ABSTRACT
PTP1B and TC-PTP are highly related protein-tyrosine phosphatases (PTPs) that regulate the JAK/STAT signaling cascade essential for cytokine-receptor activation in immune cells. Here, we describe a novel immunotherapy approach whereby monocyte-derived dendritic cell (moDC) function is enhanced by modulating the enzymatic activities of PTP1B and TC-PTP. To downregulate or delete the activity/expression of these PTPs, we generated mice with PTP-specific deletions in the dendritic cell compartment or used PTP1B and TC-PTP specific inhibitor. While total ablation of PTP1B or TC-PTP expression leads to tolerogenic DCs via STAT3 hyperactivation, downregulation of either phosphatase remarkably shifts the balance toward an immunogenic DC phenotype due to hyperactivation of STAT4, STAT1 and Src kinase. The resulting increase in IL-12 and IFNγ production subsequently amplifies the IL-12/STAT4/IFNγ/STAT1/IL-12 positive autocrine loop and enhances the therapeutic potential of mature moDCs in tumor-bearing mice. Furthermore, pharmacological inhibition of both PTPs improves the maturation of defective moDCs derived from pancreatic cancer (PaC) patients. Our study provides a new advance in the use of DC-based cancer immunotherapy that is complementary to current cancer therapeutics.
Introduction
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway plays a crucial role in the processes of hematopoiesis, immune cell proliferation, differentiation and immunological functions.Citation1 Two highly related protein-tyrosine phosphatases (PTPs), TC-PTP and PTP1B are major negative regulators of the JAK/STAT signaling cascade. These enzymes display over 75% sequence identity in their catalytic domain and overlap in some of their substrate recognition profiles. In vitro analysis with substrate-trapping mutants indicates that PTP1B stably interacts with JAK2 and TYK2Citation2 leading to the inhibition of STAT4 and STAT3 activation,Citation3,4 whereas TC-PTP interacts with JAK1 and JAK3 leading to inhibition of STAT1, STAT3 and STAT5 activation.Citation5-7
JAK/STAT signaling pathways are crucial regulators of dendritic cell (DC) differentiation, cytokine production, and DC-mediated T helper 1 (TH1) development required for an effective antitumor response.Citation8 Optimal interleukin 12 (IL-12) production constitutes the third DC-derived signal essentially required for T cell activation and differentiation into Th1 cells.Citation9 IL-12 induces tyrosine phosphorylation and activation of IL-12 receptor through JAK2 and TYK2, which phosphorylates and activates STAT4. This signaling event leads to DC activation in an autocrine manner, as well as IL-12-dependent IFNγ production by DCs.Citation10
Previous studies have reported the activation of Src tyrosine kinases as part of the signaling cascade downstream TLR activation in DCs. As evidence, the treatment with a Src-specific inhibitor significantly affects the production of pro-inflammatory cytokines without altering the costimulatory molecule expression in DCs stimulated with TLR agonists. Consequently, DCs with impaired Src activation fail to induce efficient Th1 cell differentiation.Citation11,12 Src tyrosine kinase has been identified as an interacting protein of PTP1B in myeloid DCs, in which, Src dysregulation in the absence of PTP1B is associated with low podosome numbers and focal contacts resulting in defects of migration and T cell stimulation after DC maturation stimulus.Citation13
Cancer patients display a significant reduction in mature and functional DCs and have an aberrant accumulation of immature myeloid cells.Citation14,15 DCs derived from cancer patients express very low levels of co-stimulatory molecules CD80 and CD86, promoting T cell tolerance or anergy.Citation16 Thus, tumor-associated defects in DC function count as major factors responsible for tumor escape from immune surveillance.
Here, we report that differential PTP1B and TC-PTP activity influences the maturation and activation state of myeloid DCs through regulation of JAK/STAT and Src signaling. The downregulation (heterozygous, Het) of PTP1B and TC-PTP induce highly immunogenic DCs, whereas gene deletion (knockout, KO) of these PTPs gives rise to DCs with an impaired capacity for CD4+ T cell activation. Importantly, simultaneous downregulation of both PTPs through genetic or pharmacological means restores antigen-presentation capacity and cytokine production in defective moDCs from pancreatic cancer (PaC) patients. In conclusion, activity of these two phosphatases can alter the degree of antitumor immune response by regulating JAK/STAT and Src signaling pathways in myeloid DCs.
Results
PTP1B differential expression influence DC maturation and antitumor response
We set out to determine the contribution of PTP1B to the immune response using a B cell lymphoma transgenic mouse model (Eμ-myc), which mimics human Burkitt's lymphoma. We generated Eμ-myc mice that were PTP1B null and Het (Eμ-myc:1B−/− and Eμ-myc:1B+/−, respectively) by breeding the transgenic myc and PTPN1 strains and monitoring tumor growth and survival overtime. Eμ-myc:1B+/− mice displayed a significant delay in tumor development and showed increased survival compared with Eμ-myc:1B−/− (p = 0.0003) and Eμ-myc:1B+/+(p <0.0001). By 80 d of age, 95% of Eμ-myc:1B+/− mice were tumor-free compared with 65% of Eμ-myc:1B−/− and 49% of Eμ-myc:1B+/+. By 120 d of age, 46% of Eμ-myc:1B+/− mice were tumor-free compared 11% of Eμ-myc:1B−/− and 7% of Eμ-myc:1B+/+. No significant differences in survival were observed between Eμ-myc:1B−/− and Eμ-myc:1B+/+ in this tumor model ().
Figure 1. Effect of PTP1B phosphatase on the antitumor immune response in Eμ-myc mouse model of B cell lymphoma. (A) Tumor-free mice curve of Eμ-myc mice crossed with full body PTP1B KO (Eμ-myc:1B−/−), Het (Eμ-myc:1B+/−) or wt mice (n = 22 per group). Quantification of immune cells infiltrated at the tumor site: (B) Absolute number of immune cells infiltrated in the tumor site. DCs (CD11c+), granulocyte-derived myeloid suppressor cells (G-MDSC: CD11b+/Gr-1+/Ly-6G+), regulatory T cells (Treg: CD4+/FoxP3+), CD4+ T cells (CD4+/CD3+), CD8+ T cells (CD8+/CD3+), NK cells (NK1.1+/CD3−) and macrophages (F4/80) (n = 4). Expression of MHCI/II on tumor-infiltrated DCs. (C) MHC class I. (D) MHC class II (n = 4). (E) PTP1B expression in CD11c+ cells isolated from the bone marrow of Eμ-myc:1B−/−, Eμ-myc:1B+/−or wt mice. The results are representative of three independent experiments. The comparisons were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric. p values are indicated.
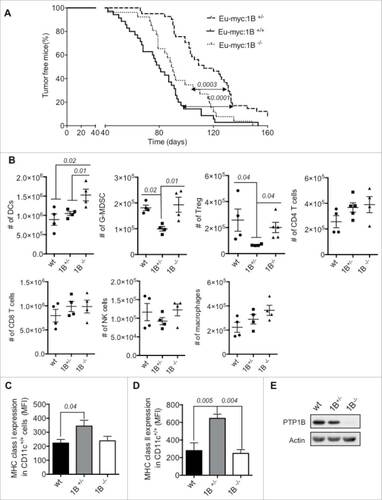
To characterize the antitumor immune response in the context of PTPN1 gene loss (PTP1B knockout) or downregulation (PTP1B heterozygous), we quantified the number of immune cell types infiltrated in the tumor site. Here, wild type (wt), PTP1B Het (1B+/−) and PTP1B KO (1B−/−) mice received subcutaneous injections of 106 Eμ-myc B cell lymphomas mixed in matrigel. Compared with wt and 1B+/−, the 1B−/− implants contain a significantly higher number of dendritic cells based on the expression of CD11c, whereas the number of the rest of immune cell types analyzed were similar to ones in the wt implants. In contrast, the tumor implants from 1B+/− mice display a similar number of DCs compared with the WT implants. However, we observe a decrease in the numbers of immune suppressor cells, such as: granulocyte myeloid-derived suppressor cells (G-MDSC: CD11b+/Gr-1+/Ly6G+) and regulatory T cells (Treg: CD4+/Foxp3+) compared with wt and 1B−/− implants ().
Since we observed a significantly higher number of DCs in the implants from 1B−/− mice but a significant decrease in survival rate compared with 1B+/− mice, we investigated the maturation state of the tumor-infiltrated DCs (CD11c cells) in the three groups.
Tumor-infiltrated DCs extracted from 1B+/− mice displayed significantly higher MHC class I expression than wt DCs and higher MHC class II expression than wt and 1B−/− DCs, which indicates a more immunogenic phenotype ( and and S1). We confirmed the expression of PTP1B in the CD11c+ cells (DCs) isolated from these mice (). These results suggest that differential PTP1B expression/activity can influence the maturation state of DCs and consequently the antitumor response in vivo.
We hypothesized that PTP1B downregulation would affect cytokine receptor signaling, which enhances DC maturation and activation leading to a more effective antitumor response and increased survival. To validate our hypothesis, we generated mice carrying a DC-specific deletion of PTP1B and its highly related phosphatase, TC-PTP. Mature monocyte-derived DCs (moDCs) were generated from tissue-specific PTP1B-deficient CD11c-Cre mice. Approximately 94–95% purity was achieved for both immature and mature moDCs. The efficiency of PTPN1 (PTP1B) and PTPN2 (TC-PTP) deletion in the CD11c-Cre mice was assessed by western blot with mature moDCs. As a result of these crosses, we obtained mice that were knockout and heterozygous for PTP1B (1Bfl/fl and 1Bfl/wt) and TC-PTP (TCfl/fl and TCfl/wt) respectively only in the dendritic cell compartment (CD11c+ cells) (Fig. S2).
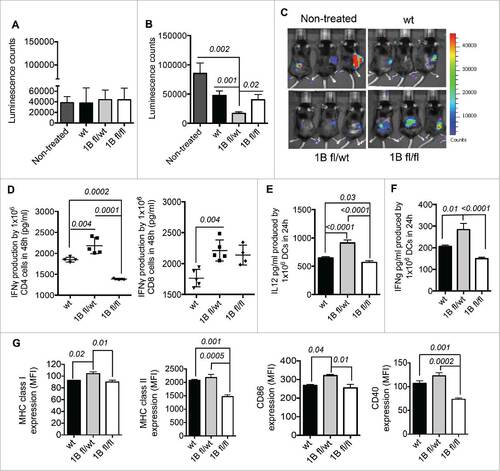
The lymphoma mouse model E.G7-OVA expressing luciferase was used as a proof of concept to specifically determine the magnitude of the antitumor response and therapeutic properties of PTP1B-deficient (1Bfl/wt and 1Bfl/fl) moDCs. Mice with pre-established tumors () were treated with one intraperitoneal (IP) injection of 5 × 106 OVA-pulsed mature PTP1B-deficient moDCs or mature wt (Cre− wt/wt) moDCs. 1Bfl/wt moDC treatment in tumor-bearing mice provides a greater therapeutic benefit compared with 1Bfl/fl or wt moDC treatments, based on the significant delay of tumor growth over time ( and ). Based on the previous results, we examined the ability of chicken ovalbumin (OVA)-pulsed PTP-deficient moDCs to activate CD8+ or CD4+ T cells derived from OT-I and OT-II transgenic mice, respectively. OVA-pulsed mature moDCs were co-cultured with CD8+ or CD4+ T cells for 48 h and IFNγ secreted as an indicator of T cell activation was quantified by ELISA. 1Bfl/wt moDCs acted as significantly more potent antigen-presenting cells to CD4+ and CD8+ T cells than wt or 1Bfl/fl moDCs, respectively. In contrast, 1Bfl/fl moDCs displayed an impaired capacity for CD4+ T cell activation, (). As the most relevant phenotypic feature, mature 1Bfl/wt moDCs secrete higher levels of Th1-polarizing cytokines IL-12 and IFNγ than wt moDCs. 1Bfl/fl moDCs produced reduced levels of IFNγ and IL-12 ( and ). Furthermore, matured 1Bfl/wt moDCs display more immunogenic phenotype than wt moDCs characterized by higher MHC class I and CD86 expression ().
Figure 2. Characterization of PTP1B deficient moDCs. (A) Therapeutic effect of PTP1B deficient moDCs in a mouse model of lymphoma E.G7-OVA: Tumor volume before moDC treatments (10 d after implantation of 5 × 105 E.G7-OVA cells) and (B) 18 d after tumor implantation and 8 d after intraperitoneal (IP) injections of 5 × 106 1B-deficient moDCs compared with control group (tumor-bearing mice without moDC treatment). (C) In vivo images of tumor-bearing mice 8 d after moDCs treatments (n = 5). (D) Quantification of IFNγ produced by activated OT-II CD4+ T and OT-I CD8+ T cells respectively co-cultured with mature and OVA-pulsed 1Bfl/fl, 1B fl/wt or wt moDCs during 48 h (n = 4–5). The amount of IFNγ produced by mature PTP1B-deficient or wt moDCs was subtracted for both OT-II CD4+ and OT-I CD8+ co-cultures. Production of Th1 polarizing cytokines (n = 5): (E) IL-12 and (F) IFNγ. (G) Mean Fluorescent Intensity (MFI) values of the expression of MHC class I, MHC class II. CD86 and CD40 (n = 3). The comparisons were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric. The results are representative of at least three independent experiments. p values are indicated.
The downregulation of TC-PTP, a highly related PTP1B phosphatase, similarly affects DC maturation state
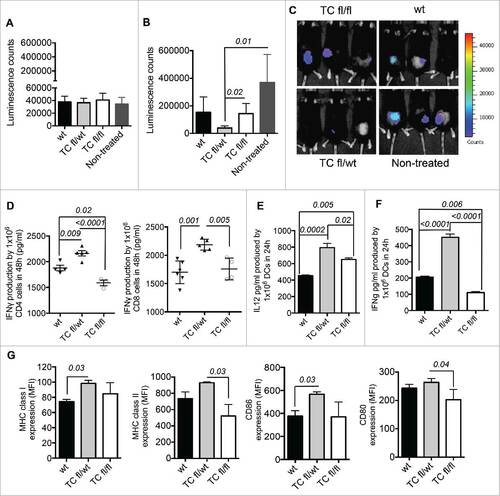
As described previously, mice with pre-established tumors () were treated with one IP injection of 5 × 106 OVA-pulsed mature TC-PTP-deficient moDCs or mature wt moDCs. Eight days post-TCfl/wt moDC treatment, we observed a significant delay in tumor growth compared with untreated and TCfl/fl moDC treated groups of mice ( and ). Similarly, mature TCfl/wt moDC induce greater CD4+ and CD8+ T cell activation in vitro and produce significant amount of IL-12 and IFNγ. TCfl/fl moDCs display a dramatic decrease in the capability for CD4+ T cell activation and IFNγ production in comparison with wt moDCs (). As previously observed in mature 1Bfl/wt moDC, mature TCfl/wt moDCs also exhibit a more immunogenic phenotype based on higher MHC class I and CD86 expression than wt moDCs ().
Figure 3. Characterization of TC-PTP deficient moDCs. (A) Therapeutic effect of TC-PTP deficient moDCs in a mouse model of lymphoma E.G7-OVA: Tumor volume before moDC treatment (10 d after implantation of 5 × 105 E.G7-OVA cells) and (B) after IP injections of 5 × 106 TC-deficient moDC compared with control group (tumor-bearing mice without moDC treatment). The IP injections with moDCs were given 18 d after tumor implantation. (C) In vivo images of tumor-bearing mice 8 d after moDC treatment. (D) Quantification of IFNγ produced by activated OT-II CD4+ T and OT-I CD8+ T cells respectively co-cultured with mature and OVA-pulsed TCfl/fl, TC fl/wt or wt moDCs during 48 h (n = 3–5) and (n = 6–4). The amount of IFNγ produced by mature TC-PTP-deficient or wt moDCs was subtracted for both OT-II CD4+ and OT-I CD8+ co-cultures. Production of Th1 polarizing cytokines (n = 5): (E) IL-12 and (F) IFNγ. (G) MFI values of the expression of MHC class I and II, CD86 and CD80 (n = 3). The comparisons were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric. The results are representative of three independent experiments. p values are indicated in the figures.
Both PTP1B and TC-PTP independently potentiate moDC functions through their substrate activation
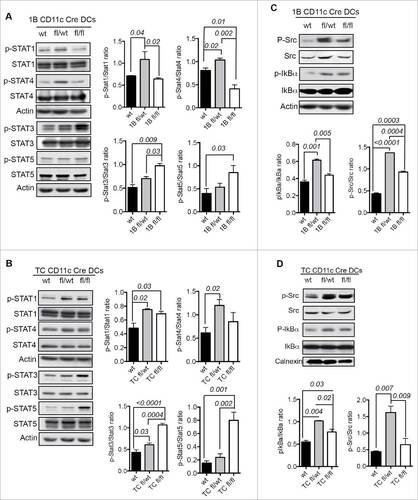
We analyzed the activation status of PTP1B and TC-PTP substrates in the JAK/STAT signaling pathway and specifically the activation status of STAT molecules downstream of cytokine receptors in terminally differentiated and mature moDCs. STAT4 and STAT1 were significantly more phosphorylated in 1Bfl/wt moDCs and TCfl/wt moDCs compared with wt moDCs, whereas STAT3 and STAT5 appeared to be hyper-phosphorylated in both 1Bfl/fl moDCs and TCfl/fl moDCs ( and ). We determined the activation status of the PTP1B-regulated kinase, Src, associated with TLR4.Citation13 Compared with wt moDCs, both 1Bfl/wt and TCfl/wt moDCs exhibit significantly increased phosphorylation of Src (Y416) that upregulates the enzyme activity, as well as higher IkBα phosphorylation, an indicator of NF-kB activation downstream of TLR4 engagement ( and ). On the other hand, we determined noticeable differences between 1Bfl/fl moDCs and TCfl/fl moDCs for the Src (Y416), NF-kB and STAT1 signaling. In 1Bfl/fl moDCs, Src signaling is more activated than in wt DCs whereas in TCfl/fl moDCs, IkBα and STAT1 are higher phosphorylated than the ones in wt DCs (). These results suggest that PTP1B and TC-PTP do not play redundant roles in DC functions.
Figure 4. Molecular mechanisms of PTP1B and TC-PTP downregulation in moDCs. (A) Activation status of STAT proteins in PTP1B deficient moDCs (n = 4). (B) Activation status of STAT proteins in TC-PTP deficient moDCs (n = 3). (C) Activation status of Src kinase and IkBα downstream of TLR4 in PTP1B deficient moDCs (n = 4). (D) Activation status of Src kinase and IkBα in TC-PTP deficient moDCs (n = 3). The results are representative of three independent experiments. p values are indicated in the figures.
Simultaneous downregulation of PTP1B and TC-PTP exerts additive effect on DC functions
To investigate how contemporaneous downregulation of both PTP1B and TC-PTP affects DC functions, we generated PTP1B and TC-PTP double heterozygous CD11c Cre mice (1B:TCfl/wt) (Fig. S3A). 1B:TCfl/wt moDCs exhibited an improved maturation state with significant upregulation of MHC class I, CD86 and CD80 compared with wt moDCs and the highest production of IL-12 and IFNγ ( and S3B). The additive effect due to simultaneous downregulation of both phosphatases was also noticeable with regard to more robust CD4+ and CD8+ T cell activation than the one induced by wt moDCs ( and ). As molecular mechanism, 1B:TCfl/wt moDCs display significantly higher phosphorylation of Src (Y416) and IkBα than wt moDCs. In comparison with single heterozygous moDCs, Src phosphorylation levels in 1B:TCfl/wt moDCs were intermediary between 1Bfl/wt moDCs and TCfl/wt moDCs, whereas the highest levels of IkBα phosphorylation were observed in mature 1B:TCfl/wt moDCs (). To assess the contribution of STAT1 and STAT4 as substrates of PTP1B and TC-PTP in the regulation of DC maturation and activation, we treated PTP-deficient or wt moDCs with a STAT1 inhibitor (fludarabine) and a STAT4 inhibitor (lisofylline) during the maturation process. As expected, STAT1 and STAT4 phosphorylation levels were significantly higher in 1Bfl/wt moDCs, TCfl/wt moDCs and 1B:TCfl/wt moDCs than in wt moDCs. Both STAT1 and STAT4 specific inhibitors reciprocally affect STAT1 or STAT4 phosphorylation in 1Bfl/wt moDCs, TCfl/wt moDCs and 1B:TCfl/wt moDCs, but not in the control wt moDCs, in which each STAT inhibitor specifically acts on their target molecules (STAT1 inhibitor affects STAT1 phosphorylation whereas STAT4 inhibitor impairs STAT4 phophorylation). These results suggest a positive autocrine loop operating in moDCs deficient for these two phosphatases (). Consequently, IFNγ production, which is highly dependent on IL-12-induced STAT4 activation, not only decreased in lisofylline-treated TCfl/wt moDCs, 1Bfl/wt moDCs and 1B:TCfl/wt moDCs, but also in these cells treated with fludarabine compared with non-treated cells. IL-12 production was not affected by inhibition of STAT1 activation (fludarabine treatment) but was significantly decreased in lisofylline-treated TCfl/wt moDCs and 1Bfl/wt moDCs. Wild-type moDCs produced lower amounts of IFNγ and IL-12 and this cytokine production was not affected by either fludarabine or lisofylline treatments ( and ).
Figure 5. Simultaneous downregulation of PTP1B and TC-PTP enhances moDCs maturation. (A) Comparisons between double heterozygous (1B:TCfl/wt) and TCfl/wt moDCs, 1Bfl/wt moDCs and wt moDCs respectively. MFI values of the expression of MHC class I, CD86 and CD80 (n = 5). (B) Production of Th1 polarizing cytokines (n = 5): (B) IL-12 and (C) IFNγ. (D) Quantification of IFNγ produced by activated OT-I CD8+ T cells co-cultured with mature and OVA-pulsed 1B:TCfl/wt, 1Bfl/wt, TCfl/wt or wt moDCs for 48 h (n = 8–9). (E) Quantification of IFNγ produced by activated OT-II CD4+ T cells co-cultured with mature and OVA-pulsed 1B:TCfl/wt, 1Bfl/wt, TCfl/wt or wt moDCs for 48 h (n = 5). (F) Activation status of Src kinase and IkBa downstream of TLR4 (n = 3). Significant differences are represented by p values and (#) indicate significant differences between wt DCs and the rest of the groups. (G) Activation status of STAT1, STAT4 and Src in STAT1 inhibitor (fludarabine) or STAT4 inhibitor (lisofylline) treated or non-treated 1B:TCfl/wt, 1Bfl/wt, TCfl/wt or wt moDCs (n = 3). Production of Th1-polarizing cytokines by 1B:TCfl/wt, 1Bfl/wt, TCfl/wt or wt moDCs previously treated with STAT1- or STAT4-inhibitor during the maturation process (n = 3): (H) IL-12 and (I) IFNγ. The results are representative of at least three independent experiments. Significant differences among the groups are represented by p values and (#) indicate significant differences within a group. The comparisons were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric.
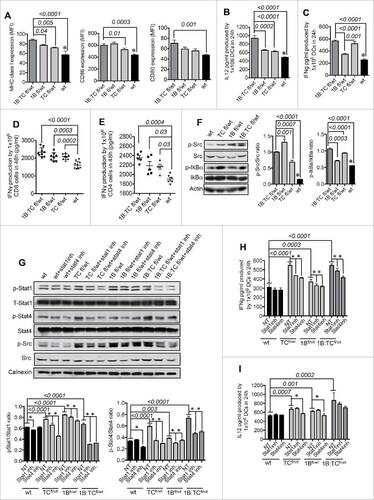
To support potential clinical applications of these findings, the activities of these two phosphatases were also simultaneously inhibited by pharmacological means using a specific inhibitor (1B/TC inh). The specificity of 1B/TC inh at 50 μM was determined based on the hydrolysis DiFMUP assay using different tyrosine phosphatases as controls and was found to be specific for PTP1B and TC-PTP (). In addition, a lower dose of the 1B/TC inh (0.094–24 μM) was tested against PTP1B and TC-PTP in a hydrolysis DiFMUP assay and the inhibitory effect of 1B/TC inh was only lost at the concentrations lower than 1.5 μM (Fig. S4A).
Figure 6. Pharmacological inhibition of both PTP1B and TC-PTP with specific inhibitor. (A) Specificity of 1B/TC inh (50 μM) to inhibit PTP1B and TC-PTP dephosphorylation (n = 3). (B) Titration of 1B/TC inh in mouse moDCs using IL-12 production as indicator of activation (n = 3). (C) IFNγ production by 1B/TC inh-treated mature moDCs (4 μM dose) (n = 4). (D) Expression levels (MFI) of CD40, CD80, CD86, MHC class I and II on 1B/TC inh-treated mature moDCs (4 μM dose) (n = 3). (E) Activation status STAT1 and STAT4 (n = 3). (F) Activation status of Src kinase and IkBα (n = 4). Therapeutic properties of 1B/TC inh-treated moDCs in a mouse model of E.G7 lymphoma: (G) Tumor volume before moDC treatment (10 d after implantation of 5 × 105 E.G7-OVA cells) and (H) after intraperitoneal (IP) injections of 5 × 106 1B/TC inh-treated or non-treated moDCs compared with control group (tumor-bearing mice without moDC treatments). The IP injections with moDCs were given 18 d after tumor implantation. (I) Images represent tumor-bearing mice 8 d after of moDC treatment. The results are representative of at least three independent experiments. The differences in more than two groups were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric. The differences between two groups were determined with unpaired t test (two tails of distribution). Significant differences are represented by p values <0.05.
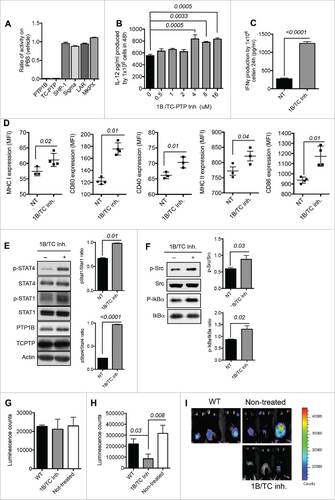
To determine the dose at which 1B/TC inh is able to decrease PTP1B and TC-PTP activities to similar levels as the 1Bfl/wt moDCs and TCfl/wt moDCs, mouse monocytes were cultured in the presence of different concentrations during moDC differentiation and maturation. We did not observe any inhibitor-related cytotoxicity effect on moDCs at the highest inhibitor concentration tested (512 μM) based on the living moDC count at the end of the incubation period (Fig. S4B). IL-12 production was used as a marker of DC maturation and activation. We obtained the highest IL-12 production with a 4 µM dose of 1B/TC inh (). moDCs treated with 4 µM of 1B/TC inh produced significant higher amount of IFNγ () and exhibited greater immunogenic phenotype as characterized by increased expression of MHC class I, MHC class II, CD40, CD80 and CD86 compared with non-treated moDCs (). However, 1B/TC inh treatment did not affect the cell surface expression of several chemokine receptors including CCR3, CCR5, CCR6 and CCR7 (data not shown). 1B/TC inh also induces significantly higher STAT1, STAT4, Src and IkBα phosphorylation ( and ).
We investigated the therapeutic potential of 1B/TC inh-treated DCs in the mouse E.G7 lymphoma model. After 10 days, tumor-bearing mice were determined to have similar tumor burdens as measured by bioluminescence (). Mice were then divided into three groups and received injections of 1B/TC inh-treated moDCs, control non-treated moDCs or no injections. Eleven days after therapeutic intervention, mice that received 1B/TC inh-treated moDCs exhibited a significant delay in tumor growth ( and ).
1B/TC inh restores the maturation and activation state of DCs from pancreatic cancer (PaC) patients
We investigated whether the treatment of monocytes derived from PaC patients with 1B/TC inh would enhance moDC maturation and activation. Human peripheral blood monocytes from PaC patients and healthy controls were isolated as described in the Materials and methods section. We obtained 3–4 × 106 human moDCs from 20–30 mL of patient blood with 98–99% purity that were CD14− (Fig. S5A and B). 1B/TC inh was also titrated on human moDCs to define the optimal concentration to induce the highest IL-12 production (Fig. S5C). As observed in mouse moDCs, we obtained the highest activation state using a 4 µM dose, which was significantly different from non-treated DCs (p = 0.009). PaC-derived moDCs treated with 4 µM of 1B/TC inh displayed higher STAT1 and STAT4 phosphorylation than non-treated cells (). Additionally, STAT3 activation levels decreased in the 1B/TC inh-treated human moDCs compared with non-treated cells. However, no differences in Stat3 activation were observed at the concentration range from 2 to 32 μM of the inhibitor (Fig. S5D).
Figure 7. Application of 1B/TC inh-treated human DCs in cancer immunotherapy. (A) Activation status of STAT1 and STAT4 in 1B/TC inh-treated human moDCs derived from a pancreatic cancer (PaC) patient (n = 3). (B) IL-12 production by 1B/TC inh-treated human moDCs from four PaC patients. (C) Frequency of antigen-specific (CEA and CA19–9) activated T cells measured based on IFNγ production detected in ELISpot assay. The differences in more than two groups were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric. The differences between two groups were determined with unpaired t-test (two tails of distribution). Significant differences are represented by p values <0.05.
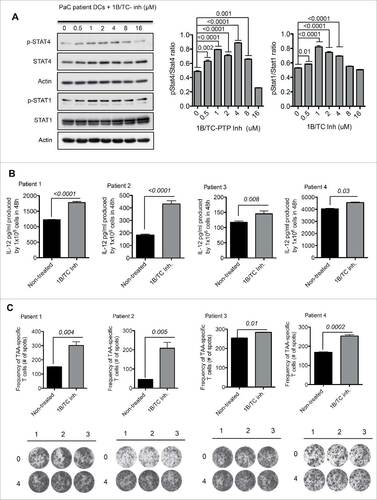
The antigen-presenting properties of human 1B/TC inh-treated moDCs were assessed by IFNγ ELISpot assays. The highly expressed PaC proteins carcinoembryonic antigen (CEA)Citation17 and cancer antigen 19–9 (CA19–9)Citation18 were used as TAAs, and autologous patient T cells were used as responder cells. 1B/TC inh-treated moDCs induced a higher number of IFNγ-producing T cells than the ones stimulated by non-treated DCs ( and ).
Discussion
We have shown how PTP1B activity contributes to the development of the antitumor response against B cell lymphomas in the Eμ-myc mouse model. Tumors in Eμ-myc:PTP1B+/− mice grow with a significant delay compared with the tumor progression observed in Eμ-myc:PTP1B−/− and Eμ-myc:PTP1B+/+ mice. To assess the contribution of PTP1B deficiency in the antitumor response, we quantified the number of immune cell types driven to the tumor site at 7 d post-tumor cell implantation. At this time point, the tumor's burden is extremely low. Thus, the quantity and quality of the first immune cells in the tumor site are more influenced by the effect of differential PTP expression/activity than by tumor-induced immunosuppression typically observed at high tumor burden.
In contrast to the tumor microenvironment in both Eμ-myc:PTP1B−/− and Eμ-myc:PTP1B+/+ mice, Eμ-myc:PTP1B+/− tumors exhibit significantly lower levels of G-MDSC and Treg as well as more immunogenic DCs (based on higher MHC class I/II expression).
These results led us to investigate the effect of differential PTP1B and the highly related TC-PTP activities in the functions of DCs as major orchestrators of the immune response. With this objective, we generated mice with PTP-specific deletions in the DC compartment.
As a cell-based therapy for cancer, treatment with TCfl/wt moDCs or 1Bfl/wt moDCs promotes a significant delay in cancer progression in mice with pre-established tumors. The improvement in the therapeutic potential of DC-based treatment is supported by major phenotypic changes acquired as a result of PTP1B and TC-PTP downregulation. Both 1Bfl/wt moDCs and TCfl/wt moDCs exhibit significantly greater expression of MHC class I, CD86 and IL-12 production, and consequently higher capacity for CD4+ and CD8+ T cell activation. IL-12 (IL-12p70) is the predominant cytokine for inducing the differentiation of Th1 cells that produce high amounts of IFNγ. IL-12 also blocks the activity of immune suppressor cells such as G-MDSC and M2 macrophages. In MDSC, IL-12 alters the suppressive function by downregulating the expression of ArgI and Nos (nitric oxide synthase) while promoting the upregulation of markers of DC maturation.Citation19,20 In agreement with our observations, IL-12 derived from immunogenic DCs may reduce the number of MDSC in the tumor site of 1Bfl/wt mice. In contrast, 1Bfl/fl and wt tumors display an elevated number of MDSCs that act as tolerogenic antigen-presenting cells capable of promoting the expansion of antigen-specific regulatory T cells in lymphomas.Citation20
moDCs heterozygous for both PTPs exhibit hyperphosphorylation of Src, STAT4, STAT1 and NF-kB signaling cascades. STAT1 activation in DCs is important to achieve optimal IL-12 production, IL-12 receptor expression and promotes the upregulation of co-stimulatory and MHC molecules during the maturation process. STAT1−/− DCs exhibit impaired upregulation of CD40, CD80, CD86 and MHC class II and consequently reduce antigen-presentation capacity and T cell priming.Citation21 STAT4 is essential for IFNγ production, as well as for the autocrine activation of DCs by IL-12 signaling.Citation22 In turn, IFNγ induces IL-12 production via STAT1, creating a positive feedback loop that sustains the production of these two Th1-polarizing cytokines.Citation21,23 On the other hand, Src activation regulates important features associated with DC maturation such as pro-inflammatory cytokine production and Th1 cell differentiation.Citation12
In contrast, mature 1Bfl/fl and TCfl/fl moDCs share phenotypic characteristics of tolerogenic DCs, the most noticeable being a significant decrease in MHC class II expression and consequently an impaired ability for CD4+ T cell activation. Depletion of both PTP1B and TC-PTP in moDCs leads to hyperactivation of STAT5 and STAT3 in moDCs. Although TCfl/fl moDCs also display higher STAT1 phosphorylation than wt DCs, STAT3 hyperactivation overcomes STAT1-mediated effects as essential signal transducer for DC maturation. In accordance with previous reports, simultaneous DC treatment with IL-10 (inducer of STAT3 phosphorylation) and IFNγ (inducer of STAT1 phosphorylation) promotes significant increase of iNOS (inducible nitric oxide synthase) and IDO (indoleamine 2,3-dioxygenase) production by bone marrow-derived DCs and consequently an impaired DC ability to activate CD4+ T cells.Citation24
In line with previous studies, STAT3 hyperactivation was associated with impaired DC maturation characterized by low MHC class II and CD40 expression, along with significant reduction of IL-12.Citation13,25 STAT3 activation is induced by most of the tumor-derived factors to promote abnormal DC differentiation and maturation.Citation26 Activated STAT3 decreases intracellular major histocompatibility complex II (MHCII) α/β dimers, and H2-DM levels in DCs by increasing cathepsin S activityCitation27 and affects NF-kB recruitment to the IL-12p40 promoter,Citation28 leading to a build-up of functionally impaired and immature myeloid cells with a high immunosuppressive potential.
Simultaneous downregulation of both PTP1B and TC-PTP in moDCs by genetic (double het 1B:TCfl/wt moDCs) or pharmacological approaches (1B/TC inh) gives rise to a highly immunogenic DC phenotype. The use of specific inhibitors for STAT1 and STAT4 activation in 1Bfl/wt, TCfl/wt and 1B:TCfl/wt moDCs but not in wt moDCs impairs the phosphorylation of both STAT1 and STAT4, which indicate the present of a positive feedback mechanism that operates in single and double heterozygous moDCs for these PTPs but not in wt moDCs. Consequently, treatment with either fludarabine or lisofylline interrupts the positive activation loop and impairs the production of IFNγ by 1Bfl/wt moDCs, TCfl/wt moDCs and 1B:TCfl/wt moDCs, whereas IFNγ production by wt moDCs is not affected. On the other hand, IL-12 production that mainly relies on NF-kB activation downstream of TLR4Citation29 and IFNγ induced by IL-12-dependent STAT4 activation was only affected by STAT4 inhibition in 1Bfl/wt moDCs and TCfl/wt moDCs, but not in 1B:TCfl/wt moDCs which suggest that this positive feedback mechanism of DC activation is more amplified in the double heterozygous moDCs due to the downregulation of both PTPs.
The inhibition of both phosphatases by pharmacological means was a more effective way to achieve an optimal DC maturation and activation since it is possible to titrate the inhibitor concentration to fine-tune both phosphatase activities. With the inhibitor, we were able not only to recapitulate the phenotype of the double het but also we observed additional upregulation of MHC class II and CD40 expression.
This is clinically relevant since patient moDCs can be activated ex vivo to improve DC function and subsequently provide antitumor responses once the cells are re-introduced to the patient. In conclusion, these phosphatases target the IL-12/IFNγ axis in moDCs and downregulation of their activities potentiates antitumor immune responses.
Our results indicate that it is possible to restore and enhance the maturation and activation of dysfunctional DCs derived from PaC patients by modulating the activity of PTP1B and TC-PTP. Consequently, moDCs with reduced PTP1B and TC-PTP activities will act as more effective APCs to induce potent antigen-specific T cell activation and antitumor responses for cancer immunotherapy. These studies provide considerable insight into the signaling pathways that govern the maturation and activation of DCs. Our findings may lead to a new generation of DC-based vaccines that would be complementary to existing immunotherapies.
Materials and methods
Mice
All animal procedures were performed using 6–10 week old male C57Bl/6n mice according to the Canadian Council on Animal Care ethical regulations and were approved by the McGill University Research and Ethics Animal committee. Eμ-myc mice were kindly supplied by Dr. Jerry Pelletier and crossed with Ptpn1fl/fl mice that were obtained from the laboratory of Dr. Gerard Karsenty.Citation30 Mice with the floxed Ptpn2 allele were generated in our laboratory.Citation40 Mice with PTP-specific deletions in the dendritic cell compartment were obtained by crossing Ptpn1fl/fl or Ptpn2fl/fl mice with CD11c-Cre transgenic mice purchased from Jackson Laboratory. OT-I (C57BL/6-Tg(Tcra Tcrb) 1100Mjb/J, stock #003831) and OT-II (B6.Cg-Tg(Tcra Tcrb) 425Cbn/J, stock#004194) transgenic mice were purchased from The Jackson Laboratory.
Generation of murine and human DCs: Murine moDCs
Murine monocytes were isolated from the bone marrow of tissue-specific PTP-deficient mice or corresponding wt littermates by CD11b+ selection using the EasySep® mouse monocyte CD11b+ selection kit (StemCell Technologies, cat# 18770). Mouse monocytes were cultured with GM-CSF and IL-4 (40 ng/mL each one) (Peprotech, cat# 315–03 and 214–14 respectively) for 6 d to allow their differentiation into immature moDCs. On day 6, immature moDCs were stimulated with 1 μg/mL LPS (Sigma, cat# L2630) for an additional 24 h to induce maturation. LPS was used as TLR4 ligand to generate immunogenic moDCs. For antigen-presentation assays, immature moDCs were pulsed with chicken ovalbumin (OVA) as the tumor-associated antigens (TAAs) and LPS and co-cultured with OT-I and OT-II T cells for 48 hours. Both supernatants and cells were collected after the incubation period. For the generation of 1B/TC-inhibitor treated DCs, 1B/TC inh was added in the culture media during the processes of differentiation and maturation of moDCs. 1B/TC inh was kindly provided by Dr. Brian Kennedy, Merck Frosst, Pointe-Claire, QC). The originally report of this compound was made by Merck-Frosst, Inc. in a paper by Montalibet et al.Citation31 Named difluoromethyl phosphonate (DFMP) inhibitor 7-bromo-6-phosphono(difluoro-methyl)-3-napthalenonitrile (PTPi), it is used to inhibit PTP1B and TC-PTP at the concentration used in this manuscript. This inhibitor was also used in previous manuscripts from the laboratory, by Julien et al.Citation32 and more recently by Pike et al.Citation4
Human moDCs
To obtain human moDCs, blood samples were obtained from advanced stage PaC patients after providing written informed consent (Dr. George Zogopolous, McGill University Health Centre). Patient moDCs were generated from peripheral blood monocytes isolated by positive selection of CD14+ cells (StemCell Technologies, Inc., cat# 18058). Human monocytes were cultured in monocyte attachment media for 1 h. After 1 h, the media was replaced with DC differentiation (StemXVivo serum-free DC base media, cat# CCM003) supplemented with GM-CSF (50 ng/mL, cat# 300–03) and IL-4 (35 ng/mL, cat# 200–04) and 1B/TC inh. Monocytes were cultured for 6 d in the differentiation media. On day 3, half the volume of the differentiation media was replaced with new media and cytokines. On the day 6, the media was replaced with maturation media containing monophosphoryl lipid A (MPLA-SM, 2 μg/mL, InvivoGen, cat# vac-mpla),Citation33 IFNγ (1000 U/mL, PeproTech, cat# 315–05) and 1B/TC inhibitor (4 μM).
Immune cell infiltration in the tumor site
To analyze the immune cell types infiltrated in the tumor site, 1 × 106 Eμ-myc B cell lymphoma cells expressing green fluorescence protein (GFP) were mixed with 500 μL Matrigel (BD Biosciences, cat# 354234) at 4 °C and injected subcutaneously in PTP1B−/−, PTP1B+/− or wt mice. All the implants were surgically removed 7 d after implantation and enzymatically dissociated with 1.6 mg/mL of collagenase type IV (Sigma-Aldrich, cat#M9445) in PBS as previously reported.Citation34 Infiltrated cells were collected and incubated with anti-FcR III/II antibodies for 30 min and with specific cell type or isotypic control antibodies for 1 h at 4 °C. The expression of surface markers was determined by flow cytometry.
Western blot analysis
For western blot analyses, mature moDCs were lysed in radioimmunoprecipitation assay buffer. Protein samples were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblotting. The following proteins were detected using antibodies from Cell Signaling Technologies (CST) phospho-specific and total STAT1 (total-stat1 CST cat# 9172 and phospho-stat1 CST cat# 9134), STAT3 (total-stat3 CST cat# 9139 and phosphor-stat3 CST cat# 9145), STAT5 (total-stat5 CST cat# 9310 and phosphor-stat5 CST cat# 9356), STAT4 (total-stat4 CST cat# 2653 and phosphor-stat4 CST cat# 5267), Src (total-Src CST cat# 2109 and phospho-Src CST cat# 2101), IkBα (total-IkBα CST #4812 and phospho-IkBα CST #2859), ERK (total-ERK CST cat# 9102 and phospho-ERK CST cat# 9106, p38 (total-p38 CST #9212 and phospho-p38 CST cat# 9211) and c-Jun (total-cJun CST cat# 9165 and phospho-cJun CST cat# 9261) proteins were detected using polyclonal antibodies (Cell Signaling Technology). β-Actin and Calnexin (a gift from Dr. John J.M. Bergeron) were used as a loading control and PTP1B or TC-PTP was detected with a monoclonal antibody (BD Biosciences cat# 610140 and Calbiochem cat# PH03L). Primary antibodies were followed by horseradish peroxidase-conjugated goat anti-rabbit or mouse IgG (Jackson Immuno Research Laboratories cat# 111–035-003 and cat# 115–035-062). Blots were revealed using Western lightning chemiluminescent substrate (PerkinElmer cat# NEL105001EA). The density of the bands was quantified with ImageJ software.
STAT1 and STAT4 inhibition
Mouse PTP-deficient moDCs were generated as described previously and maturation was induced for additional 24 h in the presence of LPS and 50 μM of STAT1 inhibitor (Fludarabine, R&D Systems, cat# 3495)Citation35 or 50 μM of STAT4 inhibitor (Lisofyllin, Santa Cruz Biotechnology, cat# sc-201055).Citation36 Mature moDCs were collected for western blot analysis with specific antibodies for phosphorylated and full length STAT1, STAT4 and Src.
Generation of a stable luciferase expressing lymphoma cell line
EG7-OVA lymphoma cells (ATCC) were placed in culture and subsequently infected, as described,Citation37 with a murine stem cell virus (MSCV) based IRES-GFP vector (Addgene #19360), subcloned with a firefly luciferase gene (Addgene #18751) into the multi-cloning site using BglII and XhoI restriction sites. GFP-positive lymphomas were isolated using fluorescence activated cell sorting (FACS) (BD FACSaria sorter). Pure populations of GFP-luciferase expressing cells were expanded after sorting and frozen until needed.
In vivo studies
For the tumor implantation, 5 × 105 GFP-luciferase expressing lymphoma cells were suspended in 100 μL PBS and subcutaneously injected into syngeneic C57Bl/6n 6–10 week old males. Intraperitoneal injections of mature and OVA-pulsed moDCs derived from tissue-specific PTP-deficient mice or wt moDCs were performed as indicated in the figure legends. Mice were then monitored for lymphoma development by whole body luminescence imaging (as indicated) using an IVIS 100 in vivo imaging system (PerkinElmer).
Phenotypic analysis of moDCs
Mature moDC cell culture supernatants were tested for the levels of IL-12 (mouse IL-12 ELISA cat# 433607 and human IL-12 cat# 431704) and IFNγ (mouse IFNγ ELISA cat# 430804 and human IFNγ cat# 430104) using enzyme-linked immunosorbent assay (ELISA) kits (BioLegend). Mature DCs were stained immediately for flow cytometry. moDC differentiation and maturation was determined via labeling with fluorescence-conjugated antibodies specific for MHC class I (cat# 311403) and class II (cat# 361706), CD80 (cat# 305219), CD252 (cat# 326307), CD40 (cat# 334309), CD86 (cat# 305421), CD83 (cat# 305305), CCR7 (cat# 353203), CCR5 (cat# 313707), CCR6 (cat# 353415), CCR3 (cat# 310707) and CD205 (cat# 342203) (BioLegend). The expression of these cell surface markers was determined by flow cytometry and analyzed using FlowJo software (TreeStar).
PTP inhibitor specificity assay
The hydrolysis of DiFMUP as a substrate for the indicated PTPs was conducted in black 96-well plates (Corning) in a final volume of 100 μL at 25 °C essentially as described.Citation31 The reaction was monitored by measuring the excitation/emission (358/450 nm) using a Varioskan plate reader (Thermo Electron). The enzyme concentration was determined by choosing a reaction rate comprised in a fluorescence range of 5–20 FU/min and reaction was conducted at the specific Km for each enzyme against DiFMUP. Km was previously determined from rates at various substrate concentrations using Michaelis–Menten equation. For the kinetic assay, the fluorescence was monitored over 10 min in 30 sec intervals and rates were calculated using a non-linear least-square fitting procedure. The 1B/TC inhibitor was diluted in PBS and reaction was performed at a final concentration of 50 μM. PBS was used as control (vehicle) and to determine the ratio of phosphatase activity to DiFMUP in presence or not of inhibitor.
ELISpot assays
ELISpot assay for the detection of human activated antigen-specific T cells was performed as described previously,38,39 and according to the manufacturer's specifications (R&D Systems, cat# EL285). Briefly, patient moDCs cultured in the presence or absence of 1B/TC inh (4 μM) were pulsed with tumor antigens CEA (BioMart cat# CDA008) and CA19–9 (LEE Biosolutions cat# 151–99) during the maturation. The pool of antigen-specific T cells was enriched by culturing total PBMC with CEA and CA19–9 proteins during 5 d in media without serum. Mature, inhibitor-treated moDCs were then co-cultured with autologous patient T cells previously isolated using CD3+ magnetic bead selection (StemCell Technologies, cat# 17851). DCs and T cells were co-cultured in a 2:1 ratio (5 × 105 T cells: 2.5 × 105 DCs) for 48 h. The frequency of IFN-γ producing T cells was quantified using an ELISpot plate reader (BD Biosciences).
Statistics
The differences in tumor growth over time among the groups were analyzed using Log-rank (Mantel–Cox) Chi Square test. The differences in more than two groups were determined using One-Way ANOVA (Holm–Sidak multiple comparison test) for parametric and Dunn's multiple test for non-parametric. The differences between two groups were determined with an unpaired t-test (two tails of distribution). Shapiro–Wilk normality test was applied to define parametric versus non-parametric values. The statistical analyses were performed using Prism 6 (Graph-Pad Software) and p values < 0.05 were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KONI_A_1321185_Supplementaryfigures.pptx
Download MS Power Point (17.3 MB)Acknowledgments
The authors are grateful to Noriko Uetani for artwork, Cameron Black and Teri Hatzihristidis for critical reviews of the manuscript. C.P. is a recipient of a Canadian Institute of Health Research (CIHR) post-doctoral research award, M.F. is a recipient of a doctoral research studentship from CIHR, J.P. is a holder of a James McGill Chair Professor Award, G.Z is a Clinician Scientist Scholar of the Fonds de recherche du Québec – Santé (FRQS), M.L.T. is the holder of the Jeanne and Jean-Louis Lévesque Chair in Cancer Research.
Funding
This work was supported in parts by grants from the National Institutes of Health to G.K., a Canadian Institutes of Health Research operating award (CIHR FDN-148366) to J. Pelletier, and by the following operating grants from the Rob Lutterman- CRS research program, “the ACLON-Strauss Foundation,” the Canadian Cancer Society Innovation award program, a Canadian Institutes of Health Research operating award (MOP-62887) and a CIHR Targeting High Fatality Cancers - Innovation Grant (MOP- 361853) to M.L.T.
References
- Li HS, Watowich SS. Innate immune regulation by STAT-mediated transcriptional mechanisms. Immunol Rev 2014; 261:84-101; PMID:25123278; https://doi.org/https://doi.org/10.1111/imr.12198
- Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem 2001; 276:47771-4; PMID:11694501; https://doi.org/https://doi.org/10.1074/jbc.M009472200
- Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci USA 2014; 111:9887-92; PMID:24958858; https://doi.org/https://doi.org/10.1073/pnas.1402468111
- Pike KA, Hutchins AP, Vinette V, Théberge JF, Sabbagh L, Tremblay ML, Miranda-Saavedra D. Protein tyrosine phosphatase 1B is a regulator of the interleukin-10-induced transcriptional program in macrophages. Sci Signal 2014; 7:ra43; PMID:24803538; https://doi.org/https://doi.org/10.1126/scisignal.2005020
- Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr Biol 2002; 12:446-53; PMID:11909529; https://doi.org/https://doi.org/10.1016/S0960-9822(02)00697-8
- Pike KA, Tremblay ML. Regulating naive and memory CD8 T cell homeostasis – a role for protein tyrosine phosphatases. FEBS J 2013; 280:432-44; PMID:22458809; https://doi.org/https://doi.org/10.1111/j.1742-4658.2012.08587.x
- Scharl M, Hruz P, McCole DF. Protein tyrosine phosphatase non-receptor Type 2 regulates IFN-gamma-induced cytokine signaling in THP-1 monocytes. Inflamm Bowel Dis 2010; 16:2055-64; PMID:20848498; https://doi.org/https://doi.org/10.1002/ibd.21325
- Li HS, Watowich SS. Diversification of dendritic cell subsets: emerging roles for STAT proteins. JAKSTAT 2013; 2:e25112; PMID:24416644; https://doi.org/https://doi.org/10.4161/jkst.25112
- Lutz MB. Induction of CD4(+) Regulatory and polarized effector/helper T cells by dendritic cells. Immune Netw 2016; 16:13-25; PMID:26937228; https://doi.org/https://doi.org/10.4110/in.2016.16.1.13
- Tokumasa N, Suto A, Kagami S, Furuta S, Hirose K, Watanabe N, Saito Y, Shimoda K, Iwamoto I, Nakajima H. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood 2007; 110:553-60; PMID:17395783; https://doi.org/https://doi.org/10.1182/blood-2006-11-059246
- Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D'Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol 2003; 33:2832-41; PMID:14515267; https://doi.org/https://doi.org/10.1002/eji.200324073
- Kuka M, Baronio R, Valentini S, Monaci E, Muzzi A, Aprea S, De Gregorio E, D'Oro U. Src kinases are required for a balanced production of IL-12/IL-23 in human dendritic cells activated by Toll-like receptor agonists. PLoS One 2010; 5:e11491; PMID:20634889; https://doi.org/https://doi.org/10.1371/journal.pone.0011491
- Martin-Granados C, Prescott AR, Le Sommer S, Klaska IP, Yu T, Muckersie E, Giuraniuc CV, Grant L, Delibegovic M, Forrester JV. A key role for PTP1B in dendritic cell maturation, migration, and T cell activation. J Mol Cell Biol 2015; 7:517-28; PMID:26063615; https://doi.org/https://doi.org/10.1093/jmcb/mjv032
- Gabrilovich D. Mechanisms and functional significance of tumor-induced dendritic-cell defects. Nat Rev Immunol 2004; 4:941-52; PMID:15573129; https://doi.org/https://doi.org/10.1038/nri1498
- Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 2000; 6:1755-66; PMID:10815894
- Whiteside TL. Inhibiting the inhibitors: evaluating agents targeting cancer immunosuppression. Expert Opin Biol Ther 2010; 10:1019-35; PMID:20415597; https://doi.org/https://doi.org/10.1517/14712598.2010.482207
- Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma K et al. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol 2011; 2011:267539; PMID:21922022; https://doi.org/https://doi.org/10.1155/2011/267539
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 2012; 3:105-19; PMID:22811878; https://doi.org/https://doi.org/10.3978/j.issn.2078-6891.2011.021
- Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology 2011; 133:221-38; PMID:21453419; https://doi.org/https://doi.org/10.1111/j.1365-2567.2011.03429.x
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 2008; 68:5439-49; PMID:18593947; https://doi.org/https://doi.org/10.1158/0008-5472.CAN-07-6621
- Johnson LM, Scott P. STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J Immunol 2007; 178:7259-66; https://doi.org/https://doi.org/10.4049/jimmunol.178.11.7259
- Chiang PH, Wang L, Bonham CA, Liang X, Fung JJ, Lu L, Qian S. Mechanistic insights into impaired dendritic cell function by rapamycin: inhibition of Jak2/Stat4 signaling pathway. J Immunol 2004; 172:1355-63; https://doi.org/https://doi.org/10.4049/jimmunol.172.3.1355
- Conzelmann M, Wagner AH, Hildebrandt A, Rodionova E, Hess M, Zota A, Giese T, Falk CS, Ho AD, Dreger P et al. IFN-gamma activated JAK1 shifts CD40-induced cytokine profiles in human antigen-presenting cells toward high IL-12p70 and low IL-10 production. Biochem Pharmacol 2010; 80:2074-86; PMID:20709027; https://doi.org/https://doi.org/10.1016/j.bcp.2010.07.040
- Yanagawa Y, Iwabuchi K, Onoe K. Co-operative action of interleukin-10 and interferon-gamma to regulate dendritic cell functions. Immunology 2009; 127:345-53; PMID:19191915; https://doi.org/https://doi.org/10.1111/j.1365-2567.2008.02986.x
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 2009; 15:114-23; PMID:19185846; https://doi.org/https://doi.org/10.1016/j.ccr.2008.12.018
- Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol 2004; 172:464-74; https://doi.org/https://doi.org/10.4049/jimmunol.172.1.464
- Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity 2005; 23:491-502; PMID:16286017; https://doi.org/https://doi.org/10.1016/j.immuni.2005.09.010
- Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood 2005; 105:689-96; PMID:15251981; https://doi.org/https://doi.org/10.1182/blood-2004-04-1309
- Pfeiffer IA, Hoyer S, Gerer KF, Voll RE, Knippertz I, Gückel E, Schuler G, Schaft N, Dörrie J. Triggering of NF-kappaB in cytokine-matured human DCs generates superior DCs for T-cell priming in cancer immunotherapy. Eur J Immunol 2014; 44:3413-28; PMID:25100611; https://doi.org/https://doi.org/10.1002/eji.201344417
- Zee T, Settembre C, Levine RL, Karsenty G. T-cell protein tyrosine phosphatase regulates bone resorption and whole-body insulin sensitivity through its expression in osteoblasts. Mol Cell Biol 2012; 32:1080-8; PMID:22252315; https://doi.org/https://doi.org/10.1128/MCB.06279-11
- Montalibet J, Skorey K, McKay D, Scapin G, Asante-Appiah E, Kennedy BP. Residues distant from the active site influence protein-tyrosine phosphatase 1B inhibitor binding. J Biol Chem 2006; 281:5258-66; PMID:16332678; https://doi.org/https://doi.org/10.1074/jbc.M511546200
- Julien SG, Dubé N, Read M, Penney J, Paquet M, Han Y, Kennedy BP, Muller WJ, Tremblay ML. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet 2007; 39:338-46; PMID:17259984; https://doi.org/https://doi.org/10.1038/ng1963
- Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 2008; 65:3231-40; PMID:18668203; https://doi.org/https://doi.org/10.1007/s00018-008-8228-6
- Stagg J, Wu JH, Bouganim N, Galipeau J. Granulocyte-macrophage colony-stimulating factor and interleukin-2 fusion cDNA for cancer gene immunotherapy. Cancer Res 2004; 64:8795-9; PMID:15604233; https://doi.org/https://doi.org/10.1158/0008-5472.CAN-04-1776
- Frank DA, Mahajan S, Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med 1999; 5:444-7; PMID:10202937; https://doi.org/https://doi.org/10.1038/7445
- Coon ME, Diegel M, Leshinsky N, Klaus SJ. Selective pharmacologic inhibition of murine and human IL-12-dependent Th1 differentiation and IL-12 signaling. J Immunol 1999; 163:6567-74; PMID:10586050
- Schmitt CA, Lowe SW. Bcl-2 mediates chemoresistance in matched pairs of primary E(mu)-myc lymphomas in vivo. Blood Cells Mol Dis 2001; 27:206-16; https://doi.org/https://doi.org/10.1006/bcmd.2000.0372
- Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest 2000; 105:R9-R14; PMID:10727452; https://doi.org/https://doi.org/10.1172/JCI9051
- Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest 1999; 104:173-80; PMID:10411546; https://doi.org/https://doi.org/10.1172/JCI6909
- Bussières-Marmen S, Vinette V, Gungabeesoon J, Aubry I, Pérez-Quintero LA, Tremblay ML. Loss of T-cell protein tyrosine phosphatase in the intestinal epithelium promotes local inflammation by increasing colonic stem cell proliferation. Cell Mol Immunol 2017; PMID:28287113; https://doi.org/https://doi.org/10.1038/cmi.2016.72
