013; 72(10):1691-5; PMID:23396641; https://doi.org/https://doi.org/10.1136/annrheumdis-2012-202542
- Arnaud L, Hervier B, Neel A, Hamidou MA, Kahn JE, Wechsler B, Pérez-Pastor G, Blomberg B, Fuzibet JG, Dubourguet F et al. CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim–Chester disease: a multicenter survival analysis of 53 patients. Blood 2011; 117(10):2778-82; PMID:21239701; https://doi.org/https://doi.org/10.1182/blood-2010-06-294108
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375-90; PMID:26980727; https://doi.org/https://doi.org/10.1182/blood-2016-01-643569
- Haroche J, Amoura Z, Trad SG, Wechsler B, Cluzel P, Grenier PA, Piette JC. Variability in the efficacy of interferon-alpha in Erdheim–Chester disease by patient and site of involvement: results in eight patients. Arthritis Rheum 2006; 54(10):3330-6; PMID:17009306; https://doi.org/https://doi.org/10.1002/art.22165
- Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ et al. Consensus guidelines for the diagnosis and clinical management of Erdheim–Chester disease. Blood 2014; 124(4):483-92; PMID:24850756; https://doi.org/https://doi.org/10.1182/blood-2014-03-561381
- Diamond EL, Durham BH, Haroche J, Yao Z, Ma J, Parikh SA, Wang Z, Choi J, Kim E, Cohen-Aubart F et al. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov 2016; 6(2):154-65; PMID:26566875; https://doi.org/https://doi.org/10.1158/2159-8290.CD-15-0913
- Haroche J, Charlotte F, Arnaud L, von Deimling A, Helias-Rodzewicz Z, Hervier B, Cohen-Aubart F, Launay D, Lesot A, Mokhtari K et al. High prevalence of BRAF V600E mutations in Erdheim–Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012; 120(13):2700-3; PMID:22879539; https://doi.org/https://doi.org/10.1182/blood-2012-05-430140
- Cangi MG, Biavasco R, Cavalli G, Grassini G, Dal-Cin E, Campochiaro C, Guglielmi B, Berti A, Lampasona V, von Deimling A et al. BRAFV600E-mutation is invariably present and associated to oncogene-induced senescence in Erdheim–Chester disease. Ann Rheum Dis 2015; 74(8):1596-602; PMID:24671772; https://doi.org/https://doi.org/10.1136/annrheumdis-2013-204924
- Cavalli G, De Luca G, Dagna L. Advances in potential targeted therapies for Erdheim–Chester disease. Expert Opin Orphan Drugs 2017; 5(3):253-60.
- Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol 2015; 7(2):122-36; PMID:25755684; https://doi.org/https://doi.org/10.1177/1758834014566428
- Stoppacciaro A, Ferrarini M, Salmaggi C, Colarossi C, Praderio L, Tresoldi M, Beretta AA, Sabbadini MG. Immunohistochemical evidence of a cytokine and chemokine network in three patients with Erdheim–Chester disease: implications for pathogenesis. Arthritis Rheum 2006; 54(12):4018-22; PMID:17133532; https://doi.org/https://doi.org/10.1002/art.22280
- Arnaud L, Gorochov G, Charlotte F, Lvovschi V, Parizot C, Larsen M, Ghillani-Dalbin P, Hervier B, Kahn JE, Deback C et al. Systemic perturbation of cytokine and chemokine networks in Erdheim–Chester disease: a single-center series of 37 patients. Blood 2011; 117(10):2783-90; PMID:21205927; https://doi.org/https://doi.org/10.1182/blood-2010-10-313510
- Mossetti G, Rendina D, Numis FG, Somma P, Postiglione L, Nunziata V. Biochemical markers of bone turnover, serum levels of interleukin-6/interleukin-6 soluble receptor and bisphosphonate treatment in Erdheim–Chester disease. Clin Exp Rheumatol 2003; 21(2):232-6; PMID:12747282
- Haroche J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Toledano D, Barete S, Charlotte F, Cluzel P, Donadieu J et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim–Chester disease. J Clin Oncol 2015; 33(5):411-8; PMID:25422482; https://doi.org/https://doi.org/10.1200/JCO.2014.57.1950
- Dagna L, Corti A, Langheim S, Guglielmi B, De Cobelli F, Doglioni C, Fragasso G, Sabbadini MG, Ferrarini M. Tumor necrosis factor alpha as a master regulator of inflammation in Erdheim–Chester disease: rationale for the treatment of patients with infliximab. J Clin Oncol 2012; 30(28):e286-90; PMID:22869874; https://doi.org/https://doi.org/10.1200/JCO.2012.41.9911
- Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008; 112(10):3959-64; PMID:18784373; https://doi.org/https://doi.org/10.1182/blood-2008-05-155846
- Berti A, Ferrarini M, Ferrero E, Dagna L. Cardiovascular manifestations of Erdheim–Chester disease. Clin Exp Rheumatol 2015; 33(2 Suppl 89):S-155-63; PMID:25738753
- Pardridge WM. Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv 2015; 12(2):207-22; PMID:25138991; https://doi.org/https://doi.org/10.1517/17425247.2014.952627
- Cavalli G, Biavasco R, Borgiani B, Dagna L. Oncogene-induced senescence as a new mechanism of disease: the paradigm of Erdheim–Chester disease. Front Immunol 2014; 5:281; PMID:24982657; https://doi.org/https://doi.org/10.3389/fimmu.2014.00281
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008; 133(6):1019-31; PMID:18555778; https://doi.org/https://doi.org/10.1016/j.cell.2008.03.039
- Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology (Oxford) 2015; 54(12):2134-44; PMID:26209330; https://doi.org/https://doi.org/10.1093/rheumatology/kev269
