ABSTRACT
Therapeutic strategies aiming for the induction of an effective immune response at the tumor site can be severely hampered by the encounter of an immunosuppressive microenvironment. We investigated here the potential of concerted costimulation by tumor-directed antibody-fusion proteins with B7.1, 4–1BBL and OX40L to enforce bispecific antibody-induced T cell stimulation in presence of recognized immunosuppressive factors including IL-10, TGF-β, indoleamine 2,3-dioxygenase (IDO), PD-L1 and regulatory T cells. The expression and activity of these factors was demonstrated in the HT1080-FAP/PBMC co-culture setting, where individual and combined costimulation were still capable to enhance T cell stimulation, even though the general activation level was reduced. Additional blockade of TGF-ß or PD-1 resulted especially effective in further enhancing the degree of T cell activation. Here, best outcome was achieved by combined costimulation of targeted 4–1BBL and B7.1. Furthermore, their individual impact on the proliferation of naïve, memory and effector CD8+ and CD4+ T cell subsets, suggest the coverage of a comprehensive T cell response. Thus, our costimulatory antibody-fusion proteins show great potential to support T cell activation in adverse conditions dictated by the tumor microenvironment.
| Abbreviations | ||
| ScFv | = | single-chain fragment variable |
| TNFSF | = | tumor necrosis factor superfamily |
| EDG | = | endoglin |
| MAb | = | monoclonal antibody |
| FAP | = | fibroblast activation protein |
| IgSF | = | Immunoglobulin superfamily |
Introduction
In the context of cancer immunotherapy, modulating the antitumor immune response by interfering with the regulatory network of ligands and receptors of the TNF- and Ig- superfamily is attracting great attention.Citation1,2 In particular the approval of antagonistic antibodies against regulatory checkpoints (e.g. CTLA-4, PD-1, PD-L1) is considered a breakthrough in the field and also the development of agonistic antibodies against costimulatory receptors (e.g., 4–1BB, OX40, CD40, CD27, GITR) is intensively pursued.Citation3-5 To avoid the risk of systemic activity by focusing the activity to the tumor site, targeted approaches with antibody-fusion proteins directed against tumor associated antigens are being developed.Citation6 Antibody-mediated binding is expected to accumulate the costimulatory ligand at the tumor site, where it is presented on the tumor cell surface, mimicking the physiologic active transmembrane form of these proteins. Thus, the surrounding can be influenced, creating conditions that support and drive the development of an effective antitumor response. This becomes particularly important, since tumor development is known to be characterized by building up an immune hostile environment.Citation7 Besides evasion mechanisms that affect the tumor recognition by T cells and trafficking of T cells into the tumor, there are also immunosuppressive factors that can directly influence tumor infiltrating lymphocyte (TIL) activity. These include cytokines, (e.g., TGF-β and IL-10 that can suppress proliferation, cytokine release and cytotoxicity), enzymes (e.g., indoleamine-2,3- dioxygenase (IDO) that interferes metabolically, depleting and converting tryptophan into toxic metabolites), coinhibitory ligands (e.g., PD-L1 and PD-L2 that induce T-cell anergy or death) and the presence of immune suppressor cells (e.g., regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) that inhibit the immune reaction) (for review, see ref.Citation8). Inevitable, a dynamic interaction of the network of immunosuppressive factors created by the tumor and the regulatory network of the immune system aiming at an antitumor response has to take place. For the latter, members of the B7- and TNF-superfamily are known to have a pivotal role in T cell biology, controlling activation, differentiation and function of a T cell response in a context-dependent manner.Citation1 Thus, the immune system disposes of a highly elaborated regulatory spectrum that can be interfered and mechanistically exploited for therapeutic purpose.Citation2,9 Although the negative impact of the immunosuppressive factors on immunotherapeutic approaches in general is taken for granted the potential of counteracting particular conditions by costimulatory means remains to be explored.
We have reported previously improved antitumor activity in vitro and in vivo by a targeted approach in a model system, combining a bispecific antibody that retargets T cells to tumor cells with antibody-fusion proteins presenting different costimulatory ligands of the Ig- and TNF-superfamily.Citation10,11 Here, we could show differences in the proliferation of T cell subsets in response to costimulation by the ligands 4–1BBL, OX40L and B7.1 applied either individually or in combination. We further analyzed the expression and activity of immunosuppressive factors TGF-β, IL-10, IDO and Tregs in our co-culture system and demonstrated the potential of the combinatorial fusion protein setting to counteract these conditions, promoting T cell stimulation. Moreover, the additional blockade of TGF-β, IL-10 and IDO activity as well as combination with the CTLA-4 and PD-1 checkpoint inhibitors revealed further options for combinatorial strategies.
Results
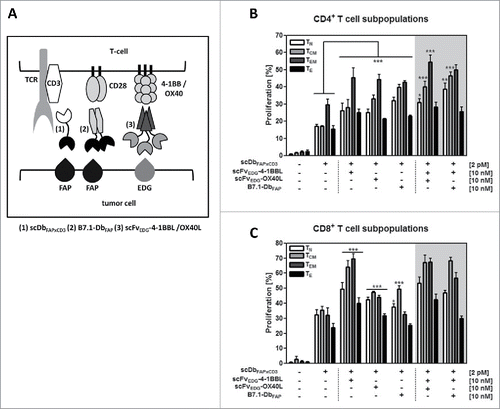
In our model system (), tumor cells expressing the fibroblast activation protein (FAP) and endoglin (EDG) (HT1080-FAP cell line) were co-cultured with PBMCs in presence of a FAP-directed bispecific antibody (scDbFAPxCD3) and EDG- or FAP-directed antibody-fusion proteins with costimulatory members of the TNFSF (scFvEDG-4–1BBL, scFvEDG-OX40L) and IgSF (B7.1-DbFAP), respectively.Citation11 The bispecific antibody binds to FAP and CD3, retargeting T cells to tumor cells, inducing polyclonal T cell stimulation in a tumor targeting-dependent, but MHC-independent manner. At suboptimal concentrations of the bispecific antibody, T cell stimulation can be further enhanced by the costimulatory activity of the tumor-directed antibody-fusion proteins, where individual or combinatorial effects can be monitored for example by T cell proliferation and cytokine release. We analyzed here the effect of defined costimulatory constellations on the proliferation response of different T cell subpopulations (,). Naïve, central memory, effector memory and effector CD4+ and CD8+ T cells, respectively, could be activated by the bispecific antibody. Costimulatory activity of scFvEDG-4–1BBL, scFvEDG-OX40L and B7.1-DbFAP, could enhance the proliferation of all CD4+ T cell subtypes. Here, in general, strongest proliferation was observed on CD4+ effector memory cells. Central memory and naïve CD4+ T cells were most effectively stimulated by the B7.1 fusion protein. These subpopulations also benefited from the combined costimulation of B7.1 with 4–1BBL or OX40L with 4–1BBL (). In terms of CD8+ T cell subpopulations, all of them, in particular the memory phenotypes could be effectively costimulated by 4–1BBL and to a lesser extent by OX40L. B7.1 was effective reinforcing the proliferation of naïve and central memory, but not of effector memory and effector CD8+ T cells. The activity of 4–1BBL was notably dominant and generally not further enhanced by combination with neither OX40L nor B7.1 (). Thus, differential influence on T cell subsets by the costimulatory fusion proteins and their combinations was shown, empathizing the role of 4–1BBL, especially in the CD8+ T cell context.
Figure 1. (A) Cartoon illustrating the model system of the co-culture (tumor cells/T-cells) with the bispecific antibody (scDbFAPxCD3) and the costimulatory fusion proteins (B7.1-DbFAP, scFvEDG-4–1BBL/OX40L). FAP: fibroblast activation protein, EDG: endoglin, TCR: T cell receptor. Proliferation of (B) CD4+ and (C) CD8+ T cell subpopulations in response to stimulation by the bispecific antibody and costimulatory antibody-fusion proteins. HT1080-FAP cells were co-cultured with CFSE-labeled PBMCs in presence of recombinant protein combinations for 6 d. Naïve (CD45RA+,CCR7+)(TN), central memory (CD45RA−,CCR7+)(TCM), effector memory (CD45RA−,CCR7−)(TEM) and effector (CD45RA+,CCR7−)(TE) CD4+ and CD8+ T cells were identified and proliferation measured by flow cytometry. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistic comparison refers either to the effect of the scDb alone or in case of costimulatory fusion protein combinations to the highest individual costimulatory effect. Gray background points out the combination of 2 costimulatory fusion proteins.
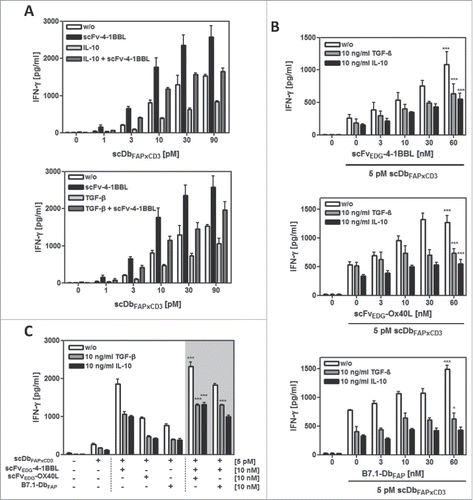
Next, we investigated the influence of the immunosuppressive factors IL-10 and TGF-β on the performance of the combinatorial setting. First, we measured the expression of these cytokines in the co-culture of HT1080-FAP and PBMCs with and without T cell stimulation via the bispecific antibody (scDbFAPxCD3) (). Both cytokines were observed to be already present in the unstimulated co-culture system. TGF-β was expressed at low levels by the HT1080-FAP cells. Here, the expression was not further increased by the addition of unstimulated or stimulated PBMCs. On the other hand, IL-10 was not expressed by the tumor cells, but secreted by unstimulated PBMCs, where IL-10 release was significantly enhanced by the bispecific antibody-mediated T cell stimulation. Blocking the cytokine activity with antagonistic IL-10R and TGF-β-specific antibodies, respectively, enhanced significantly the bispecific antibody-induced T cell stimulation in terms of IFN-γ release, thus demonstrating indirectly the immunosuppressive activity of the endogenous expressed IL-10 and TGF-β (). Consequently, the costimulatory effects we had observed in our model system so far were accomplished in an immunosuppressive environment. Indeed, we could show that the costimulatory effects of all 3 antibody-fusion proteins (scFvEDG-4–1BBL, scFvEDG-OX40L and B7.1-DbFAP) were further enhanced by blocking the IL-10 and TGF-β activity, respectively (). Only the IFN-γ release obtained by the combined costimulation of 4–1BBL with OX40L or B7.1 was not affected by the IL-10 activity blockade. However, combined costimulation still benefited from TGF-β neutralization, leading to the strongest overall effects. To assess the costimulatory capacity of the fusion proteins under more stringent conditions, exogenous cytokine was used. Addition of 10 ng/ml recombinant IL-10 and TGF-β, respectively, to the co-culture setting confirmed the immunosuppressive properties of these cytokines, since bispecific antibody-mediated IFN-γ release was reduced up to approximately 50% (). In both cases, costimulation by 10 nM 4–1BBL could efficiently restore the primary effect of the bispecific antibody (i.e. the effect in absence of recombinant IL-10/TGF-β) (). Furthermore, it could be shown, that in this case the counteracting costimulatory effect was fusion protein concentration-dependent (). Also costimulation by OX40L was able to enhance the bispecific antibody induced stimulation against the immunosuppressive effect of recombinant TGF-β and IL-10 (). Thus, in presence of an approximately 20-fold (IL-10) and 100-fold (TGF-β) higher basal level of immunosuppressive cytokine, the costimulation provided by the antibody-fusion proteins scFvEDG-4–1BBL and scFvEDG-OX40L could still restore and even exceed the primary activation effect of the bispecific antibody in absence of recombinant IL-10 and TGF-β (). In addition, TGF-β but not IL-10 was shown to reduce the cytotoxic potential of bispecific antibody-stimulated T cells in terms of granzyme B expression. Also in this case, the effect could be counteract by costimulation mediated by 4–1BB, either alone or even stronger in combination with OX-40 (Suppl. 4). Although costimulation was clearly effective in counteracting the immunosuppressive effect imposed by the environment, overall signal outcome remained in general reduced in comparison to the unrestricted costimulatory effect.
Figure 2. Expression and activity of endogenous IL-10 and TGF-β in the HT1080-FAP/PBMC co-culture setting. (A) Release of IL-10 and TGF-β into the co-culture supernatant (HT1080-FAP/PBMC unstimulated or stimulated) was determined after 24 h by sandwich-ELISA. (B) Immunosuppressive activity of IL-10 and TGF-β was demonstrated by blockade with antagonistic antibodies. T cells were stimulated via scDbFAPxCD3 in the HT1080-FAP/PBMC co-culture setting +/− anti-IL-10R mAb (10 µg/ml) and anti-TGF-β mAb (5 µg/ml), respectively. IFN-γ release was measured after 48 h by sandwich-ELISA. (C) Stimulation of T cells by the combinatorial setting of bispecific antibody and costimulatory fusion proteins +/− blocking IL-10 and TGF-β activity with 10 µg/ml anti-IL-10R mAb and 5 µg/ml anti-TGF-β mAb, respectively. IFN-γ release was measured after 48 h by Sandwich-ELISA. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Gray background points out the combination of 2 costimulatory fusion proteins.
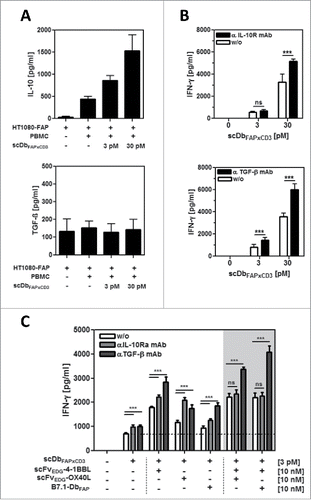
Figure 3. Effect of exogenous IL-10 and TGF-β on PBMC stimulation by fusion protein combinations in the HT1080-FAP/PBMC co-culture. HT1080-FAP cells were co-cultured with PBMCs +/− 10 µg/ml recombinant IL-10 or TGF-β in presence of (A) bispecific antibody titrated +/− 10 nM scFv-4–1BBL, (B) 5 pM bispecific antibody +/− costimulatory fusion protein titrated and (C) 5 pM bispecific antibody +/− 10 nM costimulatory fusion protein single or combined. After 48 h, IFN-γ concentration in the supernatant was measured in ELISA. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistic comparison refers to the corresponding effect of the scDb alone. Gray background points out the combination of 2 costimulatory fusion proteins.
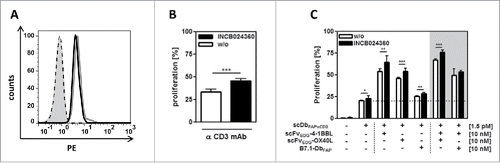
We also analyzed the expression and immunosuppressive effect of indolamin-2,3-dioxygenase (IDO) in the co-culture system. IDO-expression was clearly demonstrated in HT1080-FAP cells by intracellular flow cytometry analysis (). Blocking IDO-activity with the INCB024360 inhibitor led to enhanced anti-CD3 mAb-induced T cell proliferation, confirming the immunosuppressive activity of the endogenous IDO (). Hence, in the co-culture setting costimulatory effects of 4–1BBL, OX40L and B7.1 were achieved in presence of immunosuppressive IDO and these effects could be further enhanced by co-application of the IDO-inhibitor (). Thus, IDO was shown to contribute to the complex suppressive environment in our setting that was encountered efficiently by the costimulatory fusion proteins.
Figure 4. IDO expression and activity in the HT1080-FAP/PBMC co-culture setting. (A) IDO expression in HT1080-FAP cells was detected via flow cytometry by intracellular staining with FITC-conjugated anti-IDO mAb after 24 h co-culture with stimulated (0,2 µg/ml anti-CD3-mAb) (gray line) or unstimulated (black line) PBMC. Isotype control (dotted line), cells only (gray filled). (B) IDO activity inhibits PBMC proliferation. CFSE-labeled PBMCs in co-culture with HT1080-FAP cells were stimulated with 0,1 µg/ml cross-linked α-CD3 mAb for 6 d in presence or absence of 5 nM INCB024360. Proliferation was measured by flow cytometry. (C) Blocking IDO activity in the HT1080-FAP/PBMC co-culture enhances the stimulation induced by the combinatorial setting of recombinant fusion proteins. HT1080-FAP cells were incubated with combinations of bispecific antibody and costimulatory fusion proteins in presence of 5 nM INCB024360. CFSE-labeled PBMCs were added and after 6 d proliferation of PBMCs measured by flow cytometry. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Gray background points out the combination of 2 costimulatory fusion proteins.
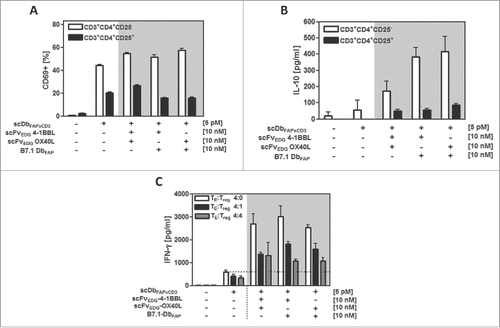
Another relevant immunosuppressive factor in the tumor microenvironment is the presence of regulatory T cells (Tregs). Here we investigated the costimulatory potential of our fusion protein combinations to influence the activation of Tregs and their suppressive activity. Isolated Tregs (CD4+CD25+) were activated by the bispecific antibody although to a much lesser extent than the fraction of T helper (CD4+CD25−) cells. The enhancement effect of costimulatory fusion protein combinations on the activation marker CD69 expression was only minor or absent on Tregs (). IL-10 expression by Tregs was supported to similar extent by all costimulatory fusion protein combinations, but also here the expression levels were clearly inferior to that induced on the CD4+ T helper cell population (). Thus, the support provided by costimulation for CD4+ T cell activation and IL-10 release is clearly less pronounced on Tregs than on CD4+ T helper cells. Furthermore, we investigated the costimulatory effect of antibody fusion protein combinations on the bispecific antibody-induced stimulation of CD4+ T helper cells in presence of activated Tregs at ratios of 4:1 and 4:4 (TE:Tregs) (). In presence of Tregs, costimulation was statistically significant and still clearly effective (enhancement factor 3–4), although in comparison to effector cells only (enhancement factor 4–5) the signal obtained was reduced. The combination of scFv-4–1BBL and scFv-OX40L, scFv-4–1BBL and B7.1-Db as well as scFv-OX40L and B7.1-Db showed similar potential to enhance the IFN-γ release by the effector cells. Thus, the combinatorial setting with antibody-fusion proteins was able to provide different costimulatory options that defy the suppressive activity of regulatory T cells.
Figure 5. Costimulatory potential of the combinatorial fusion protein setting in presence of Tregs. HT1080-FAP cells were incubated with the recombinant fusion proteins for 1 h. (A,B) Freshly isolated CD4+CD25−T cells and CD4+CD25+ Tregs were added and after 24 h activation measured by (A) CD69 expression via flow cytometry and (B) IL-10 release by ELISA. (C) Suppressive activity of activated and expanded Tregs on CD4+ T cells was assayed by adding them at the TE: Treg ratios of 4:0, 4:1 and 4:4. After 48 h supernatant was harvested and IFN-γ measured in ELISA. Graphics show mean ± SD, n = 3. Gray background points out the combination of 2 costimulatory fusion proteins.
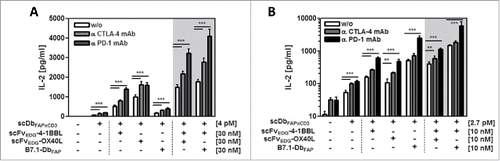
Moreover, we analyzed the possibility to further extent the immune stimulatory effect of the combinatorial setting of bispecific antibody and costimulatory fusion proteins by the addition of CTLA-4 and PD-1 checkpoint inhibitors. Activation of T cells was measured in terms of IL-2 release. Here, the release of IL-2 induced by the bispecific antibody and costimulated by the fusion proteins with 4–1BBL and OX40L, either individually or combined, was enhanced effectively in the range of factor 1.4 – 1.5 and 1.6 – 2.7 by combination with the antagonistic anti-CTLA-4 and the anti-PD-1 mAb, respectively (). In this assay, single B7.1-mediated costimulation was reduced due to target-directed competition with the bispecific antibody. Nevertheless, co-administration of scFv-4–1BBL led to a synergistic effect, triplicating the signal obtained by single scFv-4–1BBL costimulation, which was further enhanced by factor 1.6 and 2.3 by additional CTLA-4 and PD-1 blockade, respectively. In general, dual costimulation was most effectively combined with PD-1 blockade and to less degree with CTLA-4 blockade. A similar, but less pronounced pattern was observed in the IFN-γ release assay (Suppl. 1). Next, we investigated if restimulation of activated T cells could also benefit from this combinatorial approach. Therefore, T cells were pre-stimulated with anti-CD3 mAb for 6 d before being introduced into the assay (). Also in this case, costimulation via scFv-4–1BBL, scFv-OX40L and in particular B7.1-Db was still effective individually and in combination. Additional combination with anti-CTLA-4 mAb further enhanced the signal of scFv-4–1BBL and scFv-OX40L by factor 1.4 – 2.0, but did not further increase the effect of B7-Db costimulation. On the other hand, combination with anti-PD-1 mAb was clearly more effective than with the anti-CTLA-4 mAb. Anti-PD-1 mAb strongly improved the effect of all costimulatory constellations with a considerably stronger enhancement (factor 2.8 – 4.8) than that observed in the case with primary stimulated PBMCs. Hence, stimulation of resting T cells as well as reactivation of T cells can be achieved efficiently by the proposed combinatorial setting of bispecific antibody and costimulatory antibody fusion proteins. Here the pairing of scFv-4–1BBL and B7.1-Db in combination with the PD-1 checkpoint inhibitor resulted particularly effective.
Figure 6. Stimulation of resting (A) and activated (B) T cells by the combinatorial fusion protein setting in presence of CTLA-4 and PD-1 checkpoint inhibitors. HT1080-FAP cells were incubated with the combinatorial setting of bispecific antibody and costimulatory fusion proteins for 1h, followed by the addition of 10 µg/ml anti-CTLA-4 or anti-PD-1 mAb, respectively. Then, resting or previously activated (0.1 µg/ml cross-linked anti-CD3 mAb for 6 days) PBMCs were added. After 24 h IL-2 concentration was determined by ELISA. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Gray background points out the combination of 2 costimulatory fusion proteins.
In summary, the combinatory setting composed of a bispecific antibody (scDbFAPxCD3) and costimulatory antibody-fusion proteins (scFvEDG-4–1BBL, scFvEDG-OX40L and B7.1-DbFAP) was shown to perform efficiently at diverse costimulatory constellations in presence of the immunosuppressive factors IL-10, TGF-β, IDO and regulatory T cells. The capacity of costimulation to counteract particular suppressive factors was demonstrated, as well as its potential to contribute to further combination strategies (e.g., with checkpoint inhibitors) aiming to improve the immune response at the tumor site.
Discussion
Within the costimulatory network, the activation of the receptors CD28, 4–1BB and OX40 have shown promising antitumor potential in different mouse models, whereby the activity of diverse receptor combinations resulted more effective than their individual activity.Citation12-14 It stands to reason that for further evaluation of costimulatory combinations not only mechanistic aspects at the molecular level, but also the immune cell type distribution and the influence of the conditions encountered in the microenvironment at the tumor site needs to be taken into consideration. Several studies in colorectal carcinoma have demonstrated a positive prognostic value for tumor infiltrating lymphocytes (TILs), where immune cell density was measured for CD3+, CD8+ and memory (CD45RO+) lymphocytes.Citation15-18 Memory T cells can be further specified in central memory (TCM) and effector memory (TEM), where TCM has been described to traffic to lymphoid tissues and exhibit higher proliferative capacity than TEM, that localize in peripheral tissues and respond rapidly exerting effector functions.Citation19 In renal cell carcinoma patients tumor infiltrating lymphocyte (TIL) subset distribution showed predominance of the TEM phenotype, followed by TE and TCM phenotype for CD8+ and CD4+ T cells, respectively.Citation20 In our fusion protein setting, we observed that TEM were strongly responsive to 4–1BBL costimulation, where the effect was clearly dominant on CD8+ TEM, and benefited additionally from the combination with OX40L on CD4+ TEM. On the other hand CD4+ TCM were highly responsive to B7.1, and the proliferation effect was further enhanced by the combination with 4–1BBL, which as a single agent was also very effective in costimulating CD8+ TCM. Thus, costimulation via 4–1BBL appears as favorite option to support the expansion of CD8+ memory T cells, while combination with OX40L or B7.1 further increases the impact on CD4+ T cell memory subsets. Combinations might be advantageous, since CD4+ T cells are not only involved in antitumor immunity orchestrating the response, but have shown also cytotoxic activity themselves.Citation21,22 Thus, costimulation by OX40 has been reported to drive cytotoxic CD4Th1 differentiation where clonal expansion was maximized by 4–1BB costimulation.Citation23 Bispecific antibodies have also shown to induce Granzyme B expression in CD4+ T cells, acquiring cytotoxic potential,Citation24 which could be further enhanced by B7.1, 4–1BB or OX40 costimulation.Citation25 In addition, it was observed that bispecific antibody-mediated tumor cell lysis by CD4+ and CD8+ T cells was more effective for the TEM than for the naïve T cell subset.Citation26 Thus, the combinatory setting of the bispecific antibody and the costimulatory antibody-fusion protein pairings scFv-4–1BBL/B7.1-Db and scFv-4–1BBL/scFv-OX40L showed suitable properties for retargeting and expansion of memory T cells to be found at the tumor site.
In the co-culture setting (HT1080-FAP/PBMC) endogenous expression and immunosuppressive activity was demonstrated for TGF-β, IL-10, IDO and PDL-1. This evidences the complexity of negative forces that T cells confront in presence of tumor cells, a situation widely recognized to hamper an appropriate immune response at the tumor site.Citation8 We observed that costimulation mediated by each of the antibody-fusion proteins was still capable to clearly enhance the stimulation induced by the bispecific antibody under these conditions, indicating the potential of the costimulatory receptors CD28, OX40 and 4–1BB to counter an unfavorable, mixed microenvironment. Since activation of T cells can potentially contribute either directly (e.g., induction of IL-10 or TGF-β by T cell subsets) or indirectly (e.g., IFN-γ induced expression of IDO and PD-L1 by tumor cells) to the presence of immunosuppressive factors, the situation becomes certainly an act of balance. We observed that IL-10 was expressed only by the PBMCs and that secretion was enhanced in response to CD3-mediated T cell activation (). Here, costimulation by B7, but not 4–1BBL nor OX40L supported IL-10 secretion (Suppl. 2). Blocking IL-10 activity increased T cell activation in terms of IFN-γ release by the individual but not the 4–1BB combined fusion protein-mediated costimulation (), emphasizing the importance of 4–1BB. Others have reported that 4–1BB costimulation of CD3/CD28 activated TDLN reduced IL-10 secretionCitation27 and studies with chimeric antigen receptor (CAR) T cells bearing endodomains of CD3ζ, CD28 and OX40, have shown that IL-10 secretion was induced by CD28 costimulation, but effectively repressed by co-signaling with OX40 in activated CD4+ helper and regulatory T cells.Citation28 Also, it has been reported that IL-10 can inhibit CD28 tyrosine phosphorylation, blocking CD28 signaling and thereby T cell proliferation at a low TCR signal, an effect that can be overruled by enforcing the strength of TCR triggering.Citation29 Thus, costimulation by 4–1BB, that can contribute also independently from CD28 to T cell stimulation might be here of advantage. Indeed, by adding recombinant IL-10 to our setting we observed that costimulation via 4–1BB was more effective than via CD28 ().
In terms of TGF-β, we observed basal expression levels by the HT1080-FAP cells that were not further increased by the presence of activated T cells (). 4–1BB costimulation showed the ability to promote T cell stimulation even in presence of high TGF-β levels, an effect that was further enhanced by the combination with OX40 and CD28 costimulation (). In detail, interplay of TGF-β and costimulation might affect T cells at different levels. Combined costimulation of CD28 and 4–1BB has been described to reduce the production of TGF-β by T cells,Citation30 abrogate TGF-β1-mediated suppression of CTL differentiationCitation31 and inhibit TGF-β1-mediated generation of regulatory T cells.Citation32 On the other hand TGF-β1 was reported to inhibit 4–1BB expression.Citation33 Here, in general terms, we could show that our combination strategy was significantly improved by the additional blockade of TGF-β, suggesting the possibility of an extended combination strategy. For this purpose, TGF-β-neutralizing antibodies or TGF-β receptor kinase inhibitors could be applied, which have already shown antitumor potential in preclinical studies and are being evaluated in clinical trials.Citation34 So far, treatment with an agonistic OX40 antibody in combination with a TGF-β receptor signaling inhibitor (SM16) has shown to result in tumor regression in diverse mouse models, supporting the idea of combination strategies with costimulatory TNFSF members.Citation35,36
HT1080-FAP cells showed clear IDO expression that was not further influenced by the presence of activated T cells (). Thus, although IFN-γ has been described to induce IDO expression,Citation37 in our setting the level of IFN-γ released by fusion protein-mediated T cell stimulation was apparently not sufficient to promote the negative IDO effect on T cell proliferation. Since all costimulatory constellations benefited from the addition of INCB024360 (Epacadostat), a second-generation IDO1 inhibitor that is currently being evaluated in several clinical trials with cancer patients,Citation38 this combination strategy holds potential as well.
Currently, many of the cancer clinical trial designs include the combination with checkpoint inhibitors,Citation37 illustrating the priority given these days to immune regulators as combination partners in therapeutic strategies. Addition of antagonistic CTLA-4 or PD-1 antibodies to the bispecific antibody/costimulatory fusion protein setting further enhanced the stimulatory effect on T cells for all fusion protein constellations. Blocking PD-1 resulted here especially effective, which might be attributed in part to the high PD-L1 expression on the tumor cell line (HT1080-FAP) that was even further increased by the presence of activated, IFN-γ secreting T cells (Suppl. 3). Strongest IL-2 release was achieved by the combination of PD-1 blockade with B7.1 and 4–1BB costimulation. Cooperation of CD28 costimulation and PD-1 blockade might here result specially favorable, since it was shown recently, that PD-1 suppresses T cell function primarily by inactivating CD28 signalingCitation39 and that costimulation via the CD28/B7 pathway was essential for an effective anti-PD-1 therapy in tumor bearing mice.Citation40 This axis could also be expected to be strengthened by the interaction of the B7.1 fusion protein and PD-L1, thus interfering with PD-1 suppression, as has been shown for B7.1-Fc fusion proteins.Citation41 This might enforce CD28-supported T cell activation, especially on prestimulated T cells with induced CD28, PD-1 and PD-L1 expression, overruling the effect of CTLA-4 blockade. On the other hand costimulation via 4–1BB was further enhanced in either constellation (with both checkpoint inhibitors) which is in accordance with the report of synergistic antitumor effects by combination therapies with agonistic 4–1BB and antagonistic PD-1or CTLA-4 mAbs in diverse mouse models.Citation42-44 Clinical studies with PD-1 and CTLA-4 checkpoint inhibitors have shown differences in their toxicity profile, that required the careful adjustment of dose and schedule for combinatorial applications.Citation45 Thus, the successful clinical translation of combinatorial approaches will not only depend on improved immune stimulation, but also on the feasibility to reach the right balance that warrants safety standards.
Here, in our targeted approach with the recombinant fusion proteins, costimulation via 4–1BB has shown promising properties to figure as key element for combinatory strategies either with other costimulatory molecules or alternative approaches, aiming for the support of relevant T cell subpopulations in an adverse tumor microenvironment.
Material and methods
Materials
Antibodies were purchased from BioLegend (anti-human CD3 PE/PerCPCy5.5, 317336; anti-human CCR7 PE, 353204; anti-human CD45RA APC, 304112; anti-human IL-10R(CD210), 308806; anti-human PD-1, 329912), KPL (goat anti-mouse IgG H+L; 01–10–06), Miltenyi Biotec (anti- human CD4 Vioblue, 130–097–333; anti-human CD8 PEVio770, 130–096–556; anti-human granzyme B PE, 130–101–351) and R&D Systems (anti-human CD3ϵ, MAB100; anti-human TGF-β1,2,3, MAB1835; anti-IDO Alexa488, IC6030G). IL-10 (11340103), IL-2 (11340025) and IFN-γ (11343536) were purchased from Immunotools and TGF-β (100–21) from Peprotech. INCB024360 (S7587) and rapamycin (S1039) were obtained from Sellek Chemicals and Absource Diagnostics, respectively. CellTrace™ CFSE cell proliferation kit (C34554) was obtained from Life Technologies. Human IFN-γ (DY285), IL-2 (DY202), IL-10 (DY217B) and TGF-β (DY240) DuoSet® ELISA kits were purchased from R&D Systems. CD4+CD25+ regulatory T cell isolation kit (130–091–301), Treg expansion kit (130–095–345) and TexMACS Medium (130–097–196) were purchased from Miltenyi. HT1080-FAP cells (stable transfectants with human FAP) were kindly provided by Prof. Klaus Pfizenmaier (IZI) and cultured in RPMI 1640 (Life Technologies, 11875), 5% FBS (PAN Biotech, 3302-P121707), supplemented with 200 µg/ml of G418 (Sigma, G8168). Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coat of healthy donors (Klinikum Stuttgart, Germany) by Ficoll density gradient centrifugation (Lymphocyte Separation Medium 1077, Promocell, C-44010) and cultivated in RPMI 1640, 10% FBS.
Generation, purification and characterization of the bispecific antibody and the costimulatory antibody fusion proteins had been described previously.Citation11
T cell proliferation assays
Two × 104 HT1080-FAP cells/well were seeded in 96-well-plates. PBMC were thawed, incubated for 2 h for monocyte plastic-adherence and remaining cells in suspension transferred to a fresh flask for overnight culture. The next day, PBMCs were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) at 625 nM/1 × 106 cells /ml following the instructions of the manufacturer. HT1080-FAP cells were incubated for 1 h with the bispecific antibody +/− the corresponding antibody-fusion proteins before addition of 2 × 105 PBMCs/well. After 6 days, cells were stained with fluorescence labeled anti-cell surface marker antibodies and proliferation of cell populations measured in a MACSQuant flow cytometer (Miltenyi, Bergisch-Gladbach, Germany). Data was analyzed using FlowJo (Tree Star, Ashland, USA).
Subsets of naïve (TN), central memory (TCM), effector memory (TEM) and effector (TE) T cells were identified according to the cell-surface marker panel proposed for standardized human immunophenotyping for the human immunology project.Citation46 Accordingly, T cell (CD4+ or CD8+) subsets were identified as follows: naïve: CD45RA+ CCR7+, central memory: CD45RA− CCR7+, effector memory: CD45RA-CCR7−, effector: CD45RA+ CCR7−.
Cytokine release assays
Two × 104 HT1080-FAP cells/well were seeded in 96-well-plates. In parallel, PBMCs were thawed and cultured overnight. The next day, HT1080-FAP cells were preincubated for 1 h with the recombinant fusion proteins before the addition of blocking monoclonal antibody, cytokines or inhibitors, according to the corresponding assay design. After 1 h incubation, 2 × 105 PBMCs/well were added. Supernatants were harvested after 24 or 48 h and concentration of IL-2 or IFN-γ, respectively, determined by sandwich ELISA.
Regulatory T cell assays
Regulatory T cells were separated from freshly isolated PBMCs using the CD4+CD25+ regulatory T cell isolation kit. For Treg activation studies, 2 × 105 cells of the isolated fractions of CD4+CD25+ and CD4+CD25− T cells were directly applied to the co-culture assay with HT1080-FAP cells, preincubated for 1 h with the respective fusion proteins. After 24 h, T cell activation was analyzed measuring CD69 expression by flow cytometry and after 48 h IL-10 release was determined by sandwich-ELISA. To analyze the suppressive activity of Tregs, Tregs were first isolated as described above and then consecutively expanded for 2 weeks with the Treg expansion kit (Miltenyi) in TexMACS Medium supplied with 500 U/ml IL-2 and 100 µg/ml rapamycin, according to the instructions of the manufacturer. CD4+CD25− T cells separated during the Treg isolation process were frozen and reposited for further studies with Tregs of the same donor. For Treg suppressor studies, the setting of cytokine release was performed as indicated above, in which PBMCs were replaced by 2 × 105 CD4+CD25− T cells/well, either alone or in combination with 5 × 104 or 2 × 105 Treg cells/well, respectively. Suppressor activity was detected in terms of decreased IFN-γ release.
Statistical analysis
Unless otherwise stated, all data are represented as mean ± SD of 3 independent experiments. Block shift correction was performed according to the formula: X’n = Xn – (Yn – Y) with X’n being the corrected value of X from the experiment n, Y the average of the X values from all experiments performed and Yn the average of the duplicate values of X from experiment n. Statistical significance was determined using one-way ANOVA followed by Tukey's post test (Graphpad Prism, Graphpad Software Inc., La Jolla, USA). P values below 0.05 were considered statistically significant (*** P < 0.001, ** P < 0.01, * P < 0.05).
Disclosure of interest
The authors report no conflict of interest.
supplimentary_files.zip
Download Zip (483.5 KB)Funding
This work was supported by the German Cancer Aid (Grant 110679).
References
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227-42. doi:10.1038/nri3405. PMID:23470321
- Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15(8):457-72. doi:10.1038/nrc3973. PMID:26205340
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432-3. doi:10.1126/science.342.6165.1432. PMID:24357284
- Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology 2014;3(1):e27297. doi:10.4161/onci.27297. PMID:24701370
- Callahan MK, Postow MA, Wolchok JD. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44(5):1069-78. doi:10.1016/j.immuni.2016.04.023. PMID:27192570
- Müller D. Antibody fusions with immunomodulatory proteins for cancer therapy. Pharmacol Ther. 2015;154:57-66. doi:10.1016/j.pharmthera.2015.07.001. PMID:26145167
- Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61-73. doi:10.1016/j.immuni.2013.07.005. PMID:23890064
- Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4(7):e1016700. doi:10.1080/2162402X.2015.1016700. PMID:26140242
- Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12(2):147-68. doi:10.1038/nrd3930. PMID:23334208
- Hornig N, Kermer V, Frey K, Diebolder P, Kontermann RE, Müller D. Combination of a bispecific antibody and costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. J Immunother. 2012;35(5):418-29. doi:10.1097/CJI.0b013e3182594387. PMID:22576347
- Hornig N, Reinhardt K, Kermer V, Kontermann RE, Müller D. Evaluating combinations of costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. Cancer Immunol Immunother. 2013;62(8):1369-80. doi:10.1007/s00262-013-1441-7. PMID:23715927
- Li G, Wu X, Zhang F, Li X, Sun B, Yu Y, Yin A, Deng L, Yin J, Wang X. Triple expression of B7-1, B7-2 and 4-1BBL enhanced antitumor immune response against mouse H22 hepatocellular carcinoma. J Cancer Res Clin Oncol. 2011;137(4):695-703. doi:10.1007/s00432-010-0905-9. PMID:20563597
- Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38(9):2499-511. doi:10.1002/eji.200838208. PMID:18792403
- Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgström P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116(6):934-43. doi:10.1002/ijc.21098. PMID:15856473
- Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654-66. doi:10.1056/NEJMoa051424. PMID:16371631
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960-4. doi:10.1126/science.1129139. PMID:17008531
- Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944-51. doi:10.1200/JCO.2008.19.6147. PMID:19858404
- De la Cruz-Merino L, Henao Carrasco F, Vicente Baz D, Nogales Fernández E, Reina Zoilo JJ, Codes Manuel de Villena M, Pulido EG. Immune microenvironment in colorectal cancer: a new hallmark to change old paradigms. Clin Dev Immunol. 2011;2011:174149. doi:10.1155/2011/174149. PMID:22162710
- Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14(1):24-35. doi:10.1038/nri3567. PMID:24336101
- Attig S, Hennenlotter J, Pawelec G, Klein G, Koch SD, Pircher H, Feyerabend S, Wernet D, Stenzl A, Rammensee HG, et al. Simultaneous infiltration of polyfunctional effector and suppressor T cells into renal cell carcinomas. Cancer Res. 2009;69(21):8412-9. doi:10.1158/0008-5472.CAN-09-0852. PMID:19843860
- Dobrzanski MJ. Expanding roles for CD4T cells and their subpopulations in tumor immunity and therapy. Front Oncol. 2013;3:63. doi:10.3389/fonc.2013.00063. PMID:23533029
- Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y, et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med. 2012;209(11):2113-26. doi:10.1084/jem.20120532. PMID:23008334
- Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Ménoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187(7):3555-64. doi:10.4049/jimmunol.1101244. PMID:21880986
- Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T, et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43(8):1129-43. doi:10.1016/j.molimm.2005.07.034. PMID:16139892
- Hornig N. Combinations of costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy [dissertation]. Stuttgart (Germany): University of Stuttgart; 2013.
- Kischel R, Hausmann S, Klinger M, Baeuerle PA, Kufer P. Effector memory T cells make a major contribution to redirected target cell lysis by T cell-engaging BiTE antibody MT110. Poster presented at: AACR Annual Meeting; 2009 April; Denver, USA.
- Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003;63(10):2546-52. PMID:12750278
- Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1(4):458-66. doi:10.4161/onci.19855. PMID:22754764
- Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28co-stimulation pathway. Eur J Immunol. 2000;30(6):1683-90. doi:10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. PMID:10898505
- Kim YJ, Broxmeyer HE. Therapeutic potential of 4-1BB (CD137) as a regulator for effector CD8(+) T cells. J Hematother Stem Cell Res. 2001;10(4):441-9. doi:10.1089/15258160152509064. PMID:11522228
- Kim YJ, Stringfield TM, Chen Y, Broxmeyer HE. Modulation of cord blood CD8+ T-cell effector differentiation by TGF-beta1 and 4-1BB costimulation. Blood. 2005;105(1):274-81. doi:10.1182/blood-2003-12-4343. PMID:15353478
- Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-γ. PLoS One. 2012;7(8):e42459. doi:10.1371/journal.pone.0042459. PMID:22870329
- Kim YJ, Han MK, Broxmeyer HE. 4-1BB regulates NKG2D costimulation in human cord blood CD8+ T cells. Blood. 2008;111(3):1378-86. doi:10.1182/blood-2007-01-069450. PMID:18024793
- Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-β pathway for cancer therapy. Clin Cancer Res. 2012;18(17):4514-21. doi:10.1158/1078-0432.CCR-11-3224. PMID:22711703
- Garrison K, Hahn T, Lee WC, Ling LE, Weinberg AD, Akporiaye ET. The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol Immunother. 2012;61(4):511-21. doi:10.1007/s00262-011-1119-y. PMID:21971588
- Triplett TA, Tucker CG, Triplett KC, Alderman Z, Sun L, Ling LE, Akporiaye ET, Weinberg AD. STAT3 Signaling Is Required for Optimal Regression of Large Established Tumors in Mice Treated with Anti-OX40 and TGFβ Receptor Blockade. Cancer Immunol Res. 2015;3(5):526-35. doi:10.1158/2326-6066.CIR-14-0187. PMID:25627655
- Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016;37(3):193-207. doi:10.1016/j.it.2016.01.002. PMID:26839260
- Vacchelli E, Aranda F, Eggermont A, Sautès-Fridman C, Tartour E, Kennedy EP, Platten M, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3(10):e957994. doi:10.4161/21624011.2014.957994. PMID:25941578
- Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428-1433. doi:10.1126/science.aaf1292. PMID:28280247
- Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, et al. Rescue of exhausted CD8T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355(6332):1423-1427. doi:10.1126/science.aaf0683. PMID:28280249
- Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191(5):2829-36. doi:10.4049/jimmunol.1202777. PMID:23918985
- Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, Logronio K, Tu GH, Tsaparikos K, Li X, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2014;3(2):149-60. doi:10.1158/2326-6066.CIR-14-0118. PMID:25387892
- Azpilikueta A, Agorreta J, Labiano S, Pérez-Gracia JL, Sánchez-Paulete AR, Aznar MA, Ajona D, Gil-Bazo I, Larrayoz M, Teijeira A, et al. Successful Immunotherapy against a Transplantable Mouse Squamous Lung Carcinoma with Anti-PD-1 and Anti-CD137 Monoclonal Antibodies. J Thorac Oncol. 2016;11(4):524-36. doi:10.1016/j.jtho.2016.01.013. PMID:26845193
- Kocak E, Lute K, Chang X, May KF Jr, Exten KR, Zhang H, Abdessalam SF, Lehman AM, Jarjoura D, Zheng P, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66(14):7276-84. doi:10.1158/0008-5472.CAN-05-2128. PMID:16849577
- Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. doi:10.1186/s40425-017-0218-5. PMID:28239469
- Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12(3):191-200. PMID:22343568
