ABSTRACT
Tumor-associated macrophages (TAMs) play a role in tumor development and progression. We hypothesized that abundance of TAMs might modify efficacy of 5-fluorouracil chemotherapy in colorectal cancer. We measured the density of CD68+ TAMs at the invasive front of primary tumor of colorectal carcinoma (PT-TAMs; n = 208), at available matched metastatic lymph node (LN-TAMs; n = 149), and in an independent set of primary colorectal cancers (PT-TAMs, n = 111). The hazard ratios for disease-free survival were computed by Cox proportional-hazards model. In exploratory analysis, the interaction between TAMs and 5-fluorouracil adjuvant therapy was significant (PT-TAMs, p = 0.02; LN-TAMs, p = 0.005). High TAMs were independently associated with better disease-free survival only in 5-fluorouracil-treated patients (PT-TAMs, HR 0.23; 95%CI, 0.08–0.65; p = 0.005; LN-TAMs, HR 0.13; 95%CI, 0.04–0.43; p = 0.001). The independent predictive value of PT-TAMs was replicated in the external set (HR, 0.14; 95%CI 0.02–1.00; p = 0.05). In an in vitro experiment, 5-fluorouracil and macrophages showed a synergistic effect and increased colorectal cancer cell death. High densities of TAMs, particularly in metastatic lymph-nodes, identify stage III colorectal cancer patients benefitting from 5-fluorouracil adjuvant therapy.
| Abbreviations | ||
| TAMs | = | Tumor-associated macrophages |
| TANs | = | tumor-associated neutrophils |
| PT-TAMs | = | TAMs at the invasive front of primary tumors |
| LN-TAMs | = | () TAMs in the nodal metastasis |
| IRA | = | immune-reactive area |
Introduction
Adjuvant chemotherapy is the standard post-surgical treatment of patients with stage III colorectal cancer. In this setting, fluoropyrimidines improve 5-year survival rates by 10–15%, an additional 4–6% being contributed by their combination with oxaliplatin.Citation1-3 Even among patients receiving adjuvant therapy, one third still progresses to metastatic disease,Citation4-6 a rate not improved by biologic agents.Citation5,7-9 These facts indicate that mechanisms of disease eradication achieved by chemotherapy are unlikely to cooperate with those of disease control exerted by biologic agents,Citation10 putting forward the lack of markers predicting the efficacy of chemotherapy in stage III colorectal cancer. Molecular features which define colorectal cancer subtypes with heterogeneous prognosis (such as KRAS/BRAF and microsatellite status), do not predict responsiveness to adjuvant chemotherapyCitation10,11 as they do in metastatic setting, or alike PI3K-mutations toward aspirin responsiveness in adjuvant setting.Citation12 Also tumor-infiltrating lymphocytes (TILs), which effectively forecast the outcome of patients with early colorectal cancer, are unlikely to act as predictive factor in node-positive patients.Citation13-16 Under this respect, other key players of the tumor microenvironment deserving consideration are the tumor-associated macrophages (TAMs). Acting as promoters of cancer progression,Citation17,18 their high density usually correlates with worse prognosis, with the notable exceptions of colorectal cancer.Citation19-24 However, in some neoplastic disease, such as follicular lymphomas, TAM prognostic impact heavily depends upon treatment.Citation25-27 Preclinical studies have also depicted a complex dual role for TAMs in modulating the response to anticancer therapies.Citation28-30 In some cancers, selected chemotherapeutic agents enhance the anti-tumor activity of TAMs by eliciting adaptive responses able to determine immunogenic cell death,Citation31,32 by depleting immunosuppressive TAMs,Citation33,34 or by reprogramming macrophages to an antitumor phenotype.Citation35,36 Although scanty clinical data are available on interactions of TAMs with chemotherapy efficacy in solid tumors, high loads of TAMs correlated with prolonged survival in 5-fluorouracil-treated patients with gastric cancer,Citation37 and partially corrected the poor prognosis of patients resected for pancreatic cancer and treated with adjuvant gemcitabine.Citation38 In keeping with these data, we recently reported the favorable prognostic interaction of 5-fluorouracil adjuvant therapy in colorectal cancer patients with high densities of tumor-associated neutrophils (TANs), a native component of the tumor infiltrate otherwise associated with poor clinical outcome.Citation39-41
This study was aimed to see whether the favorable prognostic association between TAMs densities and colorectal cancer outcome reflects a beneficial interaction of macrophages with 5-fluorouracil-based adjuvant chemotherapy. To this aim, the predictive value of TAMs at the invasive front of primary tumors (PT-TAMs) and of nodal metastasis (LN-TAMs) was tested in a consecutive series of stage III colorectal cancer, and in external set of primary tumors. In vitro we conducted experiments investigating the interplay of 5-fluorouracil with macrophages in determining colorectal cancer cell death.
Results
Densities of TAMs in primary tumors and in metastatic lymph nodes
Patient demographics, clinico-pathological, and molecular features of 208 stage III colorectal cancers are detailed in the Table S1. CD68-immunoreactive cells were present in most of tumors samples, both at the invasive front of primary cancers (186 of 208; 89.4%), and in metastatic lymph nodes (141 of 149; 94.6%). The more dense PT-TAM (median IRA%, 3.95 %; 2nd-3rd quartile, 1.65–8.38) and the less dense LN-TAM (median IRA%, 2.25 %; 2nd-3rd quartile, 0.75–5.24; p < 0.001) contents were significantly related (linear correlation p < 0.001, Pearson's r = 0.37). Overall, PT-TAM densities in the institutional cohort (n = 208; median 3.80%; 2nd-3rd quartile, 1.47–8.19) were not different (p = 0.50) from those of the external set of primary cancers (n = 111; median 4.36 %; 2nd-3rd quartile, 2.12–6.99).
Table 1. Risk of postoperative recurrence in stage III colorectal cancer by TAM densities, clinical-pathological features, and 5-fluorouracil (5FU) adjuvant therapy.
As to pathological features (Table S2), lower densities of PT-TAMs were associated with deeper tumor local invasion (pT4 vs. pT3, p = 0.01), atypical tumor histology (mucinous or medullary vs. adenocarcinoma, p = 0.03), and low amounts of CD3-TILs (p<0.001).
Table 2. Outcome predictors in 5-fluorouracil-treated patients with stage III colorectal cancer (Cox univariate analysis).
However, TAM densities were similar for the 3rd (PT-TAM median, 4.74%) and 4th (5.57%; p = 0.88) TIL quartiles.
The density of TAMs was not significantly associated with patient demographics, with the administration adjuvant therapy, nor with tumor molecular features.
No significant association between PT-TAMs densities and clinical-pathological variables was detected in the external validation set (CD3+ TILs not determined).
Prognostic interaction of tumor-associated macrophages and neutrophils with 5-fluorouracil adjuvant therapy
The impact of TAM density on the risk of postoperative recurrences is shown in . At univariate Cox analysis, high densities of both PT- and LN-TAMs were significantly associated with a lower risk of postsurgical recurrence (p = 0.006 and p = 0.001, respectively). Other variables significantly associated with a more favorable outcome included age <70 y (p = 0.01), N1 nodal status (p = <0.001), absence of vascular invasion (p<0.001), and postoperative treatment with 5-fluorouracil (p = 0.04).
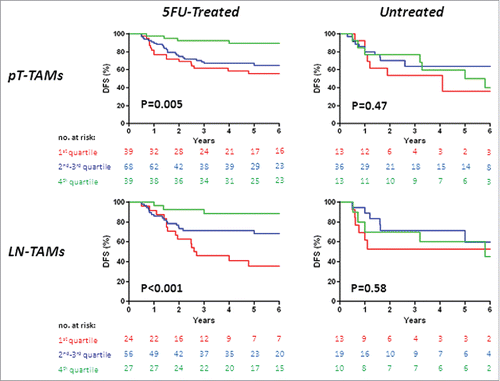
Interaction analysis revealed significant prognostic interactions of 5-fluorouracil adjuvant therapy with PT-TAMs (p = 0.02), with LN-TAMs (p = 0.005), and with density of intra-tumoral neutrophils (TANs, p = 0.01). In accordance with the Cox model, Kaplan-Meier curves of disease-free survival () showed that increasing densities of PT-TAMs (p = 0.005) and of LN-TAMs (p< 0.001) were significantly associated with a better prognosis only in patients treated with 5-fluorouracil adjuvant therapy. In particular, 5-fluorouracil-treated patients with highest (4th quartile) densities of TAMs had a 5-year disease-free survival much better than that of corresponding patients operated for cancers with lower densities of macrophages (PT-TAMs, 89.7% vs. 60.8%, p = 0.001; LN-TAMs, 88.6% vs. 58.0%, p = 0.008). Also the 4th quartile of intra-tumoral neutrophils (TANs) was weakly (p = 0.04) associated with better disease-free survival in 5-fluorouracil-treated patients (Fig. S1). In contrast, no association of TAMs and TANs densities with disease-free survival was observed in the subset of patients not treated with adjuvant therapy.
Figure 1. Disease-free survival (DFS) in stage III colorectal cancer, by TAM-density quartiles. Kaplan-Meier Curves. Patients were stratified by quartile distribution of TAMs densities at the invasive front of their primary tumor (PT-TAMs, upper panels) or of metastatic lymph-nodes (LN-TAMs, lower panels). Higher densities of both PT-TAMs and LN-TAMs were significantly associated with better disease-free survival in patients treated with 5-fluorouracil (left panels), but not in the subset of untreated patients (right panels). Also higher densities of tumor-associated neutrophils (PT-TANs) were weakly associated with the survival of 5-fluorouracil -treated patients (see Fig. S1). P values are for Log-Rank test.
Predictive value of tumor-associated macrophages and neutrophils
Receiving Operator Characteristic (ROC) curves (Fig. S2) were used to determine optimal cutoffs of TAMs and TANs densities predicting colorectal cancer recurrences in 5-fluorouracil- treated patients. Optimal cut-offs were 8.0% immune-reactive area for PT-TAMS, 3.7% for LN-TAMs, and 1.16 % for TANs, values slightly lower than the inferior limits of 4th-quartile subclasses (9.2%, 5.5%, and 1.35%, respectively). According to the cut-off values, patients were grouped in high or low densities of TAMs and TANs. At Kaplan-Meier curves (), 5-fluorouracil-treated patients with high PT-TAMs, or high LN-TAMs, showed a significantly better disease-free survival than patients with low densities of macrophages (5-year disease-free survival: by PT-TAMs, 90.2% vs. 60.0%, p = 0.0008; by LN-TAMs, 92.2% vs. 50.2%, p<0.0001). Also high TAN densities were associated with better outcome (p = 0.04; Fig. S3).
Figure 2. Disease-free survival (DFS) in 5-fluorouracil-treated patients with stage III colorectal cancer, by high/low TAMs. Kaplan-Meier Curves. Patients were classified by high/low density of TAMs, measured at the invasive front of their primary colorectal cancer (PT-TAMs) or of metastatic lymph-nodes (LN-TAMs), and defined by optimal cut-offs at receiver operator characteristic (ROC) curves (Fig. S2). P values are for Log-Rank test. Also high densities of intra-tumoral neutrophils (PT-TANs) were weakly (p = 0.04) associated with better disease-free survival (Fig. S3). The association of high PT-TAMs with better disease-specific survival was confirmed in the external validation set (Fig. S4).
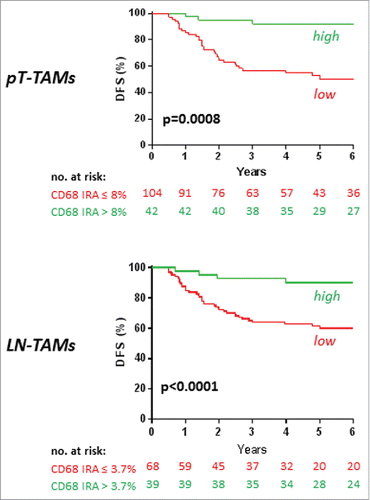
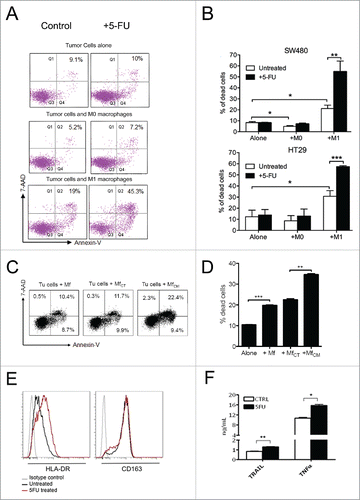
Figure 3. Chemotherapy and macrophage synergism in cytotoxic function in vitro. A) Facs plots of SW480 colorectal cancer cells co-cultured with un-polarized (M0) or macrophages polarized toward a cytotoxic phenotype by IFNg/LPS stimulation (M1). Tumor cell death was evaluated by Annexin V/7-AAD staining. 24-hour 5-fluorouracil treatment on colorectal cancer cells alone did not significantly increase tumor cell death compared with untreated cells (top). Macrophages induced detectable tumor cell death, which was further enhanced by treatment with 5-fluorouracil (bottom). One representative of 5 experiments is shown. B) Histograms representative of 3 independent experiments performed with 2 MSS cell lines (SW480 and HT29): cytotoxicity of M1 macrophages toward colorectal cancer cells (*; P ≤ 0.05) was significantly increased by the addition of 5-fluorouracil in the 2 microsatellite stable cell lines, SW480 (**; P ≤ 0.005) and HT29 (***P ≤ 0.001). Histograms show means ± standard error. C) Culture of macrophages with supernatant of 5-fluorouracil-treated colorectal cancer cells (MfCM) significantly increased macrophage cytotoxicity (right) compared with control macrophages (left) or macrophages cultured with supernatant of control colorectal cancer cells (MfCT) (middle). One representative of 2 experiments is shown. D) Histograms representative of 2 independent experiments (***; P ≤ 0.001; **; p ≤ 0.01). E) Effect of chemotherapy on macrophage polarization markers. 5-Fluorouracil treatment of M0 macrophages increased the expression of the M1-marker HLA-DR, while the M2-marker CD163 did not shown any change. F) Effect of chemotherapy on macrophages-M1 cytotoxicity. TRAIL and TNFa expression was measured by ELISA in cellular extracts from macrophages stimulated with LPS/IFNg and subsequently exposed to 5-fluorouracil for 24 hours. One representative of 2 experiments is shown. Bars represent means ± standard error. (*: p <0.05; **: p ≤ 0.01).
Survival curves of patients from the external validation set (for which disease-specific survival data were available) confirmed the better outcome of 5-fluorouracil-treated patients with high densities of PT-TAMs (p = 0.03), together with the lack of any impact on the outcome of untreated subjects (p = 0.62) (Fig. S4).
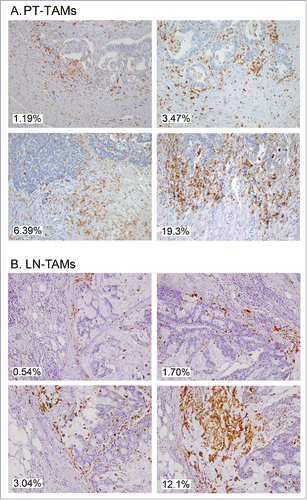
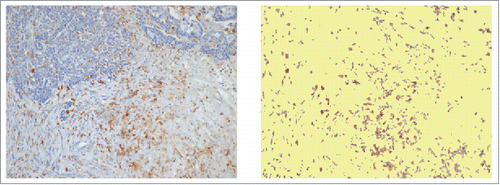
Figure 4. Immunostaining of CD68+ TAMs at the invasive margin of primary colorectal cancer (A) and of metastatic lymph-nodes (B). Examples of tissue areas with variable extent of CD68-immunoreative area (% IRA in white boxes).
At Cox univariate analysis (), high PT-TAMs (p = 0.002), high LN-TAMs (p = 0.001), high TANs (p = 0.04), N1 nodal status (p<0.001), and absence of vascular invasion (p = 0.008), were associated with better disease-free survival of 5-fluorouracil-treated patients from the institutional cohort. A favorable predictive value of high PT-TAMs (p = 0.05), N1 nodal status (p = 0.03), and absence of vascular invasion (p = 0.05) was also observed in the external set. In multivariate models adjusted by nodal status and vascular invasion (), only PT-TAMs (p = 0.005) and LN-TAMs (p = 0.001) maintained an independent predictive value. PT-TAMs resulted to be an independent outcome predictor also in the external validation set (p = 0.05).
Table 3. Cox multivariate analysis of TAM and TAN densities, and other pathological features predicting the outcome of stage III colorectal cancer treated with 5-fluorouracil adjuvant therapya
Synergic colorectal cancer cell killing by macrophages and 5-fluorouracil
At fluorescence-activated cell sorting (FACS), the death rate of SW480 cells was not increased by short-term (24-hour) exposure to 10 mM 5-fluorouracil (, top panels), even if cancer cells were co-cultured with un-polarized macrophages (, middle panels). In contrast, co-culture of tumor cells with M1-polarized macrophages (, bottom panels) almost doubled the cell death rate, which was further increased by exposure to 5-fluorouracil.
In independent experiments with SW480 and HT29 cells, the tumoricidal activity induced by co-culture with M1-macrophages and 5-fluorouracil exposure exceeded the sum of the cytotoxic effects of macrophages and 5-fluorouracil alone (). Cancer cells co-cultured wit M1-macrophages and exposed to 5-fluorouracil had a death rate significantly superior to that of corresponding 5-fluorouracil -untreated cells (SW480, p<0.01; HT29, p<0.001) which, in turn, had a death rate significantly higher than that of cells exposed to 5-fluorouracil in the absence of macrophages or after co-culturing with un-polarized macrophages (p<0.05 for both SW480 and HT29).
Macrophages preliminarily cultured with the supernatant of 5-fluorouracil-treated SW480 colorectal cancer cells and then co-cultured with tumor cells displayed a cytotoxicity significantly greater (p<0.01) than that of control macrophages or of macrophages pre-cultured with conditioned medium from 5-fluorouracil-untreated colorectal cancer cells ( and ).
The exposure of un-polarized (M0) macrophages to 5-fluorouracil increased the expression of the M1-marker HLA-DR but not that of the M2-marker CD163 (Panel E), while drug-exposure of M1-macrophages was accompanied by a significant release of TRAIL (p<0.01) and TNFa (p<0.05) pro-apoptotic cytokines (Panel F).
Discussion
We first report that densities of TAMs in the primary tumor and in metastatic lymph nodes act as an independent predictive factor in patients resected for stage III colorectal cancer and treated with 5-fluorouracil adjuvant therapy. Together with the in-vitro evidence that macrophage greatly enhance colorectal cancer cell killing by exposure to 5-fluorouracil, clinical data strongly support a synergism of TAMs with fluoro-pyrimidines.
In contrast to their association with poor prognosis in several tumors, high densities of TAMs favorably influence the postsurgical clinical outcome of colorectal cancer.Citation19-24 The present study clarifies that the association between high TAMs densities and better survival in patients with stage III colorectal cancer results from their interaction with 5-fluorouracil, the drug at the basis of adjuvant therapy for decades. Given that such an association has been shown to be stage-dependent and virtually limited to stage III disease,Citation22-24 the synergism with 5-fluorouracil likely accounts for the entire prognostic value of TAMs in colorectal cancer. Consistently, we found no correlation between TAMs and outcome in our institutional cohort of more than 2 hundred stage II patients (HR 0.98; 95% CI 0.88–1.08; p = 0.66; data not shown).
Our results draw considerable strength from the fact that TAMs exhibited their predictive role when measured at distinct anatomic sites. Actually, high densities of macrophages in metastatic lymph nodes (LN-TAMs) were associated with disease-free survival of 5-fluorouracil-treated patients more closely than high loads of TAMs measured in primary tumors (PT-TAMs), which might reflect a more accurate identification of high-TAM infiltrates at the nodal invasive front. As to the density of LN-TAMs, Onishi and coll., reported that a high density of CD169+, but not of CD68+, macrophages was associated with better survival of patients with stage I-IV colorectal cancer. The lack of association of CD68+ LN-TAMs with prognosis is likely due to small study population, and to the absence of stage stratification with respect to chemotherapy treatment.Citation42 The prognostic interaction of LN-TAMs anyhow supports the concept that macrophages can synergize with 5-fluorouracil in metastatic microenvironment, where cytotoxic drugs are expected to act. Supporting this interpretation, Cavnar et al. recently reported the association between high-TAM infiltration of colorectal cancer liver metastases and better survival, in a cohort of patients treated with peri-surgical chemotherapy.Citation43 Noticeably, the overall predictive value of TAMs essentially resulted from the excellent outcome of cancers with very high densities of CD68+ cells. Such cancers represented approximately 30% of the entire series (28.8 % by high PT-TAMs, 36.4% by high LN-TAMS), a fraction roughly equaling the reduction in the relative risk of death that is expected from 5-fluorouracil adjuvant therapy in stage III colorectal cancer.
The densities of tumor-infiltrating CD3+ lymphocytes (TILs), although weakly correlated with PT-TAMs loads, had no prognostic value in our series of stage III colorectal cancer. This finding challenges the concept of a stage-independent correlation between TILs and colorectal cancer prognosis,Citation44 rather indicating that the established protective role of TILs in stage II diseaseCitation13, 15, 44, 45 is lost once nodal metastases develop. In addition, the lack of prognostic impact for TILs densities in stage III colorectal cancer implies that their correlation with PT-TAMs is not extended to the very dense infiltrates of TAMs that account for the prediction of 5-fluorouracil responsiveness. Taken together, results from combined analysis of CD3+ and CD68+ cells support a disease model in which native immune evasion accompanies the development of micro-metastases, that can be best eradicated by adjuvant therapy in patients with high TAM loads.
CD68 pan-macrophage marker does not allow the sub-classification of TAMs. However, in-vitro experiments indicate that 5-fluorouracil exposure favor macrophage polarization toward a M1 anti-tumor role. These findings are in keeping with in-vivo studies showing that the skewing of TAMs to M1-like phenotype contributes to the anti-tumor and anti-angiogenic effects of pharmacological agents in mice.Citation35,36 Furthermore, the unique exposure of colorectal cancer microenvironment to commensal enteric microbiota, which has been recently shown to shape the immune cells response to cytotoxic agents,Citation46,47 might contribute to TAMs polarization and eventually account for the predictive value of macrophages in colorectal cancer. On the other hand, TAMs subpopulations are variably represented in distinct microenvironments of the same tumor,Citation46 and expression of polarization markers is considerably heterogeneous in colorectal cancer.Citation23 Accordingly, the polarization of TAMs in the resected primary tumor might even be irrelevant to their successive synergism with post-surgical chemotherapy, so that TAMs densities might simply predict the likelihood of a robust response toward micro-metastases. This situation somehow resembles the one occurring in follicular lymphoma, in which administered therapeutic regimes skew the predictive role of CD68+ TAMs (refs Citation24-26). In a wider perspective, our data add to those pointing to the involvement of tumor targeting immune responses in determining the clinical efficacy of conventional chemotherapy regimens, besides their cytostatic and cytotoxic effects.Citation48,49 The pathways mediating the interaction of macrophages with 5-fluorouracil remain to be identified, although the release of TNFα/TRAIL in vitro by M1 macrophages exposed to 5-fluorouracil suggests the involvement of these molecules in immunogenic cell death of colorectal cancer cells.Citation50
The study is limited by the size of the external validation set, and by the fact that most patients were treated with 5-fluorouracil alone (93.2% in the institutional cohort, 63.1% in the validation set). At any event, the prevailing impact of 5-fluorouracil in reducing the progression of stage III colorectal cancer makes it likely TAMs would maintain a strong predictive value also in cohorts of patients treated with oxaliplatin combination. Due to the relevant clinical implication of the lack of substantial survival benefit of adjuvant therapy in patients with low-TAM, our findings deserve validation in pathological archives from randomized controlled trials.
This study confirms that intra-tumoral densities of neutrophils (TANs), another native component of the immune infiltrate of colorectal cancer, are weakly associated with better outcome in 5-fluorouracil-treated patients. However, the loss of TANs predictive value at multivariate analysis endorses TAMs as the most promising marker of response to adjuvant therapy.
By revealing that high densities of TAMs strongly predict the efficacy of 5-fluorouracil in stage III colorectal cancer, we provide an explanation for the unique prognostic association of TAMs with colorectal cancer outcome, contributing a step toward the personalized management of the disease. In particular, the measurement of TAMs in metastatic lymph nodes is proposed as an independent and powerful tool to forecast chemotherapy efficacy. Our results also encourage evaluating immune-therapeutic interventions aimed at TAMs recruitment and polarization as a way to improve the response rate of node-positive colorectal cancer to 5-fluorouracil-based adjuvant therapy.
Materials and Methods
Tissue samples and patients
The study was conducted on specimens of primary tumor and of metastatic lymph nodes obtained from a consecutive series of 208 patients resected for stage III colorectal cancer at the Humanitas Research Hospital between 1997 and 2006. Tissues from patients having undergone neo-adjuvant chemo-radiotherapy for rectal cancer were excluded from the series to avoid possible interferences with TAMs. After study approval by the Hospital Ethics Committee, the informed consent to tissue analysis and data treatment was obtained from each patient by the referring physician or by a clinician involved in the study. Patient demographics and tumor pathological features were available at the hospital intranet system. The same source provided most of clinical data relative to post-surgical treatment and follow-up.
The administration of 5-fluorouracil-based adjuvant therapy was always prescribed on clinical grounds and not in the context of prospective trials. Adjuvant therapy was less frequently (p<0.001) administered to ≥70-year old patients (39/90, 43%) than to younger patients (107/118, 90.7%). Only in 10 of 146 (6.8%) treated patients oxaliplatin was part of the therapeutic regimen. The 5-year follow-up schedule, as ordered at discharge, included yearly abdominal CT scan (with abdominal US at 6 months), yearly thorax imaging (X-rays or CT scan), quarterly measurement of CEA levels, and biannual clinical evaluation. The mean ( ± SD) follow-up of patient included in the study was 4.82 ± 2.56 y. External institutions, referring physicians or patient families were contacted to obtain clinical data not available in hospital records. The database was finally completed with data on tumor microsatellite status, K-RAS (codon12 and 13), and BRAF mutation status (codon 1799 hot spot) determined by the Laboratory of Molecular Gastroenterology, as described previously.Citation51
Primary-tumor tissues were also obtained from 2 external cohorts of stage III colorectal cancer. Eighty-two tissue sections were collected from the Department of Medical Biosciences of the University of Umeå (Sweden), while 29 colorectal cancer tissue micro-arrays came from the Institute of Pathology of the University of Bern (Switzerland). Tissues from these subsets were merged to create a validation set external to the original cohort. Demographics, pathological data and disease-specific survival were made available for corresponding patients. Also among these patients, 5-fluorouracil-based adjuvant therapy was prescribed according to recommendations in force at the time of surgery, and oxaliplatin was part of the adjuvant therapy in 22 of 60 (36.7%) patients. The mean ( ± SD) follow-up in the overall external set was 4.82 ± 2.56 y.
Immunohistochemical staining
Serial sections of formalin-fixed and paraffin-embedded tissues were preliminary stained with hematoxylin-eosin to select areas adequately representing the tumor invasive front. Adequate sections were obtained from all the 208 primary tumors and from 149 matched metastatic lymph nodes, nodal micrometastasis being insufficiently represented in the archive material of 59 patients.
Immediately adjacent tissue sections were used for immunohistochemical analysis. For each specimen, 2-μm thick formalin-fixed sections were de-paraffined, rehydrated and exposed to an antigen-retrieval system (Diva Decloaker solution, Biocare Medical). After blocking endogenous peroxidase (Peroxidazed-1 and Background Sniper, Biocare Medical), slides were incubated at room temperature with 3% hydrogen peroxide for 10 minutes and then with 1:50 PBS-diluted primary monoclonal antibody (anti-CD68, KP-1 clone, Dako) for 60 minutes. After exposure to MACH 4 Universal HRP Polymer (Biocare Medical) for 30 minutes at room temperature Space Import-Export, Italy) to reveal bound antibodies, 3′-diaminobenzidine tetrahydrochloride was used as a chromogen (DABchromogen X50, ChemMate, Dako) and nuclei were lightly counterstained with a freshly made hematoxylin solution (Harris Hematoxylin, DiaPath).
Staining for TANs and CD3+ TILs was respectively performed with an anti-CD66b monoclonal antibody (clone G10F5; BD Pharmingen, CA) in 200 specimens of primary tumors and with a monoclonal antibody (clone F7.2.38, M7254; Dako) in 208 tissue sample of primary tumors, as described previouslyCitation13,39 (Fig. S5).
Figure 5. Capture of immune reactive areas at image analysis. Example of computer assisted selection of immune reactive areas spots by red green and blue (RGB) color segmentation of the original digital microphotograph. Immune-reactive area, brown. Not stained tissue, yellow. The percent ratio between immune reactive areas and total area was automatically calculated.
Computer-aided image analysis
An expert pathologist (M. R.) who was completely blind to clinical data selected 3 non-contiguous microscopic areas of CD68-stained slides. At 10x magnification, cancer tissue was to represent approximately 50% of each microscopic field both for the invasive front of primary tumors and for metastatic nodal nests (). A digital image (1280 × 960 pixels, 2.21 pixel/micron resolution) covering 0.25 mm2 of each selected area, was obtained by a 5-megapixel color camera (Olympus XC50). An ad hoc software developed at the Humanitas Research Hospital (patent WO 2005 006255), using a segmentation algorithm based on RGB (red, green and blue) scale, was used to automatically measure the immune-reactive area in digitized images.Citation13 In calculating the percent immune-reactive area (% IRA), the system corrected microscopic images for unfilled natural holes, vascular spaces, or histological artifacts (). The value of % CD68-IRA attributed to each tumor or nodal specimen was the mean of values found at analysis of 3 distinct microscopic fields. The value of % CD66b-IRA for TANs was similarly calculated as the mean of 3 distinct intra-tumoral regions.Citation39
In-vitro experiments
Human mononuclear cells were obtained from the peripheral blood of healthy volunteers by gradient centrifugation with Histopaque-1077 (Sigma-Aldrich, Milan, Italy). Monocytes were isolated using the Monocyte Isolation Kit II (MACS Miltenyi Biotec, Italy), and residual T and B cells were removed by plastic adherence. Monocytes were differentiated into macrophages by 6-day incubation with macrophage colony-stimulating factor (M-CSF, Tebu-Bio, Italy) at the concentration of 100 ng/ml. Polarization of macrophages toward the M1-like phenotype was obtained on day 6 by stimulating macrophages with interferon-gamma (IFNγ, 25 ng/ml, Tebu-Bio, Italy) for 20-hour and with lipopolysaccharides (LPS, 50 ng/ml) for 4 hours.
HT29 and SW480 colorectal cancer cells, obtained by ATCC®, were cultured in RPMI-1640 medium (Euroclone, Italy) supplemented with 10% fetal bovine serum (FBS). After labeling with the fluorescent cell tracer carboxyfluorescein diacetate succinimidyl ester (CFSE, Becton Dickinson, Italy), cells were co-cultured with M1 macrophages, at a 1:1 ratio, in 10% FBS medium for 24 hours. To test the early effect of 5-fluorouracil on cell viability, 2,4-dihydroxy-5-fluoropyrimidine(5-fluorouracil, Sigma-Aldrich, Italy) at a 10 mM concentration was added to tumor cells alone or in co-culture with M1 macrophages. To see whether the cytotoxic effect of macrophages was enhanced by cell treatment with 5-fluorouracil, the supernatants of SW480 cells treated with 20 mM 5-fluorouracil for 72 hours, or of untreated cells as a control, were added in a 1:3 ratio to the medium of differentiated macrophages, before 24-hour co-culturing on day 6. The rate of tumor cell death was assessed by flow cytometry. To restrict the analysis of dead cells to CFSE-labeled tumor cells, co-cultured macrophages were labeled with an anti-HLA-DR-PE human antibody (clone AC122, Miltenyi Biotec, Italy). After cell staining for annexin-V and 7-aminoactinomycin, dead tumor cells were quantified by a FACS Canto System (Becton Dickinson, Italy).
To assess the effect of 5-fluorouracil on macrophage polarization and on the expression of apoptosis-related cytokines, macrophages were exposed to 20 mM 5-fluorouracil for 24 hours. For subsequent analysis of HLA-DR and CD163 polarization markers, M0 macrophages were preliminary incubated with 1% human serum on ice for 20 minutes to block Fc-receptor sites. Cells were then washed with FACS buffer (PBS-/- + 0,5% BSA + 0,05% Sodium Azide), centrifuged at 1300rpm for 5 minutes, incubated with staining antibodies (mouse anti-human CD16-PercPcy5, 3G8 clone, as isotype control; HLA-DR-PE, G46–6 clone; CD163-BV421, GHI/61 clone - Becton Dickinson, Italy) on ice for 20 minutes, and washed with FACS buffer. Stained cells were finally measured using FACS Canto System and FlowJo single-cell analysis software. For analysis of 5-fluorouracil-induced changes in cytokines, M1-polarized macrophages were washed from LPS- and IFNγ and then cellular extracts were tested for protein-normalized levels of TRAIL and TNFα by specific ELISA assays (Prodotti Gianni, Italy).
Statistical analysis
The Mann-Witney test was used to analyze the associations of CD68+ TAMs densities with patient demographics and with pathological or molecular features of colorectal cancer. A receiver operating characteristic (ROC) curve was constructed to check the predictive performance of CD68-immunoreactive area in patients with stage III cancer. Cox proportional hazard models were used to evaluate TAMs densities, other molecular, clinical-pathological variables and their interactions, as outcome predictors. Cox multivariate analysis was done by entering only variables with a p value less than 0.10 at univariate analysis. By a backward step-wise elimination approach, non-significant variables were removed from the model. Disease-free-survival and disease-specific survival were calculated from diagnosis until March 1, 2014, which was the date of data censoring. Kaplan-Meier curves were plotted and log-rank test were used to analyze the survival of colorectal cancer patients grouped according to TAM densities. The Students' t test was used to compare rates of in vitro cell death in different experimental settings. All the statistical analyses were performed using STATA (version 13.1). For each statistical test, 2-sided P values < 0.05 were considered statistically significant.
Author contributions
AM and LL had full access to all data in the study and take responsibility of the integrity of the data and the accuracy of the data analysis. Study concept and design: LL, PB, AM. Acquisition, analysis, or interpretation of data: PB, FM, FG, GC, GB, GDC, TC, LL, LR. Drafting of the manuscript: AM, PB, LL, and SO. Critical revision of the manuscript for important intellectual content: AM, PB, GC, AM, SO, LL. Statistical analysis: AM, PB, LL, GC. Administrative, technical, or material support: RP, AL, VHK, MR.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Suppl_materials.zip
Download Zip (3.6 MB)Acknowledgments
Thanks to Mrs Valentina Giatti for the help in the managing of the patients database.
Funding
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Investigator Grant - IG 2014,00 Number 16092 (to L.L.), and by USA National Institute of Health grant R35CA197735 (to S.O.); Nodal Award (to S.O.) from Dana-Farber Harvard Cancer Center. The funding source did not have access to the raw data and had no role in study design; data collection, analysis, or interpretation; or writing of the report. The corresponding author had full access to all the data and final responsibility for the decision to submit the Article for publication.
References
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Eng J Med 2005; 352:2696-704; PMID:15987918; https://doi.org/10.1056/NEJMoa043116
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Eng J Med 2004; 350:2343-51; PMID:15175436; https://doi.org/10.1056/NEJMoa032709
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27:3109-16; PMID:19451431; https://doi.org/10.1200/JCO.2008.20.6771
- Yothers G, O'Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29:3768-74; PMID:21859995; https://doi.org/10.1200/JCO.2011.36.4539
- Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012; 307:1383-93; PMID:22474202; https://doi.org/10.1001/jama.2012.385
- Shi Q, Andre T, Grothey A, Yothers G, Hamilton SR, Bot BM, Haller DG, Van Cutsem E, Twelves C, Benedetti JK, et al. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database. J Clin Oncol 2013; 31:3656-63; PMID:23980089; https://doi.org/10.1200/JCO.2013.49.4344
- de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012; 13:1225-33; PMID:23168362; https://doi.org/10.1016/S1470-2045(12)70509-0
- Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Lopa SH, Wolmark N. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013; 31:359-64; PMID:23233715; https://doi.org/10.1200/JCO.2012.44.4711
- Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Petersen LN, Blons H, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15:862-73; PMID:24928083; https://doi.org/10.1016/S1470-2045(14)70227-X
- Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol 2015; 33:1787-96; PMID:25918287; https://doi.org/10.1200/JCO.2014.60.0213
- Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M, Goldberg RM, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 2015; 148:88-99; PMID:25305506; https://doi.org/10.1053/j.gastro.2014.09.041
- Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Eng J Med 2012; 367:1596-606; PMID:23094721; https://doi.org/10.1056/NEJMoa1207756
- Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol 2009; 10:877-84; PMID:19656725; https://doi.org/10.1016/S1470-2045(09)70186-X
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610-8; PMID:21245428; https://doi.org/10.1200/JCO.2010.30.5425
- Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232:199-209; PMID:24122236; https://doi.org/10.1002/path.4287
- Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010; 222:350-66; PMID:20927778; https://doi.org/10.1002/path.2774
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; https://doi.org/10.1038/nature07205
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 2013; 339:286-91; PMID:23329041; https://doi.org/10.1126/science.1232227
- Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. Plos One 2012; 7:e50946; PMID:23284651; https://doi.org/10.1371/journal.pone.0050946
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol 2013; 35:585-600; PMID:23657835; https://doi.org/10.1007/s00281-013-0367-7
- Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 2007; 13:1472-9; PMID:17332291; https://doi.org/10.1158/1078-0432.CCR-06-2073
- Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 2010; 8:13; PMID:20141634; https://doi.org/10.1186/1479-5876-8-13
- Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamäki R, Jalkanen S. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 2012; 131:864-73; PMID:21952788; https://doi.org/10.1002/ijc.26457
- Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. Plos One 2012; 7:e47045; PMID:23077543; https://doi.org/10.1371/journal.pone.0047045
- de Jong D, Koster A, Hagenbeek A, Raemaekers J, Veldhuizen D, Heisterkamp S, de Boer JP, van Glabbeke M. Impact of the tumor microenvironment on prognosis in follicular lymphoma is dependent on specific treatment protocols. Haematologica 2009; 94:70-7; PMID:19059937; https://doi.org/10.3324/haematol.13574
- Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res 2007; 13:5784-9; PMID:17908969; https://doi.org/10.1158/1078-0432.CCR-07-0778
- Kridel R, Xerri L, Gelas-Dore B, Tan K, Feugier P, Vawda A, Canioni D, Farinha P, Boussetta S, Moccia AA, et al. The prognostic impact of CD163-positive macrophages in follicular lymphoma: A study from the BC cancer agency and the lymphoma study association. Clin Cancer Res 2015; 21:3428-35; PMID:25869385; https://doi.org/10.1158/1078-0432.CCR-14-3253
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer cell 2013; 23:277-86; PMID:23518347; https://doi.org/10.1016/j.ccr.2013.02.013
- Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 2015; 212:435-45; PMID:25753580; https://doi.org/10.1084/jem.20150295
- Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; 14(7):399-416; PMID:28117416; https://doi.org/10.1038/nrclinonc.2016.217
- Mantovani A, Polentarutti N, Luini W, Peri G, Spreafico F. Role of host defense merchanisms in the antitumor activity of adriamycin and daunomycin in mice. J Natl Cancer Inst 1979; 63:61-6; PMID:286835; https://doi.org/10.1093/jnci/63.1.61
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; https://doi.org/10.1146/annurev-immunol-032712-100008
- Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res 2014; 74:2663-8; PMID:24778417; https://doi.org/10.1158/0008-5472.CAN-14-0301
- Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013; 23:249-62; PMID:23410977; https://doi.org/10.1016/j.ccr.2013.01.008
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 2010; 16:4583-94; PMID:20702612; https://doi.org/10.1158/1078-0432.CCR-10-0733
- Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 2011; 19:31-44; PMID:21215706; https://doi.org/10.1016/j.ccr.2010.11.009
- Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, Zheng L, Li L. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol 2011; 18:2585-93; PMID:21347781; https://doi.org/10.1245/s10434-011-1609-3
- Di Caro G, Cortese N, Castino GF, Grizzi F, Gavazzi F, Ridolfi C, et al. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut 2016; 65(10):1710-20; PMID:26156960; https://doi.org/10.1136/gutjnl-2015-309193
- Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer 2016; 139:446-56; PMID:26939802; https://doi.org/10.1002/ijc.30076
- Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013; 218:1402-10; PMID:23891329; https://doi.org/10.1016/j.imbio.2013.06.003
- Shen MX, Hu PP, Donskov F, Wang GH, Liu Q, Du JJ. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. Plos One 2014; 6; 9(6); PMID:24906014; https://doi.org/10.1371/journal.pone.0098259
- Ohnishi K, Komohara Y, Saito Y, Miyamoto Y, Watanabe M, Baba H, Takeya M. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer science 2013; 104:1237-44; PMID:23734742; https://doi.org/10.1111/cas.12212
- Cavnar MJ, Turcotte S, Katz SC, Kuk D, Gonen M, Shia J, Allen PJ, Balachandran VP, D'Angelica MI, Kingham TP, et al. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann Surg Oncol 2017; 24(7):1835-1842; PMID:28213791; https://doi.org/10.1245/s10434-017-5812-8
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; https://doi.org/10.1126/science.1129139
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Eng J Med 2005; 353:2654-66; PMID:16371631; https://doi.org/10.1056/NEJMoa051424
- Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res 2014; 74:24-30; PMID:24220244; https://doi.org/10.1158/0008-5472.CAN-13-1196
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971-6; PMID:24264990; https://doi.org/10.1126/science.1240537
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer cell 2015; 28:690-714; PMID:26678337; https://doi.org/10.1016/j.ccell.2015.10.012
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29:482-91; PMID:19881547; https://doi.org/10.1038/onc.2009.356
- Nagasaki E, Takahara A, Koido S, Sagawa Y, Aiba K, Tajiri H, Yagita H, Homma S. Combined Treatment With Dendritic Cells and 5-fluorouracil Elicits Augmented NK Cell-mediated Antitumor Activity Through the Tumor Necrosis Factor-alpha Pathway. J Immunother 2010; 33:467-74; PMID:20463601; https://doi.org/10.1097/CJI.0b013e3181d36726
- Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, Carnaghi C, Doci R, Rosati R, Montorsi M, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res 2007; 13:3831-9; PMID:17606714; https://doi.org/10.1158/1078-0432.CCR-07-0366
