ABSTRACT
Natural killer (NK) cells serve a critical role in the immune response against microbes and developing tumors. We have demonstrated that NK cells produce stimulatory cytokines (e.g., IFN-γ) in response to potent stimulation via immobilized IgG (to engage Fc receptors) and interleukin (IL)-12. CD25 is a component of the high-affinity IL-2R, which promotes NK cell activation in response to low doses of IL-2 such as those released by activated T cells. We hypothesized that stimulation of NK cells via IgG and IL-12 would enhance CD25 expression and promote NK cell anti-tumor activity in response to low-dose IL-2. It was confirmed that this dual stimulation strategy significantly enhanced NK cell CD25 expression compared to unstimulated cells or cells treated with IgG or IL-12 alone. Dual stimulated NK cells also were more responsive to low-dose IL-2. Dual stimulated NK cells subsequently treated with low-dose IL-2 (10 pg/mL) displayed enhanced intracellular signaling as indicated by increased pSTAT5 levels. IFN-γ production and cytotoxicity against K562 cells by NK cells stimulated with low-dose IL-2 was comparable to that of cells treated with high-dose IL-2 (10 ng/mL). Importantly, cells isolated from head and neck cancer patients receiving the mAb cetuximab and IL-12 on a clinical trial displayed increased CD25 expression following combination therapy compared to baseline. Altogether, these findings suggest that FcR and IL-12R co-stimulation induces expression of the high-affinity IL-2R and promotes NK cell anti-tumor activity.
Introduction
NK cells are large, granular lymphocytes that are capable of killing transformed cells without previous sensitization.Citation1 NK cells have the ability to directly target and kill virally-infected or transformed cells and also produce an array of stimulatory cytokines such as IFN-γ, MIP-1α, and TNF-α.Citation2 In order to modulate this robust activity, NK cells express a spectrum of activating and inhibitory surface receptors, including Fc receptors (FcR), the NKG2D receptor, a variety of killer cell immunoglobulin-like receptors (KIR), and multiple cytokine receptors.Citation3,4 Because NK cells are non-MHC-restricted and also recognize cells expressing stress-induced ligands such as MICA/B and ULBPs, they play a vital role in immune surveillance and control of tumor growth and metastasis.Citation5,6 For instance, it has been shown that in an NK cell-deficient mouse model, depletion of NK cells diminished natural killing activity and led to unrestricted tumor cell growth and invasion.Citation7 For these reasons, it is important to develop strategies to maximize NK cell activity in the treatment of cancer.
Antibody-dependent cell-mediated cytotoxicity (ADCC) is an essential NK cell effector function that is achieved via binding of the NK cell low-affinity FcγRIII (CD16) to the Fc portion of antibodies (Ab) bound to target cell-specific antigens.Citation8 FcR engagement by monoclonal antibodies (mAb) represents the dominant mechanism by which the immune system responds to Ab-coated tumor cells in vivo.Citation9 Moreover, our group has shown that stimulatory cytokines (e.g., IL-12) strongly enhance NK cell activity against Ab-coated tumor cells in vitro, in murine models, and in phase I clinical trials.Citation10-14 Given that other immune cells within the tumor microenvironment are a source of NK cell activating cytokines, it is important to investigate how NK cells engage other effector cells and determine how NK cells respond to mAb and cytokine stimulation in order to combat tumor development and aid in coordination of the adaptive immune response.
Most NK cells constitutively express a low- or intermediate-affinity IL-2 receptor (IL-2R); however, upon activation, synthesis of the IL-2Rα chain enables formation of an extremely sensitive, high-affinity IL-2R at the NK cell surface.Citation15 This high-affinity receptor is composed of three chains: the γc (CD132), β (CD122), and α (CD25). While the common γ chain is shared among related cytokine receptors (i.e., IL-4, IL-7, IL-9, IL-15, and IL-21); and the β chain is also a component of the IL-15 receptor, the α chain is unique to the IL-2R.Citation16,17 Previous studies have shown that increased CD25 expression leads to robust NK cell activation and proliferation in response to low-dose IL-2.Citation15 Further, it has been demonstrated that CD25 expression may be upregulated upon stimulation with cytokines such as IL-12.Citation15,18 Our laboratory has shown that dual stimulation of NK cells via the FcR and IL-12R leads to maximal activation of various signaling pathways (e.g., JAK/STAT and PI3-K/Akt) and promotes NK cell effector functions.Citation19 Thus, it was hypothesized that dual stimulation of NK cells via immobilized IgG and IL-12 would lead to increased expression of CD25 and ultimately promote NK cell responsiveness to low-dose IL-2. We now show that dual stimulation of NK cells via immobilized IgG and IL-12 (to engage Fc and IL-12 receptors, respectively) leads to a significant increase in CD25 expression, which enhances signaling via the high affinity IL-2R and facilitates NK cell anti-tumor activity in response to low-dose IL-2. This increase in NK cell activity and cytokine production not only has the capacity to mediate tumor cell destruction, but also may facilitate the recruitment of other immune effectors to the tumor microenvironment to mount an optimal anti-tumor response.
Results
CD25 is upregulated in response to FcR and IL-12R dual stimulation
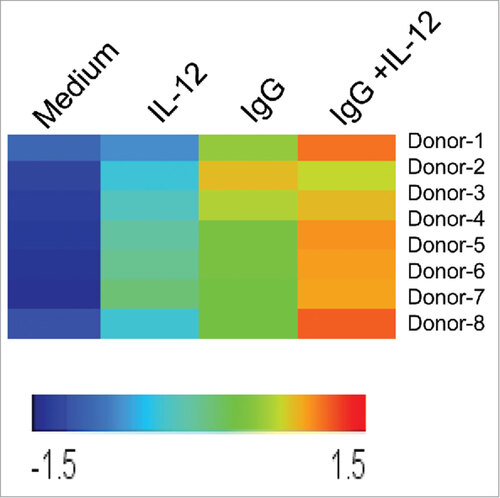
In order to examine the regulation of CD25 in activated NK cells, a powerful, clinically-relevant dual-stimulation strategy was employed to engage NK cell Fc and IL-12 receptors. In this study, NK cell CD25 expression was measured following stimulation via two pathways: FcR engagement via immobilized IgG, and IL-12R activation via the prototypic NK cell stimulatory factor, IL-12. First, NK cells from healthy donors (n = 8) were cultured in the presence of medium alone, IL-12, immobilized IgG, or the combination of IgG and IL-12. Following stimulation, the cells were harvested and CD25 expression was evaluated via microarray, Real-Time PCR, immunoblot, and flow cytometry. In the microarray experiment, CD25 expression was highest in 7/8 donors following dual-stimulation via immobilized IgG and IL-12 compared to medium or either treatment condition alone ().
Figure 1. NK cell CD25 transcript levels are upregulated following dual stimulation of Fc and IL-12 receptors. Healthy donor human NK cells were purified and cultured in medium alone or in the presence of immobilized IgG, IL-12, or IgG and IL-12 (n = 8). Following 24 h stimulation, cells were harvested and RNA was isolated, converted to cRNA, and hybridized to an Affymetrix Genechip, and CD25 expression levels were evaluated. Each row represents an individual donor, and each column represents one of the four treatment conditions in the NK cell assay. Relative hybridization intensities of CD25 expression are shown across all samples, and colors reflect the magnitude of relative expression of CD25. Brighter orange to red corresponds to higher expression and blue corresponds to lower expression.
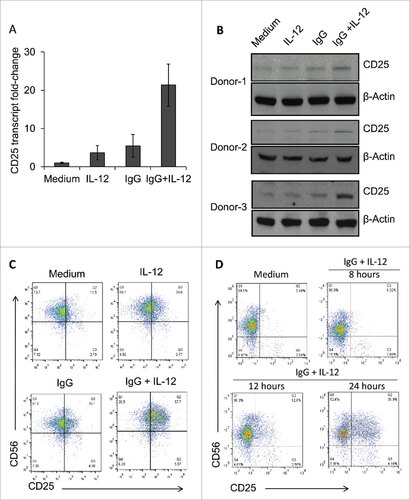
To confirm these findings, Real-Time PCR was performed to measure CD25 transcript levels. In dual-stimulated cells, CD25 transcript levels were elevated approximately 20-fold compared to cells cultured in medium alone and 4-fold greater than those stimulated via IL-12 or IgG alone (). Next, CD25 expression at the protein level was examined. Immobilized IgG and IL-12-stimulated NK cells exhibited the most robust CD25 expression in three separate donors (). Because CD25 expression on the surface of NK cells enables formation of the high-affinity IL-2R, it was important to determine the effects of this stimulation strategy on CD25 surface expression as well. NK cell surface CD25 expression was markedly upregulated in dual stimulated cells in comparison to control conditions, while surface expression of CD122 (IL-2Rβ) was not significantly altered ( C, Supplemental Figure 1). Further, it was shown that substantial CD25 expression could be observed following 24 hours of FcR and IL-12R stimulation (). Taken together, these results confirm that stimulation of NK cell Fc and IL-12 receptors leads to a significant increase in CD25 expression levels in activated NK cells.
Figure 2. Dual stimulation of NK cells via immobilized IgG and IL-12 leads to increased CD25 expression. (A) Healthy donor NK cells cultured in the presence of medium, IL-12, immobilized IgG, or IgG plus IL-12 were harvested and processed for Real-Time PCR analysis of CD25 (IL-2Rα) transcript levels. Results are given as fold increase in transcript over baseline (medium). (B) Following stimulation for 24 h, NK cells were harvested, and protein lysates were examined via western blot to detect CD25 expression. β-actin was used as a loading control. Three representative donors are shown. (C) NK cells cultured in the different treatment conditions were harvested and stained via flow cytometry to evaluate surface expression of CD56 and CD25. (D) NK cells were cultured in the presence of medium (control) or immobilized IgG plus IL-12, and were collected at sequential time points (8 h, 12 h, and 24 h) and stained via flow cytometry to evaluate surface expression of CD56 and CD25 over time.
Engagement of Fc and cytokine receptors results in increased expression of CD25
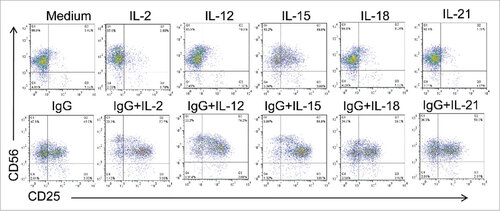
Previous studies have demonstrated that immune stimulatory cytokines such as IL-12, IL-15, and IL-18 promote NK cell activation and induce expression of the high-affinity IL-2R.Citation15,24 Thus, we investigated the impact of NK cell treatment with cytokines IL-2, IL-12, IL-15, IL-18, and IL-21 alone and in combination with immobilized IgG on CD25 surface expression. Across all conditions, dual stimulation with cytokines and immobilized IgG resulted in enhanced CD25 expression compared to the single cytokine stimuli alone. () Further, the most substantial CD25 expression was observed following stimulation via IgG and IL-2, IL-12, or IL-15. Given that numerous pre-clinical studies and clinical trials have investigated the efficacy of mAb and cytokine combination therapy for the treatment of cancer,Citation13,25 these findings affirm that FcR engagement coupled with cytokine stimulation may serve as a strategy to upregulate expression of the high-affinity IL-2R and promote anti-tumor activity.
Figure 3. Various combinations of FcR and cytokine stimulation enhance NK cell CD25 expression. Healthy donor human NK cells were isolated and cultured for 24 h in medium alone or in the presence of immobilized IgG, IL-2,-12, -15, -18, -21 or the combination of IgG and those cytokines. Cells were harvested and CD25 levels were examined by flow cytometry.
FcR and IL-12R dual stimulation alters the expression of various NK cell surface markers
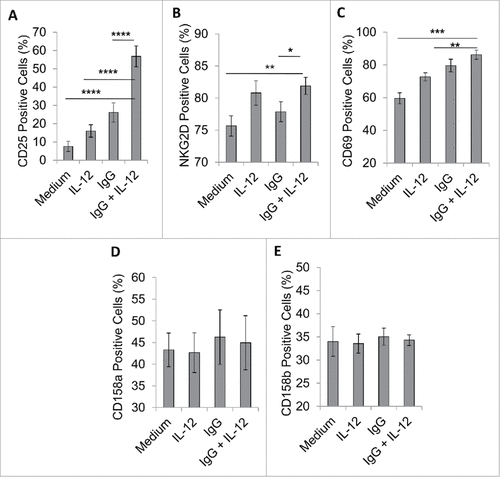
We next examined the expression of other activating and inhibitory NK cell surface markers following exposure to immobilized IgG and IL-12 in order to further characterize the effects of this treatment strategy. NK cells consistently exhibited a significant increase in CD25 expression, as expected (p < 0.0001, A). Also, compared to unstimulated cells, activated NK cells displayed significantly increased expression of NKG2D and CD69 (p < 0.01 and p < 0.001, respectively, ). However, it was determined that dual stimulation did not significantly alter the expression of NK cell inhibitory receptors CD158 a, or CD158b (also known as KIR2DL1 and KIR2DL2/L3) (). Thus, NK cells stimulated via immobilized IgG and IL-12 primarily upregulate expression of CD25 at the cell surface when activated in this manner.
Figure 4. FcR and IL-12R dual stimulation alters the expression of NK cell surface markers. NK cells were isolated and cultured in medium alone or in the presence of immobilized IgG, IL-12, or IgG and IL-12. (A) After 24 h, cells were collected and stained via flow cytometry to evaluate surface expression of CD56 and NK cell activation markers (A) CD25, (B) NKG2D and (C) CD69, or NK cell inhibitory markers (D) CD158 a, or (E) CD158b.
The IL-2R is the cytokine receptor most affected by FcR and IL-12R activation
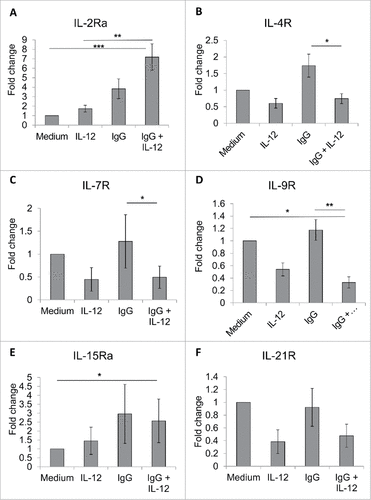
A family of cytokine receptors shares the common γ chain, including the IL-2R, IL-4R, IL-7R, IL-9R, IL-15R, and IL-21R, and the IL-2Rβ chain also is a component of the IL-15R. Given that dual stimulation significantly increases CD25 expression to impact the IL-2R, it was thought that perhaps other cytokine receptors may be affected by this stimulation strategy. Real-time PCR analysis was performed to examine expression of different NK cell cytokine receptors at the transcript level following stimulation via immobilized IgG and IL-12. In NK cells acquired from three healthy donors, it was demonstrated that though dual stimulation resulted in significantly increased IL-2Rα (p < 0.001) and IL-15Rα (p < 0.05) expression, transcript levels of the IL-4R, IL-7R, and IL-21R were not substantially altered compared to untreated NK cells (, , ). Conversely, transcript levels of IL-9R were significantly decreased with dual stimulation (, p < 0.05).
Figure 5. FcR and IL-12R dual stimulation variably modifies the expression of NK cell cytokine receptors sharing the common γ chain. NK cells were isolated and cultured in medium alone or in the presence of immobilized IgG, IL-12, or IgG and IL-12. After 24 h, cells were collected and processed for Real-Time PCR analysis of various cytokine receptor transcript levels. Results are given as average fold increase in transcript over baseline (medium) for three independent experimental donors. (A) IL-2Rα, (B) IL-4R, (C) IL-7R, (D) IL-9R (E) IL-15Rα, and (F) IL-21R.
Upregulation of CD25 enhances NK cell signaling and effector functions in response to low-dose IL-2
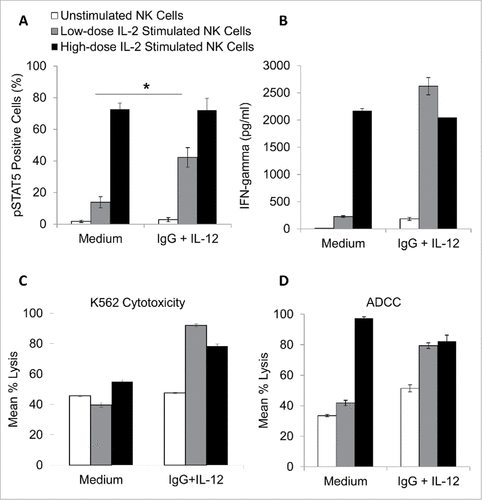
The effects of increased CD25 expression on NK cell responsiveness to IL-2 were next investigated. In order to evaluate NK cell signaling downstream of the IL-2R, phosphorylated STAT5 (pSTAT5) was measured by intracellular flow cytometry.Citation20 NK cells were cultured in medium alone or in the presence of immobilized IgG, IL-12, or the combination for 24 h. After washing and resting to allow cells to return to a state of baseline activation, NK cells were treated with medium alone or medium containing low-dose IL-2 (10 pg/mL) or high-dose IL-2 (10 ng/mL). It was demonstrated that pSTAT5 levels were negligible in untreated cells; however, stimulation via high dose (but not low dose) IL-2 resulted in increased pSTAT5. Importantly, following culture in the presence of immobilized IgG and IL-12, NK cells were much more responsive to low-dose IL-2 (compared to cells cultured in medium alone) and pSTAT5 levels approached that of NK cells stimulated with high-dose IL-2 (, p < 0.05). This response was not observed in cells pre-treated with immobilized IgG or IL-12 alone. NK cell functional activity also was examined in this system via measurement of NK cell cytokine production and lytic activity. Accordingly, it was shown that dual stimulated NK cells produced substantially more IFN-γ (the prototypic NK cell cytokine,Citation26) in response to low-dose IL-2 compared to cells that were not initially cultured in the presence of immobilized IgG and IL-12 (). Additionally, dual stimulated NK cells exhibited increased cytotoxicity against the K562 cell line in response to low-dose IL-2, as well as increased antibody-dependent cellular cytotoxicity against trastuzumab-coated SK-BR-3 cells (). As such, it was concluded that increased expression of CD25 on NK cells not only results in increased expression of the high-affinity IL-2R but also promotes responsiveness to low-dose IL-2 and facilitates IL-2-mediated NK cell effector functions.
Figure 6. NK cells pre-treated with immobilized IgG and IL-12 are more responsive to low-dose IL-2. Healthy donor NK cells were purified and cultured in the presence of medium alone, IL-12, immobilized IgG, or the combination of IgG and IL-12. After 24 hours, cells were collected, washed, and rested. Cells were left untreated (control) or treated with low dose IL-2 (10 pg/mL) or high dose IL-2 (10 ng/mL). (A) Cells were incubated for 15 minutes and were subsequently stained for pSTAT5 expression via intracellular flow cytometry. (B) For interferon-gamma analysis, NK cells were incubated in 96-well plates for 24 hours. At the end of the incubation period, culture supernatants were collected and analyzed by ELISA. Both figures depict the average of 3 independent donors ± SEM. (C) The lytic activity of untreated or immobilized IgG and IL-12 stimulated NK cells incubated with or without low dose IL-2 (10 pg/mL) or high dose IL-2 (10 ng/mL) was then assessed in a standard 4 hr chromium release assay using K562 tumor cells as targets at a 25:1 effector:target ratio or trastuzumab-coated SK-BR-3 cells at a 6.25:1 effector:target ratio (D). The percentage of lysis was calculated as previously described the graph depicts the results from one representative donor ± SD. # P < 0.05
Monoclonal antibody and cytokine combination therapy enhances CD25 expression in patients with cancer
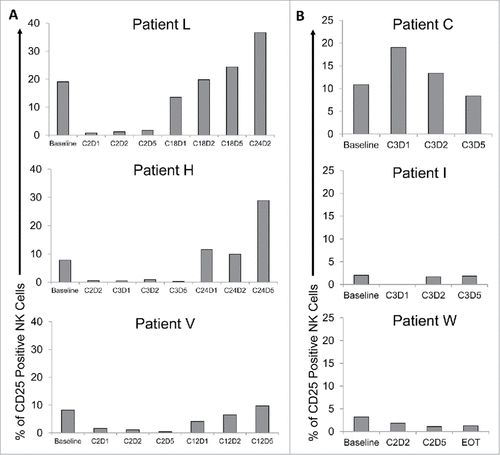
After determining that dual stimulation of NK cells via immobilized IgG and IL-12 leads to robust CD25 expression and increased NK cell activity in vitro, it was important to evaluate whether these findings might translate to the clinical setting. PBMC were isolated from head and neck cancer patients enrolled in a clinical trial in which they received cetuximab (an anti-HER1 mAb) in combination with IL-12 therapy. These cells were stained for CD56 (to identify NK cells) and CD25 to evaluate changes in expression prior to and after receiving mAb and cytokine therapy. Interestingly, in three representative patients who exhibited extended progression-free survival (PFS) ranging from 169 to 449 days, NK cell CD25 expression was elevated in later cycles following combination therapy compared to baseline (pre-therapy) levels (). However, in patients who did not respond to therapy and were required to go off-trial with a much shorter PFS (ranging from 53 to 70 days), CD25 expression levels remained stable or even decreased over the course of treatment (). Though many variables must be considered in a human system, and the data presented are preliminary, these results warrant further in vivo and prospective clinical studies regarding the role of NK cell CD25 expression in the immune response to immunotherapy for cancer.
Figure 7. Combination therapy with the monoclonal antibody cetuximab and IL-12 induces CD25 expression in patients with head and neck cancer. Patient blood was drawn at visits pre- and post-therapy (Cetuximab and IL-12 Phase I clinical trial; NCI protocol 8860; local protocol OSU 11010). Cryopreserved patient PBMC were thawed and analyzed via flow cytometry to measure CD56+ NK cell CD25 expression. Bars represent the percent CD25 positive NK cells in total PBMC at baseline and throughout various cycles (C) of cetuximab and IL-12 therapy (D1 is drawn pre-therapy, D2 after cetuximab administration, and D5 after patient has received cetuximab and IL-12 administration). (A) Three representative patients with extended PFS and elevated CD25 levels following therapy. (B) Three representative patients with short PFS and low to decreased CD25 levels following therapy. EOT = end of treatment.
Discussion
We have demonstrated that dual stimulation of NK cells via Fc and IL-12 receptors significantly increases CD25 expression, enhances IL-2-induced signal transduction and elicits robust NK cell effector functions in response to low-dose IL-2. Our group has demonstrated previously that the combination of immobilized IgG and IL-12 serves as a powerful stimulus to promote NK cell-mediated anti-tumor activity.Citation27 The present study has investigated the impact of this stimulatory strategy on NK cell cytokine signaling, specifically via the high-affinity IL-2R. The data presented herein demonstrates that increased expression of CD25 enables formation of the high-affinity IL-2R on the surface of NK cells, increases responsiveness to low-dose IL-2, and promotes NK cell pro-inflammatory activity. Since activated NK cells play an important role in the initiation of an adaptive immune response through production of stimulatory cytokines, targeting NK cell Fc and IL-12 receptors may enhance NK cell-mediated anti-tumor activity via the support of immune cell crosstalk. Further, the connection between innate and adaptive immunity may be strengthened through CD25-positive NK cells that are primed to mount an effective immune response upon exposure to T cell-derived IL-2.
IL-2 is known for its role in the development and differentiation of NK cells as well as in the regulation of NK cell functional activity.Citation17,28 Upon exposure to IL-2, NK cells exhibit increased cytotoxic activity and enhanced production of cytokines including IFN-γ.Citation29 Of note, it has been demonstrated that CD56bright NK cells express the high-affinity, heterotrimeric IL-2R; whereas CD56dim NK cells express the intermediate-affinity IL-2R and upregulate expression of the IL-2Rα chain only upon activation.Citation30-32 It has been shown in this study, as well as others, that induction of the high-affinity IL-2R leads to increased NK cell sensitivity to picomolar doses of IL-2.Citation32 This event not only promotes NK cell activity in response to therapeutic administration of low-dose IL-2, but also enhances responsiveness to endogenous IL-2 released by T cells into the surrounding microenvironment.Citation33,34 For example, Bihl et al. described a key role for antigen-primed CD4+ T cells in promoting NK cell activation, and it was demonstrated that IL-2 secreted by T cells leads to enhanced NK cell IFN-γ production in response to infection with Leishmania major.Citation35 Along those lines, a study by Horowitz et al. characterized NK cell responses following rabies virus vaccination and re-challenge.Citation36 It was shown that NK cells were critical for control of infection following vaccination, and this robust NK cell activity was dependent upon IL-2 released by memory T cells. The impact of adaptive immune influence on innate immune cell activity was highlighted in a study by Gasteiger et al. in which depletion of regulatory T cells (Treg) facilitated NK cell-CD4+ T cell crosstalk to promote NK cell expansion in response to T cell-derived IL-2.Citation37 Taken together, it may be concluded that stimulation of NK cell Fc and IL-12 receptors could promote NK cell-mediated anti-tumor activity through upregulation of the high-affinity IL-2R and may serve as a mechanism to support NK cell interactions with other immune effector cells.
Given that IL-2 is a potent stimulus for NK cell activation and proliferation, this cytokine has been used widely as a component of cancer immunotherapy. For example, Caligiuri et al. found that patients with advanced cancer who received prolonged infusions of low-dose IL-2 achieved increased circulating NK cell numbers, particularly within the CD56bright subset.Citation33 Similarly, in a study of patients with human immunodeficiency virus (HIV)-associated malignancies, daily treatment with IL-2 over a 90-day period resulted in NK cell expansion and controlled viral burden.Citation38 IL-2 also has been utilized to stimulate NK cells ex vivo prior to use in adoptive cellular therapy for melanoma and renal cell carcinoma.Citation39 Despite its anti-tumor effects, it is known that IL-2 may promote the expansion of regulatory T cell (Treg) populations that inhibit the functions of tumor-reactive lymphocytes. Nonetheless, a recent study by Su et al. demonstrated that compared to IL-15 (which does not bind IL-2Rα, and therefore does not have the same effect on Treg populations), IL-2 consistently elicited stronger anti-tumor immune reactivity mediated through the IL-2Rα.Citation40 This effect was demonstrated in lymphoreplete mice, wherein adoptively transferred IL-12-conditioned CD8+ T cells (Tc1, IL-2Rαhi) from pmel-1 T cell receptor transgenic mice preferentially expanded in response to treatment with an IL-2/mAbCD25 complex. This increase in T cell activity was shown to be a result of sustained signaling through the IL-2Rα chain even after removal of a cytokine stimulus. This was shown to be the result of IL-2Rα uniquely functioning as an extracellular IL-2 reservoir and mediating recycling of IL-2 to the cell surface. Furthermore, it was demonstrated that expression of the high-affinity IL-2R endowed donor T cells with an enhanced ability to respond to IL-2/mAb therapy and persist in a lymphoreplete setting in vivo. Given these findings, the authors called for subsequent investigation to develop methods to enhance IL-2Rα expression on immune effectors such as T cells and NK cells to promote IL-2-driven anti-tumor activity.
Though IL-2 effectively promotes immune cell activation, exogenous administration may lead to substantial toxicities including nausea, hypotension, pulmonary edema, and leukopenia, to name a few.Citation41 Thus, it is critical to determine optimal strategies to promote immune-mediated anti-tumor activity while minimizing adverse effects. Studies are underway to develop more effective IL-2 delivery vectorsCitation42 IL-2-Ab complexes called immunocytokines have been developed for tumor cell-specific targeting of IL-2, such as the huKS-IL2 conjugate that binds to EpCAM+ ovarian cancer cells and is fused to IL-2 to engage and activate NK cells in proximity of the developing tumor.Citation43 Additional methods include IL-2 structural modifications to increase IL-2 binding affinity to intermediate affinity receptors.Citation44 In the current study, it has been demonstrated that a clinically-proven treatment regimen combining FcR and IL-12R activators may serve as an alternative, powerful mechanism to upregulate IL-2Rα expression and increase NK cell responsiveness to IL-2.
The clinical utility of IL-2 as a therapeutic agent has been investigated in a variety of cancer models, and the importance of immune cell activation and high-affinity IL-2R induction is becoming increasingly clear. A few studies have described the ability of single cytokines and stimulatory cytokine combinations to upregulate NK cell CD25 expression.Citation15,18,Citation24,45 One such cytokine, IL-12 (first described as the NK cell stimulatory factor), has been shown to enhance high-affinity IL-2R expression on the surface of NK cellsCitation18 In addition, NK cell priming with other stimulatory cytokines including IL-15 and IL-18 leads to increased NK cell activation and enhanced CD25 expression.Citation15,24). Leong et al. demonstrated that treatment of human NK cells with the cytokine combination of IL-12, IL-15, and IL-18 mediated significant increases in surface expression of the high-affinity IL-2R. Further, following adoptive transfer into immunodeficient NOD-SCID-γc−/− mice, cytokine-stimulated NK cells were more sensitive to exogenous IL-2 therapy as indicated by enhanced proliferation and cytokine production compared to control NK cells that were not subjected to the triple cytokine pre-stimulation strategy. Of note, knowledge gained through studying the impact of cytokine therapy for cancer may be translated to the setting of infectious disease, as studies have shown that IL-12 and IL-18 function to upregulate CD25 and enhance NK cell IFN-γ production in response to viral and bacterial infection as wellCitation18,45 On the other hand, the role of mAb therapy and FcR stimulation in promoting immune cell responsiveness to IL-2 is not well understood. In a study by Anegón et al. in the late 1980's, it was demonstrated that FcR crosslinking led to increased expression of the high-affinity IL-2R on human NK cells.Citation46 More recent experimentation has indicated that CD16 engagement leads to upregulation of CD25 and other NK cell activation markers.Citation47 Our laboratory has demonstrated previously that stimulatory cytokines (e.g., IL-12) strongly enhance the NK cell response to FcR engagement mediated by Ab-coated tumor cells in vitro, in murine models, and in phase I clinical trials.Citation10,11,Citation14,25 Moreover, in a recent study examining the gene expression profile of dual-stimulated NK cells (following FcR and IL-12R engagement), it was determined that this stimulatory strategy resulted in expression of a unique set of genes, including genes encoding cytotoxicity receptors, intracellular signaling molecules, and cytokines that serve to mediate enhanced NK cell-mediated cytotoxicity and promote interactions with other immune cells within an inflammatory tumor microenvironment.Citation27 These studies provided a rationale for investigation of FcR activation and cytokine stimulation of NK cells.
In summary, we have employed a robust stimulation strategy to examine regulation of the high-affinity IL-2R on NK cells. It has been shown that FcR and IL-12R stimulation leads to increased CD25 expression and promotes NK cell activity in response to low-dose IL-2. Given that interactions between immune cells within the tumor microenvironment play a key role in cancer immunosurveillance, optimizing NK cell effector functions will support coordinated anti-tumor activity and improve the efficacy of anti-cancer therapies.
Materials and methods
Cytokines and antibodies
Recombinant human IL-12 (rhuIL-12) was provided by Genetics Institute, Inc. Polyclonal human IgG (huIgG) was purchased from Sigma-Aldrich Co. Recombinant human IL-15 and IL-18 were purchased from Peprotech, Inc. (Rocky Hill, NJ). Recombinant human interleukin-21 (rhuIL-21) was supplied by ZymoGenetics, Inc. Recombinant human IL-2 (rhuIL-21) was purchased from the Ohio State Medical Center pharmacy (Nation Drug Code 65483–116-07). The anti-HER2 mAb trastuzumab was provided by Genentech Inc. (San Francisco, CA).
Isolation of human NK cells
NK cells were isolated from fresh peripheral blood leukopacks (American Red Cross) by 30 minute incubation with RosetteSep cocktail (Stem Cell Technologies, 15025 C.1), followed by Ficoll-Hypaque density gradient centrifugation. NK cells were labeled with a CD56-phycoerythrin Ab (NKH1-RD1, Beckman Coulter) and checked for purity via flow cytometry. CD56+ NK cells were greater than 95% pure by flow cytometric analysis (FlowJo). Human NK cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated pooled human AB serum (HAB) (Sigma, H4522), 100 units/mL penicillin, and 100 mg/mL streptomycin (both from Life Technologies Inc., 15140-122).
In vitro co-stimulation and cytotoxicity assays
For NK cell FcR activation by immobilized IgG, wells of a 96-well flat-bottom plate were coated with 100 μg/mL of polyclonal huIgG in PBS overnight at 4 °C. Plates then were washed with cold PBS, and human NK cells were plated at 2 × 105 cells/well supplemented with IL-12 (10 ng/mL), as previously described.Citation10 Control wells consisted of NK cells in medium alone, immobilized IgG alone, or IL-12 alone. Following activation, cells were harvested and subjected to further analysis to evaluate CD25 expression. For subsequent functional analyses, cells were washed and rested for 6 hours, then treated with low dose (10 pg/mL) or high-dose IL-2 (10 ng/mL). 24 hours later, cell-free culture supernatants were collected and analyzed for IFN-γ content by ELISA (R&D Systems) as previously described.Citation10 or cells were harvested and intracellular pSTAT5 expression was examined as described previously.Citation20 For cytotoxicity assays, 51Cr-labeled K562 tumor cells were incubated with NK cells at various effector:target (E:T) ratios. For ADCC assays, 51Cr-labeled SK-BR-3 cells treated with 100 µg/mL trastuzumab for 1 hour were incubated with NK cells at various effector:target (E:T) ratios. Following a 4 hour incubation, supernatants were harvested and percent lysis was calculated as previously described.Citation21
Preparation of labeled RNA and microarray hybridization
After stimulation of NK cells via immobilized IgG, IL-12, or both, cellular RNA was obtained from individual donors (n = 8) and subjected to a cleanup protocol with RNeasy mini kits (Qiagen), according to the manufacturer's specifications. The quality of total RNA was assessed using an Agilent Bioanalyzer. cDNA was prepared and subjected to in vitro transcription in the presence of biotinylated nucleoside triphosphates. The biotinylated cRNA was fragmented to uniform sizes and the integrity of labeled cRNA was verified by gel electrophoresis. The Affymetrix GeneChip expression array U133 A was hybridized with each prepared cRNA target in duplicate, according to the manufacturer's instructions. The raw and processed microarray data has been made publicly available in GEO (GSE63038).
Real-Time PCR
IL-2Rα (CD25) expression values identified via microarray experiments were validated by Real-Time PCR. Following RNeasy purification of NK cell lysates from the immobilized IgG assay, 2 mg of total RNA was reverse transcribed and the resulting cDNA was used as a template to measure gene expression by Real-Time PCR using pre-designed primer/probe sets (Assays On Demand; Applied Biosystems) according to the manufacturer's recommendations, as previously described.Citation22 Human b-actin (Applied Biosystems, 4332645) was used as the internal control in each reaction well. Real-Time PCR data was analyzed using the ABI PRISM® 7900 Sequence Detection System.
Immunoblot Analysis
CD25 expression in NK cells was examined via immunoblot analysis. Lysates were prepared from human NK cells as previously describedCitation23 and assayed for the expression of CD25 (Santa Cruz Biotechnology) or β-Actin, as a loading control (Sigma-Aldrich, A5441).
Flow cytometry
Surface expression of NK cell CD25 was analyzed using an allophycocyanin-conjugated anti-CD56 mAb (Beckman-Coulter, IM2474U) as well as a phycoerythrin-conjugated anti-CD25 mAb (BD Biosciences, 341010), as previously described.Citation13 Other NK cell surface markers including CD69 (PeCy7, 557745), NKG2D (APC, 558071), CD122 (PE, 557323) CD158 a (FITC, 556062), CD158b (PE, 559785), and CD158 e1/e2 (PE, 556063) were examined (all from BD). The percentage of positively staining cells and mean fluorescence intensity were calculated within the specified cell population. The anti-pSTAT5 mAb (APC, 612599) used to examine phosphorylation of STAT5 was purchased from BD, as well.
Statistical analysis
Analyses of NK cell activation marker surface expression and pSTAT5 levels were performed using a random effects mixed model and student t-tests, while multiple comparisons were adjusted by Holm's method. Statistical analyses were performed using SAS 9.4 software, and the level of statistical significance was set at 0.05.
2017ONCOIMM0504R-f01-z-4c.pptx
Download MS Power Point (1.9 MB)Acknowledgments
The authors thank The Ohio State University Comprehensive Cancer Center (OSUCCC) Microarray Core Facility, the OSUCCC Real-Time Core Facility, and the OSUCCC Analytical Cytometry Shared Resource for providing equipment and assisting in data collection.
References
- Lanier LL, Phillips JH, Hackett J, Tutt, M, and Kumar V. Natural killer cells: Definition of a cell type rather than a function. J Immunol. 1986;137:2735-39.
- Badgwell B, Parihar R, Magro C, Dierksheide J, Russo T, and Carson WE 3rd. Natural killer cells contribute to the lethality of a murine model of Escherichia coli infection. Surgery. 2002;132:205-12.https://doi.org/10.1067/msy.2002.125311
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, R. Biassoni MC, and Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001;19:197-223.https://doi.org/10.1146/annurev.immunol.19.1.197
- Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359-93.https://doi.org/10.1146/annurev.immunol.16.1.359
- Lanier0 LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015;3:575-82.https://doi.org/10.1158/2326-6066.CIR-15-0098
- Hallett WH, and Murphy WJ. Positive and negative regulation of Natural Killer cells: Therapeutic implications. Semin Cancer Biol. 2006;16:367-82.https://doi.org/10.1016/j.semcancer.2006.07.003
- Kim S, Iizuka K, Aguila HL, Weissman IL, and Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000 97:2731-6.https://doi.org/10.1073/pnas.050588297
- Leibson PJ. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655-61.https://doi.org/10.1016/S1074-7613(00)80441-0
- Clynes RA, Towers TL, Presta LG, and Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443-6.https://doi.org/10.1038/74704
- Parihar, R., Dierksheide J, Hu Y, and Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983-92.https://doi.org/10.1172/JCI0215950
- Jaime-Ramirez, AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, Parihar R, Karpa V, Papenfuss TL, LaPerle KM, et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-γ production. J Immunol. 2011;186:3401-09.https://doi.org/10.4049/jimmunol.1000328
- Roda, JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, and Carson WE. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6419-28.https://doi.org/10.1158/1078-0432.CCR-07-0865
- Parihar, R., Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, VanBuskirk AM, Magro CM, Young DC, Shapiro CL, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027-37.https://doi.org/10.1158/1078-0432.CCR-04-0265
- Bekaii-Saab, TS, Roda JM, Guenterberg KD, Ramaswamy B, Young DC, Ferketich AK, Lamb TA, Grever MR, Shapiro CL, and Carson WE. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8:2983-91.https://doi.org/10.1158/1535-7163.MCT-09-0820
- Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, and Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transplant. 2014;20:463-73.https://doi.org/10.1016/j.bbmt.2014.01.006
- Smith KA. The interleukin 2 receptor. Adv. Immunol. 1988;42:165-79.https://doi.org/10.1016/S0065-2776(08)60844-5
- Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595-601.https://doi.org/10.1038/nri1901
- Lee SH, Fragoso MF, and Biron CA. Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J. Immunol. 2012;189:2712-6.https://doi.org/10.4049/jimmunol.1201528
- Kondadasula, SV, Roda JM, Parihar R, Yu, J, Lehman A Caligiuri MA, Tridandapani S, Burry RW, and Carson WE 3rd. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood. 2008:111:4173-83.https://doi.org/10.1182/blood-2007-01-068908
- Martin del Campo SE, Levine KM, Mundy-Bosse BL, Grignol VP, Fairchild ET, Campbell AR, Trikha P, Mace TA, Paul BK, Jaime-Ramirez AC, et al. The Raf Kinase Inhibitor Sorafenib Inhibits JAK-STAT Signal Transduction in Human Immune Cells. J Immunol. 2015:195:1995-2005.https://doi.org/10.4049/jimmunol.1400084
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, and Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur. J. Immunol. 2001;31:3016-25.https://doi.org/10.1002/1521-4141(2001010)31:10%3c3016::AID-IMMU3016%3e3.0.CO;2-J
- Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, and Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443-51.https://doi.org/10.1006/meth.2001.1266
- Jaime-Ramirez AC, McMichael EL, Kondadasula S, Skinner CC, Mundy-Bosse BL, Luedke E, Jones NB, Mani A, Roda J, Karpa V, et al. NK Cell-Mediated Antitumor Effects of a Folate-Conjugated Immunoglobulin Are Enhanced by Cytokines. Cancer Immunol. Res. 2016;4:323-36.https://doi.org/10.1158/2326-6066.CIR-15-0168
- Mirjačić Martinović K, Babović N. Džodić R, Jurišić V, Matković S, and Konjević G. Favorable in vitro effects of combined IL-12 and IL-18 treatment on NK cell cytotoxicity and CD25 receptor expression in metastatic melanoma patients. J. Transl. Med. 2015;13:120.https://doi.org/10.1186/s12967-015-0479-z
- Luedke E, Jaime-Ramirez AC, Bhave N,Roda J, Choudhary MM, Kumar B, Teknos TN, and Carson WE. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery. 2012;152:431-40.https://doi.org/10.1016/j.surg.2012.05.035
- Paolini R, Bernardini G, Molfetta R, and Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26:113-20.https://doi.org/10.1016/j.cytogfr.2014.11.003
- Campbell AR, Regan K, Bhave N, Pattanayak A, Parihar R, Stiff AR, Trikha P, Scoville SD, Liyanarachchi S, Kondadasula SV, et al. Gene expression profiling of the human natural killer cell response to Fc receptor activation: unique enhancement in the presence of interleukin-12. BMC Med Genomics. 2015;8:66.https://doi.org/10.1186/s12920-015-0142-9
- Becknell B, and Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005;86:209-39.https://doi.org/10.1016/S0065-2776(04)86006-1
- Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, and Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J. Exp. Med. 1984;160:1147-69.https://doi.org/10.1084/jem.160.4.1147
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461-9.https://doi.org/10.1182/blood-2007-09-077438
- Nagler A, Lanier LL, and Phillips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J. Exp. Med. 1990;171:1527-33.https://doi.org/10.1084/jem.171.5.1527
- Farag SS, and Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123-37.https://doi.org/10.1016/j.blre.2005.10.001
- Caligiuri MA. Low-dose recombinant interleukin-2 therapy: rationale and potential clinical applications. Semin. Oncol. 1993;20:3-10.
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, and Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood. 2003. 101:3052-57.https://doi.org/10.1182/blood-2002-09-2876
- Bihl F, Germain C, Luci C, and Braud VM. Mechanisms of NK cell activation: CD4(+) T cells enter the scene. Cell. Mol. Life Sci. 2011;68:3457-67.https://doi.org/10.1007/s00018-011-0796-1
- Horowitz, A, Behrens RH, Okell L, Fooks AR, and Riley EM. NK cells as effectors of acquired immune responses: Effector CD4+ T cell-dependent activation of NK cells following vaccination. J. Immunol. 2010;185:2808-18.https://doi.org/10.4049/jimmunol.1000844
- Gasteiger G, Hemmers S, Firth MA, Le Floc'h A, Huse M, Sun JC, and Rudensky AY. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J. Exp. Med. 2013;210:1167-78.https://doi.org/10.1084/jem.20122462
- Bernstein ZP, Porter MM, Gould M, Lipman B, Bluman EM, Stewart CC, Hewitt RG, Fyfe G, Poiesz B, and Caligiuri MA. Prolonged administration of low-dose interleukin-2 in human immunodeficiency virus-associated malignancy results in selective expansion of innate immune effectors without significant clinical toxicity. Blood. 1995;86:3287-94.
- Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, Sherry RM, Royal RE, Steinberg SM, and Rosenberg S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293-301.https://doi.org/10.1002/cncr.23552
- Su EW, Moore CJ, Suriano S, Johnson CB, Songalia N, Patterson A, Neitzke DJ, Andrijauskaite K, Garrett-Mayer E, Mehrotra S, et al. IL-2Rα mediates temporal regulation of IL-2 signaling and enhances immunotherapy. Sci. Transl. Med. 2015;7:311ra170.https://doi.org/10.1126/scitranslmed.aac8155
- Pachella LA, Madsen LT, and Dains JE. The Toxicity and Benefit of Various Dosing Strategies for Interleukin-2 in Metastatic Melanoma and Renal Cell Carcinoma. J. Adv. Pract. Oncol. 2015;6:212-21.
- Rosalia RA., Arenas-Ramirez N, Bouchaud G, Raeber ME, and Boyman O. Use of enhanced interleukin-2 formulations for improved immunotherapy against cancer. Curr. Opin. Chem. Biol. 2014;23:39-46.https://doi.org/10.1016/j.cbpa.2014.09.006
- Gubbels JAA, Gadbaw B, Buhtoiarov IN, Horibata S, Kapur AK, Patel D, Hank JA, Gillies SD, Sondel PM, Patankar MS, et al. Ab-IL2 fusion proteins mediate NK cell immune synapse formation by polarizing CD25 to the target cell-effector cell interface. Cancer Immunol. Immunother. 2011;60:1789-800.https://doi.org/10.1007/s00262-011-1072-9
- Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P, et al. 2012. Exploiting a natural conformational switch to engineer an interleukin-2 “superkine”. Nature. 484: 529-33.https://doi.org/10.1038/nature10975
- Stegmann KA, De Souza JB, and Riley EM. IL-18-induced expression of high-affinity IL-2R on murine NK cells is essential for NK-cell IFN-γ production during murine Plasmodium yoelii infection. Eur. J. Immunol. 2015;45:3431-40.https://doi.org/10.1002/eji.201546018
- Anegón I, Cuturi MC, Trinchieri G, and Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J. Exp. Med. 1988;167:452-72.https://doi.org/10.1084/jem.167.2.452
- Márquez ME, Millet C, Stekman H, Conesa A, Deglesne PA, Toro F, De Sanctis J, and Blanca I. CD16 cross-linking induces increased expression of CD56 and production of IL-12 in peripheral NK cells. Cell. Immunol. 2010;264:86-92.https://doi.org/10.1016/j.cellimm.2010.05.002
