ABSTRACT
Cancer is one of the leading causes of death in the industrialized world and represents a tremendous social and economic burden. As conventional therapies fail to provide a sustainable cure for most cancer patients, the emerging unique immune therapeutic approach of bacteria-mediated tumor therapy (BMTT) is marching towards a feasible solution. Although promising results have been obtained with BMTT using various preclinical tumor models, for advancement a major concern is immunity against the bacterial vector itself. Pre-exposure to the therapeutic agent under field conditions is a reasonable expectation and may limit the therapeutic efficacy of BMTT. In the present study, we investigated the therapeutic potential of Salmonella and E. coli vector strains in naïve and immunized tumor bearing mice. Pre-exposure to the therapeutic agent caused a significant aberrant phenotype of the microenvironment of colonized tumors and limited the in vivo efficacy of established BMTT vector strains Salmonella SL7207 and E. coli Symbioflor-2. Using targeted genetic engineering, we generated the optimized auxotrophic Salmonella vector strain SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF) harboring modifications in Lipid A and flagella synthesis. This combination of mutations resulted in an increased immune-stimulatory capacity and as such the strain was able to overcome the efficacy-limiting effects of pre-exposure. Thus, we conclude that any limitations of BMTT concerning anti-bacterial immunity may be countered by strategies that optimize the immune-stimulatory capacity of the attenuated vector strains.
Introduction
Bacteria-mediated tumor therapy (BMTT) is a unique form of immunotherapy and represents a promising strategy to target solid tumors.Citation1,2 Effective vector strains need to be deployed. Accordingly, the research community has fostered potent bacterial vector strains of the genera Salmonella, Clostridia, Escherichia and Listeria for more than a century. Many of the strains derived over this period of time have been successfully applied in preclinical and clinical trials.Citation1,3-14
Bacteria as therapeutic agents exhibit many advantages over conventional therapies such as surgery or chemotherapy: (i) their unique ability to specifically colonize tumors from a distant site of inoculation allows targeting of nearly all tumors present, including metastases. (ii) During the process of tumor colonization, the bacteria overcome physiological barriers which otherwise pose a limit to, for instance, chemotherapy. (iii) Because of an intrinsic tumor colonizing ability, engineered bacteria could be exploited as tumor targeting vectors for delivery of genetic cargo.Citation2,15,16
Despite these advantages, the immune system of the host could represent a major obstacle for BMTT. The intrinsic efficacy of BMTT on the one hand relies on the capability of the bacteria to induce, reactivate or amplify a preexisting immune response against the tumor. In accordance, it was shown that after systemic inoculation of bacteria, an initial strong induction of the innate immune system takes place. As such occurs, a resulting cytokine storm is required to initiate bacterial tumor colonization. Subsequently, due to bacterial adjuvant properties, an adaptive immune response consisting of cytotoxic T cells is induced and responsible for tumor clearance.Citation17,18 In addition, recent studies indicate that Salmonella and its effector molecules may act on and kill cancer cells directly.Citation10,19-22 To which extent direct effects or adjuvanticity effectuate efficacy of BMTT remains presently elusive.
Thus, within the current study we aimed to address the open question whether anti-bacterial immunity could interfere with the therapeutic benefit of the bacteria in an immune competent host. While laboratory animals, such as inbred mice kept under hygienic conditions, should not be pre-exposed to the therapeutic bacteria prior to treatment, humans, pets and livestock may already be sensitized and thus exhibit immunity against the microorganisms. Indeed, according to established statistics, approximately 20% of the human population displays an active antibody titer against Salmonella.Citation23 Similarly, many people ingest probiotic E. coli (e.g. Mutaflor or Symbioflor-2) as treatment of gastrointestinal disturbances.Citation24,25 Such prior encounters with bacteria that are related to the therapeutic strains may prohibit success of BMTT.
The present study aimed to investigate the influence of pre-exposure to S. Typhimurium or E. coli on the efficacy of BMTT in a murine transplantable tumor model. In addition, we explored the possibility to circumvent such potential immunity against the vector using alternate routes of inoculation, by comparing intravenous (i.v.) with intratumoral (i.t.) infection. Our results reveal that pre-exposure can indeed limit the efficacy of standard E. coli or Salmonella applied via either route of inoculation. As solution, we generated an optimized Salmonella strain SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). SF200 carries an optimized hexa-acylated Lipid A structure because of the deletions ΔlpxR9 ΔpagL7 and ΔpagP8 (Supplementary Figure S1). This structure is known to generate a maximally immune-stimulatory Lipid A moiety.Citation26,27 Furthermore, this strain harbors the aroA deletion, which we were recently able to show to confer simultaneous attenuation and increased immunogenicity.Citation28 Finally, modifications of flagella synthesis were introduced by the ΔydiV and ΔfliF mutations, which result in constitutive expression of intracellular flagellin and formation of outer membrane vesicles (OMVs).Citation28-30 This strain exhibited greater immunogenicity and was thus able to overcome limits of pre-exposure in comparison to non-optimized bacteria. From these data we conclude that pre-exposure to the bacterial vector indeed represents an obstacle and serious concern for BMTT. However, solutions can be found by an appropriate design of vector strains as demonstrated with SF200.
Results
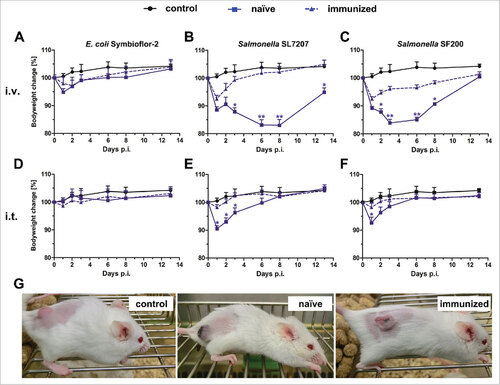
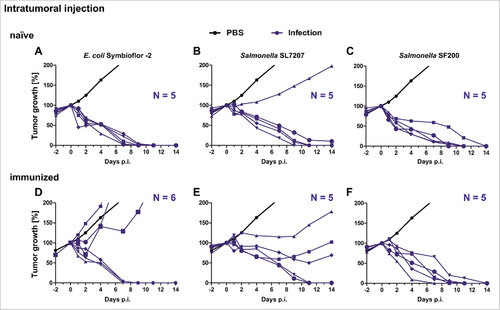
Immunized tumor bearing mice are less sensitive to bacterial infection: The influence of a bacterial pre-exposure on BMTT susceptibility was investigated by treating naïve BALB/c mice with two doses of the corresponding bacteria spaced one week apart. To this end, heat-inactivated S. Typhimurium UK-1 was administrated intravenously or live E. coli Symbioflor-2 orally. To determine whether pre-exposure had conferred immunity against a re-challenge, immunized CT26 tumor bearing mice were infected intravenously (i.v.) or intratumorally with the corresponding bacteria (). In general, pre-exposure reduced the severity of infection as judged by the body weight loss and the general appearance of the mice (). As expected, this effect was more prominent for Salmonella compared to the probiotic E. coli strain as the latter affects the mice only to a minor extend in the first place. Interestingly, intratumoral (i.t.) rather than i.v. infection did influence immunized mice to a lesser extent compared to naïve mice. This emphasizes the higher safety profile of this route of inoculation as shown previouslyCitation31 (). To exclude a strain specific effect, mice were immunized with SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF), and challenged with either Wt or SF200, respectively. No significant differences in mouse survival were observed between Wt and SF200 immunization. Therefore, immunization with UK-1 can serve to simulate pre-exposure conditions (Supplementary Figure S2). In addition, health conditions of UK-1 immunized mice challenged with SL7207 demonstrated that a general anti-Salmonella immunity had developed independent of the immunizing Salmonella strain ().
Figure 1. Health burden of naïve and immunized mice upon infection with Salmonella and E. coli. Naïve and immunized CT26 tumor-bearing mice were infected intravenously (A – C) or intratumorally (D – F) with 5#107 E. coli Symbioflor-2, 5#107 SL7207 or 5#106 SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). Bodyweight was measured with a scale and used as indicator of general health. PBS served as a negative control. (G) Photograph of mice infected intravenously with SF200 24 hpi. Displayed are values of mean ± SD. Results are representative of two independent experiments with six replicates in each group. p-Values indicate differences between infected mice under naïve and immunized conditions. #, p < 0.05; ##, p < 0.01.
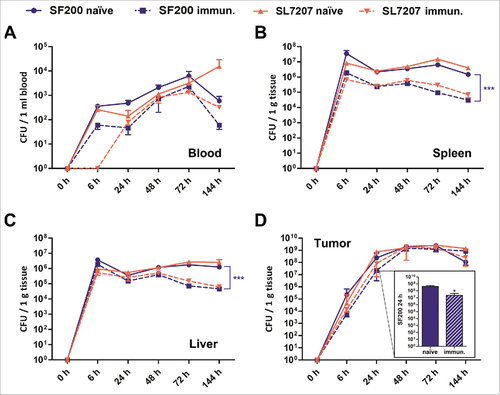
Beyond these macroscopic observations, the secondary infection induced a significantly decreased cytokine storm compared to the primary infection as revealed by measuring TNF-α levels in serum (Supplementary Figure S3). This also indicated that these mice developed immunity towards the secondary bacterial infection. To corroborate these data, the tissue-distribution of the Salmonella variants SF200 and SL7207 was determined in naïve and immunized mice upon i.v. infection (). Both Salmonella strains were equally affected by immunization. Pre-exposed mice displayed 90% less splenic colonization by salmonellae () and were less prone to splenomegaly as measured 6 dpi (Supplementary Figure S4). Interestingly, although tumor colonization was initially reduced in immunized mice, SF200 and SL7207 reached similar colonization levels 48 hpi independent on the immune state of the mice and the route of administration ( and Supplementary Figure S5). Altogether, these results demonstrate that pre-exposure simultaneously improves the safety and decreases the immune pathology elicited by the bacteria. Therefore, it may also limit the efficacy of BMTT by these bacteria. For such reasons, we further tested our new strain SF200, which was developed for improved performance in BMTT.
Figure 2. Biodistribution of Salmonella variants in naïve and immunized mice upon intravenous infection. Naïve and immunized CT26 tumor-bearing mice were infected intravenously with 5#106 SL7207 or SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). The bacterial load in blood (A), spleen (B), liver (C) and tumor (D) were determined by plating over the course of infection. Displayed are values of mean ± SD of four replicates in each group. The inset in displays enlarged the numbers of CFUs found in the tumors of both types of mice at 24 hrs upon infection with SF200. Clearly, at this time point tumors of immunized mice are colonized ten-fold lower than tumors of naïve mice. At later time points equal colonization is observed. #, p < 0.05; ###, p < 0.001.
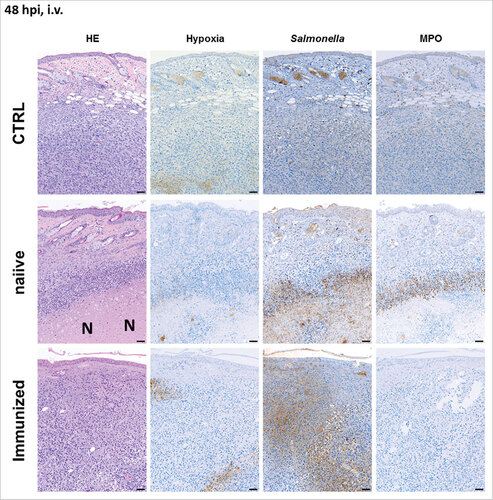
Pre-exposure influences the tumor microenvironment upon BMTT: To extend our analysis of SF200, immune histology was performed on CT26 tumors upon intravenous and intratumoral infection (i.v., and i.t., Supplementary Figure S6). As expected, tumor bearing naïve mice developed necrosis and hypoxic regions by 24 hours post infection (hpi) independent on the route of inoculation (Supplementary Figure S7 for i.v. infection and Supplementary Figure S8 for i.t. infection). This was even more prominent at 48 hpi. Necrotic areas were extensively colonized by salmonellae. Moreover, granulocytic neutrophils were abundantly present in the interphase between viable and necrotic tissue. These phenotypic characteristics were very similar to our previous findings.Citation32 However, while the microscopic profile of tumors from naïve mice upon i.t. and i.v. infection appeared identical (Supplementary Figure S7 and S8), the tumors of infected immunized mice generally displayed a profile different from the former tumors. Sparse necrotic areas and less neutrophil infiltration were found and the bacteria were distributed all over the cancerous tissue (). These results demonstrate that immunization affects the tumor microenvironment and might influence the efficacy of BMTT. However, intratumoral infections appear to be less sensitive to this effect of pre-exposure.
Figure 3. Pre-exposure reduces the formation of necrosis in the early stages of infection upon intravenous infection with Salmonella. CT26 tumor-bearing mice were infected with 5#106 SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF) via intravenous infection. 48 hpi, tumors were isolated and prepared for immune histochemical staining. Immunized mice are less prone to necrosis formation and hypoxia. Dispersion of salmonellae in and beyond necrotic center, and presence of neutrophils in immediate proximity to the salmonellae was only clearly visible in naïve mice. “N” denotes areas of necrosis. Hypoxia was stained with antibodies against metabolites of pimonidazole-HCl, otherwise administered i.v. 30 mins prior to isolation. Myeloperoxidase (MPO) denotes presence of neutrophilic granulocytes, and Salmonella was stained using a specific antibody. Differential staining was performed on consecutive sections. Scale bar corresponds to 100 μm. Images representative of at least 3 replicates are displayed.
Pre-exposure affects the efficacy of non-optimized strains upon infection: As the microenvironment of the tumor is altered also after application of SF200, the pre-sensitized host response to bacterial infection could limit the potency of BMTT. To this end, naïve and immunized CT26 tumor bearing mice were intravenously infected with 5#106 Salmonella variants or 5#107 E. coli Symbioflor-2 (). As expected, most tumors of naïve mice regressed and were cleared by 14 dpi (). However, E. coli Symbioflor-2 and the Salmonella variant SL7207 partially lost their ability to clear tumors in immunized mice in contrast to naïve mice (). In case of infection with E. coli, 20% of the tumors regrew and in case of application of SL7207 only a single tumor out of five was cleared. Thus, immunization seriously limits the therapeutic benefit of these vector strains. In contrast, the optimized Salmonella strain SF200 was able to clear all CT26 tumors also under immunized conditions (). This suggests that the increased immunogenicity of SF200 may compensate for the immunity induced by pre-exposure.
Figure 4. Tumor development upon intravenous infection with Salmonella and probiotic E. coli in naïve and immunized mice. Naïve (A – C) and immunized (D – F) CT26 tumor bearing mice were infected intravenously with 5#107 E. coli Symbioflor-2, 5#106 SL7207 or 5#106 SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). Tumor volumes were calculated on the basis of caliper measurements following infection with E. coli Symbioflor-2, SL7207 and SF200. PBS served as negative control and is depicted as mean of five replicates. Tumor progression of individual mice is displayed (blue).. Results are representative of two independent experiments.
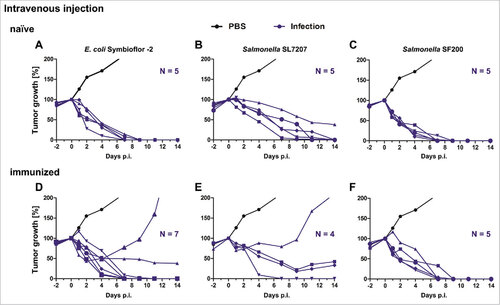
The negative influence of immunity was substantially more pronounced when the bacteria were administered intratumorally (). Both, E. coli Symbioflor-2 and Salmonella SL7207 lost more than 50% of their efficacy for tumor clearance in immunized mice compared to naïve mice (). In contrast, the efficacy of the optimized Salmonella variant SF200 was not affected by pre-exposure of the mice to Salmonella and all CT26 tumors were cleared within 14 dpi (). Importantly, when such SF200 treated, pre-sensitized tumor-bearing mice – that had cleared the CT26 tumor – were followed for more than 200 days post treatment, no tumor relapses occurred.
Figure 5. Tumor development upon intratumoral infection with Salmonella and probiotic E. coli in naïve and immunized mice. Naïve (A – C) and immunized (D – F) CT26 tumor bearing mice were infected intravenously with 5#107 E. coli Symbioflor-2, 5#106 SL7207 or 5#106 SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). Tumor volumes were calculated on the basis of caliper measurements following infection with E. coli Symbioflor-2, SL7207 and SF200. PBS served as negative control and is depicted as mean of five replicates. Tumor progression of individual mice is displayed (blue). Results are representative of two independent experiments.
We next wanted to establish that despite of anti-salmonellae immunity an immunological memory against the CT26 tumor had been established as shown before by T-cell transfer experiments.Citation31 We therefore performed a re-challenge experiment with CT26 cells. When CT26 cells were injected in such mice, no CT26 tumor formation could be observed on mice that received SF200 before while the unrelated control tumor RenCa showed uninhibited growth (Supplementary Figure 9). These results demonstrate that independent of the route of inoculation, highly potent vector strains are required to overcome the bacterial immunity in the host and to retain therapeutic efficacy. Otherwise the benefit observed under naïve conditions may be lost in a pre-sensitized population.
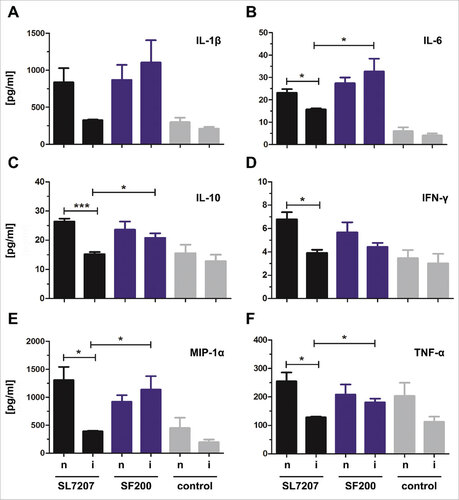
Superior immune induction by SF200 in pre-sensitized mice: In order to elucidate putative reasons for the superiority of SF200, cytokine profiles of tumors of naïve and immunized mice were analyzed 48 h after infection with SL7207 or SF200 by multiplex analysis (). In total, 23 cytokines were analyzed. Interestingly, for SF200 infected tumors, the cytokine profile was similar in naïve and immunized mice. In contrast, for SL7207 infected tumors, the cytokine levels of e.g. IL-1β, IL-6 or TNF-α were significantly lower in tumors from immunized mice compared to tumors from naïve mice. This indicates that the optimization that led to SF200 (and which are missing in SL7207), may compensate for the effect of pre-immunization. This might explain the therapeutic differences displayed in. and To confirm the immunogenic superiority of SF200, we also analyzed the cytokine profile induced in RAW264.7 macrophages as well as in sera of tumor bearing mice and compared it to an infection by UK-1 WT bacteria (Supplementary Figure 10). Again, SF200 demonstrated elevated cytokine levels in comparison to WT bacteria. These results demonstrate that the genetic modifications incorporated into SF200 significantly increased the immunogenic potency and the therapeutic power of this strain.
Figure 6. Cytokine, chemokine and growth factor detection in infected tumors of naïve and immunized mice 48 hpi. Naïve (n) and immunized (i) CT26 tumor bearing mice were infected intravenously with 5#106 SL7207 or 5#106 SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). The cytokine leves of IL-1β (A), IL-6 (B), IL-10 (C), IFN-γ (D), MIP-1α (E) and TNF-α (F) were analyzed using a Bio-PlexPro™ kit 48 hpi. Uninfected tumors from uninfected naïve and immunized mice served as control. Displayed are values of mean ± SD of four replicates in each group.
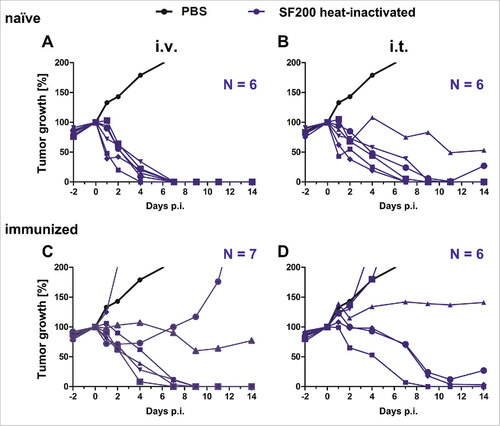
Live bacteria are required to counter immunity after pre-exposure: A fundamental question remains to be addressed in BMTT: can the therapeutic effect of bacteria be separated from traits like tumor colonization, bacterial persistence and viability? We recently demonstrated that single doses of LPS or heat-inactivated Salmonella are sufficient to induce an effective anti-tumor response in the CT26 tumor model.Citation33 As the new optimized vector strain SF200 was well tolerated after pre-exposure, we wondered whether inactivated bacteria would also be sufficiently potent to clear CT26 tumors. Thus, SF200 was heat-inactivated at 56°C and the inoculum controlled for viability via plating. To evaluate the potency of these heat-inactivated bacteria, naïve and immunized CT26 tumor bearing mice were treated intravenously and intratumorally with this preparation (). As expected, all tumors were cleared by heat-inactivated SF200 upon i.v. infection in naïve mice (). However, application of heat-inactivated bacteria i.t. reduced the efficacy of SF200 already (). Importantly, while live SF200 were able to clear all tumors in immunized mice, the heat-inactivated variant lost this capability (). 56% of the tumors were cleared upon i.v. administration, only 33% when applying the bacteria i.t. These results demonstrate that genetically optimized bacteria need to be alive to counter the induced anti-bacterial immunity after pre-exposure. The adjuvant effect of heat-inactivated bacteria per se does not provide a sufficient stimulus for a successful BMTT under these conditions.
Figure 7. Tumor development upon infection with heat-inactivated Salmonella SF200 in naïve and immunized mice. Naïve (A – B) and immunized (C – D) CT26 tumor bearing mice were infected intravenously (A and C) and intratumorally (B and D) with or 5#106 heat-inactivated SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF). Tumor volumes were calculated on the basis of caliper measurements following infection with SF200. PBS served as negative control and is depicted as mean of five replicates. Tumor progression of individual mice is displayed (blue). Results are representative of two independent experiments
Discussion
Due to their pathogenic and immunogenic nature, bacteria are exploited as versatile vehicles for vaccination and therapy such as BMTT. In BMTT, bacteria have been demonstrated to selectively target cancerous tissue, most likely by a passive mechanism.Citation34 However, it remains elusive to which extent the intrinsic ability of bacteria to induce anti-tumor activities relies on direct effector mechanisms of the bacteria such as for instance cytotoxicity, or on an exclusive immune stimulatory adjuvant effect (e.g. cytokine storm and/or T cell activation). For instance, it was recently reported that Salmonella can exhibit direct effects on the tumor microenvironment by modifying cellular transport mechanisms. However, these effects were only effective in combination with chemotherapeutics.Citation22,35 Thus, the direct effects on the therapeutic benefit of BMTT remains ambiguous.
In contrast, the adjuvant effect of bacteria is well characterized. It is known that bacteria elicit a strong cytokine storm that causes stress to the tumor microenvironment. A common phenotype is formation of necrosis and, potentially, release of tumor-antigens. Accordingly, cytotoxic T-lymphocytes are induced and are able to eradicate the tumor.Citation18 Therefore, the immune system of the host plays an essential role in BMTT. This conclusion could also be drawn from clinical trials. Patients that had already been subject to chemotherapy were generally less susceptible to BMTT, even though the therapeutic strains deployed had previously displayed potency in a number of pre-clinical animal models.Citation36,37 One could speculate that the weakened immune system under such immunocompromised conditions would generally not support BMTT anymore in terms of efficiency.
Apart from chemotherapy, accidental or intentional exposure to phylogenetic relatives of the therapeutic bacteria such as Salmonella or E. coli causes immunity in patients. Therefore, a pre-exposed patient may become less responsive to the bacterial therapy. The present study aimed to evaluate the influence of such pre-exposure on BMTT. As such, pre-exposure generally greatly affected the mice and also the therapeutic effectiveness of BMTT. As expected, mice which had previously encountered the bacteria were less sensitive to secondary infections independent of whether the bacteria had been administered systemically or directly into the tumor. Pre-exposure also led to a significantly reduced cytokine response upon secondary exposure as determined by TNF-α levels in blood. Further, pre-exposure limited bacterial survival in vivo and reduced bacteria induced inflammation upon secondary infection. These observations highlight the importance of in-depth knowledge of the immunological background of patients before assigning a particular treatment. For instance, vaccination against salmonellae is often used to protect individuals that travel to endemic countries. Suspensions of probiotic E. coli are used to treat disorders of the gastrointestinal tract. Although immunity may be transient, protection often lasts for several years before the host again displays sensitivity.Citation38 During this time, efficacy of BMTT with non-optimized bacteria may be limited due to a protective memory response.
TNF-α is a key mediator in BMTT. It induces necrosis in large parts of the tumor upon systemic infection. When we analyzed the tumor microenvironment under immunity conditions, using immune histology, we found decreased TNF-α levels, in accordance with prior findings.Citation17 Interestingly, immunity tremendously limited the extent of necrotic regions within the tumor. In addition, the typical appearance of infected tumors with salmonellae surrounding the necrotic area was also absent under these conditions. However, intratumoral application of bacteria was less prone to these alterations. A possible explanation for this observation could be that intratumorally administered bacteria are not exposed to the effector mechanisms of the immune system to a similar extent as during systemic administration. Although tumor colonization was indistinguishable between the two routes of injection after one week, we show that a hampered initial tumor colonization in immunized mice could explain the difference in histological observations. Upon systemic injection, fewer bacteria may initially reach the tumor because of a reduced cytokine response under immunity conditions. However, tumor invasion by merely a few bacteria is sufficient to ensure rapid bacterial proliferation to 109 bacteria per gram tissue after 48 hpi, as we have demonstrated in a recent study.Citation31 We conclude that tumors represent an immune-privileged niche that can be filled to a specific level dependent on the bacterial species. This also implies that a strong immune induction by the bacteria is initially required to obtain a potent anti-tumor effect that leads to clearance of the tumor rather than an efficient early tumor colonization. This could explain why a pre-sensitized mouse is less susceptible to bacterial cancer therapy using conventional strains.
However, the BMTT efficacy of our newly optimized Salmonella vector strain SF200 (ΔlpxR9 ΔpagL7 ΔpagP8 ΔaroA ΔydiV ΔfliF) was not affected under these circumstances. All tumors were successfully cleared, independent of the route of inoculation and immunization status. We constructed this strain modularly based on previous resultsCitation28,39 The homogenously hexa-acylated Lipid A structure resulting from mutations ΔlpxR9 ΔpagL7 ΔpagP8 renders the LPS molecule ideal as ligand for the TLR4-MD2 complex and is maximally immune-stimulatory.Citation26,27 The aroA deletion globally affects bacterial gene expression, and in particular two differentially expressed genes (arnT and ansB) are involved in the immune escape of Salmonella.Citation28 The modifications affecting flagella synthesis and assembly resulted in improved immunogenicity and increased formation of OMVs. The ΔydiV deletion deregulates flagella synthesis under in vivo conditions and the ΔfliF mutation prevents assembly of the flagellar export apparatus.Citation28-30 However the detailed molecular mechanism that improves bacterial performance still remains elusive.
Despite the lack of a precise mode-of-action, we were able to demonstrate that these optimizations made in SF200 result in a highly immunogenic strain that is superior to Wt and SL7207 regarding immunogenicity and safety. These optimizations were even sufficient to counter the effects of immunity and tolerance against the vector. The cytokine pattern in the tumors was almost identical in naïve and immunized mice. For instance in contrast to SL7207, the levels of IL-1β or TNF-α remained high in the tumors of immunized mice after infection with SF200. As IL-1β and TNF-α have been shown to play an important role during oncolysis,Citation40-42 our finding underscores the potency of SF200 for BMTT.
Surprisingly, tumor colonization itself may not be the driving factor for therapeutic efficacy. Both, SL7207 as well as SF200 colonized to tumor to the same extend independent of the immune status. Rather the immunogenic potential of the individual strain is decisive as indicated by the effect of immunity on the cytokine pattern elicited by the two strains. As both strains harbor an aroA deletion, the optimization of the Lipid A regarding TLR-4 affinity and activation, the changes to TLR-5 signaling by the flagella modifications and the OMV production may be reasons why SF200 is superior to SL7207 with regard to efficacy.Citation26 As the level of cytokines usually correlates with the activation of the immune system and the tumor stroma, it will be crucial to analyze infiltrating immune cell and tumor microenvironment in the future. In addition, it would be also interesting to compare the therapeutic efficacy of SF200 to other important Salmonella strains in the field of BMTT like A1-RCitation43-49 or the recently described and optimized ppGpp mutant.Citation40,50
In summary, modifications of bacterial associated immunogenicity factors are able to compensate anti-bacterial immunity caused by pre-exposure, and otherwise interfering with the efficacy of non-optimized strains such as SL7207. Thus, it strengthens our concept that it is not only sufficient to attenuate a bacterium but also necessary to improve its immune stimulating character at the very same time.Citation1 Apparently, this holds true even more in pre-exposed hosts. In this context, one could consider additional intrinsic improvements but also solutions comprising immune stimulatory genetic cargo.Citation51-56
Genetically engineered bacterial strains always raise the question of safety. A major open question was whether live bacteria are essentially required under pre-sensitized conditions or whether heat-inactivated variants with optimized immune stimulatory structures like pathogen associated molecular patterns (PAMPs) would be sufficient for therapy. An advantage of heat-inactivation is safety of treatment as already W. Coley demonstrated more than hundred years ago showing that heat-inactivated bacteria can still be effective.Citation57,58 Thus, SF200 was heat-inactivated and used for therapy. Interestingly, in naïve mice, heat-inactivated bacteria retained effectiveness against CT26 tumors, confirming observations with other types of bacteria.Citation31 However, heat-inactivated bacteria completely lost their efficacy in immunized mice. These experiments clearly demonstrate that PAMPs are able and sufficient to affect tumor growth in naïve hosts, however that pre-exposure to the same bacteria counteracts their effect. In conclusion, live bacteria are most likely required for successful tumor therapy in a pre-sensitized patient population. The exact reason why live bacteria are more potent remains unclear. We speculate that they are capable of stimulating the immune system at different levels than heat-inactivated bacteria. For instance, the type III secretion system and its effector proteins are only active in vivo in live bacteria.Citation59 Thus, their stimulative features may be missing in heat-inactivated bacteria. In addition, colonization and proliferation of live bacteria may provide a continuous stimulus unlike the case with heat-inactivated bacteria.
Taken together, a significant proportion of the human population exhibits an active titer against infectious agents. The efficacy of BMTT can be extremely limited when patients have encountered phylogenetic relatives of the therapeutic bacterial vector prior to treatment. We were able to demonstrate that the limitations conferred by bacterial immunity can be overcome by targeted genetic manipulation to increase the immune-stimulatory capacity of the bacterial vector strain used. Thus, the concern that pre-exposure represents a major disadvantage of BMTT can be countered using effective strategies including rational bacterial strain design and may facilitate reliability of BMTT in the near future.
Materials and methods
Ethics statement: All animal experiments were performed according to guidelines of the German Law for Animal Protection and with permission of the local ethics committee and the local authority LAVES (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit) under permission number 33.9-42502-04-12/0713.
Strains and preparation of inoculum: Bacterial strains are shown in Supplementary Table S1. Salmonella and E. coli strains were grown overnight and sub-cultured to mid-log phase in LB media at 37°C. Symbioflor-2 was adjusted as described previously.Citation5 In general, the bacteria were washed twice and adjusted to the desired OD600 in pyrogen free PBS. For heat-inactivation, bacteria were incubated at 56°C for one hour. Plating the inoculum served as control.
Murine tumor model: Six to seven week old BALB/c mice (Janvier) were intradermally inoculated with 5#105 syngeneic CT26 tumor cells (colorectal cancer, ATCC CRL-2638) in the right flank. Tumor development was monitored using caliper measurements. Upon reaching a tumor volume of approx. 150 mm3 after 10 days, the mice were injected intravenously into the tail vein or directly into the tumor with 5#106 Salmonella and 1#107 E. coli, respectively.
Immunization: Mice were immunized twice 5 and 4 weeks before tumor inoculation. For Salmonella, 5#106 heat-inactivated S. Typhimurium UK-1 wild-type bacteria were used to immunize the mice using an intravenous route of inoculation. For E. coli, mice were orally administered by gavage with 5#108 E. coli Symbioflor-2.
Therapeutic benefit and bacterial burden: Tumor development was monitored using caliper measurements for as long as tumors persisted or until confronted with a humane endpoint in terms of exceedingly large tumor size (∼ 1 cm3) or morbidity. Body weight as general health indicator was monitored using a scale. A loss of body weight below 80% of the original body weight was incentive to euthanize a subject. To determine the bacterial burden, blood, spleen, liver and tumor tissue were harvested at 7 days post infection and treated as described previously.Citation17 CFUs were determined by serial plating.
Histology: Mice were treated i.v. with pimonidazole 30 min before sacrifice. Tumor specimens were fixed with 4% (v/v) formalin for 24 – 48 h, embedded in paraffin. Approximately 3 μm thick sections were stained with hematoxylin/ eosin according to standard laboratory procedures. Immuno-histo-chemical staining was performed using the following antibodies: rabbit-anti-pimonidazole (HP3-100kit, Hydroxyprobe inc.), rabbit-anti-MPO (Medac/ Thermo Scientific), rabbit-anti-salmonella (US Biological) and DAB (3,3-Diaminobenzidine Zytomed Systems DAB530) as chromogen. Hematoxylin was used for counterstaining. Sections were analyzed by light microscopy blinded to the experimental groups.
Electron microscopy: SF200 was cultured in 5 ml LB overnight. On the next day, 650 µl glutaraldehyde (cf = 2%) was added to fixate the bacteria. For scanning electron microscopy fixed samples were placed onto poly-L-lysine coated cover slips (12 mm in diameter), dehydrated with a graded series of acetone, critical point dried with CO2 and sputter coated with gold-palladium. Samples were examined in a Zeiss Merlin (Oberkochen, Germany) at an acceleration voltage of 5 kV using the Everhart-Thornley SE-detector and the Inlens SE-detector in a 25:75 ratio. Contrast and brightness were adjusted with Adobe Photoshop C5.
TNF-α ELISA measurement in serum: Blood samples were collected 1.5 h post infection. The TNF-α ELISA Max™ Standard Kit (Biolegend) was used to determine the TNF-α level in serum. All steps were done according to the manufacturer's manual. Three different biological replicates were analyzed and a PBS treated group served as negative control.
Cytokine, chemokine and growth factor detection in supernatants, sera and lysates: Cytokine, chemokine and growth factor concentrations in supernatants of 264.7 RAW macrophages cells (6 hpi with MOI 10), sera (1.5 hpi, 6 hpi and 24 hpi) or tumor tissues (48 hpi) were quantified by the Luminex-based multiplex technique according to the manufacturer's instructions (Bio-Rad, USA). The tumor homogenate was adjusted to a protein concentration of 1 mg/ml using a Lowry-assay. Standard curves and concentrations were calculated with Bio-Plex Manager 6.0, the detection sensitivity of all proteins was between 1 pg/ml and 40 µg/ml.
Statistics: Significance between two groups was determined using the nonparametric Mann-Whitney test, while one-way analysis of variance (ANOVA) with Bonferroni posttest was used to compare two or more groups. Significance levels of p < 0.05, p < 0.01, or p < 0.001 were denoted with asterisks: #, ##, and ###, respectively.
Conflict of interest
Kurt Zimmermann is a general manager of Symbio Gruppe GmbH & Co KG, the company responsible for commercializing Symbioflor-2. Involvement was limited to strain provision, supportive information, and financial aid for an extended period of research.
downloadFromZipFile.pdf
Download PDF (6.4 MB)Acknowledgments
Our gratitude is extended to Susanne zur Lage, Regina Lesch, Kerstin Daemen and Jana Keil for their expert technical assistance. Moreover, we thank Roy Curtiss III for providing parental strains of Salmonella, along with expert advice concerning strain design. The study was supported in part by the Niedersächsische Krebsgesellschaft, the Deutsche Krebshilfe, the Bundesministerium für Bildung und Forschung (BMBF), Hannover Biomedical Research School (HBRS) (all to SW), Center for Infection Biology (ZIB) at Hannover Medical School (to DK and MF), a Lichtenberg Fellowship from the Niedersächsiche Ministerium für Wissenschaft und Kultur (MWK) (to SF), an Exploration Grant of the Boehringer Ingelheim Foundation (to SF), the Helmholtz Association Young Investigator grant no. VH-NG-932 (to ME) and SymbioPharm GmbH (to KZ and DK).
References
- Felgner S, Kocijancic D, Frahm M, Weiss S. Bacteria in Cancer Therapy: Renaissance of an Old Concept. Int J Microbiol. 2016;2016:1-14. doi:10.1155/2016/8451728.
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785-94. doi:10.1038/nrc2934.
- Frahm M, Felgner S, Kocijancic D, Rohde M, Hensel M, Curtiss R, Erhardt M, Weiss S. Efficiency of Conditionally Attenuated Salmonella enterica Serovar Typhimurium in Bacterium-Mediated Tumor Therapy. mBio. 2015;6:e00254-15. doi:10.1128/mBio.00254-15.
- Eisenstark A, Kazmierczak RA, Dino A, Khreis R, Newman D, Schatten H. Development of Salmonella strains as cancer therapy agents and testing in tumor cell lines. Methods Mol Biol. 2007;394:323-54.
- Kocijancic D, Felgner S, Frahm M, Komoll R-M, Iljazovic A, Pawar V, Rohde M, Heise U, Zimmermann K, Gunzer F, et al. Therapy of solid tumors using probiotic Symbioflor-2 – restraints and potential. Oncotarget;2016;7:22605-22. doi:10.18632/oncotarget.8027.
- Singh R, Paterson Y. Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev Vaccines. 2006;5:541-52. doi:10.1586/14760584.5.4.541.
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647-52. doi:10.1158/0008-5472.CAN-06-0716.
- Hoffman RM. The preclinical discovery of bacterial therapy for the treatment of metastatic cancer with unique advantages. Expert Opin Drug Discov. 2012;7:73-83. doi:10.1517/17460441.2012.644534.
- Swofford CA, Van Dessel N, Forbes NS. Quorum-sensing Salmonella selectively trigger protein expression within tumors. PNAS. 2015;112:3457-62. doi:10.1073/pnas.1414558112.
- Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. PNAS. 2007;104:10170-4. doi:10.1073/pnas.0703867104.
- Low KB, Ittensohn M, Luo X, Zheng L-M, King I, Pawelek JM, Bermudes D. Construction of VNP20009. In: Springer C.J. (eds) Suicide Gene Therapy. Methods in Molecular Medicine™, 2004;vol 90. Humana Press. doi:10.1385/1-59259-429-8:47
- Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, et al. Phase I Study of the Intravenous Administration of Attenuated Salmonella typhimurium to Patients With Metastatic Melanoma. J Clin Oncol. 2002;20:142-52. doi:10.1200/JCO.2002.20.1.142.
- Krick EL, Sorenmo KU, Rankin SC, Cheong I, Kobrin B, Thornton K, Kinzler KW, Vogelstein B, Zhou S, Diaz LA. Evaluation of Clostridium novyi-NT spores in dogs with naturally occurring tumors. Am J Vet Res. 2012;73:112-8. doi:10.2460/ajvr.73.1.112. PMID:22849671
- Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6:249ra111-249ra111. doi:10.1126/scitranslmed.3008982.
- Swofford CA, St Jean AT, Panteli JT, Brentzel ZJ, Forbes NS. Identification of Staphylococcus aureus α-hemolysin as a protein drug that is secreted by anticancer bacteria and rapidly kills cancer cells. Biotechnol Bioeng. 2014;111:1-32. doi:10.1002/bit.25184.
- Green L, Storey M, Williams E, Patterson A, Smaill J, Copp J, Ackerley D. The Flavin Reductase MsuE Is a Novel Nitroreductase that Can Efficiently Activate Two Promising Next-Generation Prodrugs for Gene-Directed Enzyme Prodrug Therapy. Cancers. 2013;5:985-97. doi:10.3390/cancers5030985.
- Leschner S, Westphal K, Dietrich N, Viegas N, Jablonska J, Lyszkiewicz M, Lienenklaus S, Falk W, Gekara N, Loessner H, et al. Tumor Invasion of Salmonella enterica Serovar Typhimurium Is Accompanied by Strong Hemorrhage Promoted by TNF-α. PLoS One. 2009;4:e6692. doi:10.1371/journal.pone.0006692. PMID:19693266
- Stern C, Kasnitz N, Kocijancic D, Trittel S, Riese P, Guzman CA, Leschner S, Weiss S. Induction of CD4+ and CD8+ anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int J Cancer. 2015;137:2019-28. doi:10.1002/ijc.29567.
- Zhao M, Yang M, Li X-M, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. PNAS. 2005;102:755-60. doi:10.1073/pnas.0408422102.
- Hiroshima Y, Zhang Y, Zhao M, Zhang N, Murakami T, Maawy A, Mii S, Uehara F, Yamamoto M, Miwa S, et al. Tumor-Targeting Salmonella typhimurium A1-R in Combination with Trastuzumab Eradicates HER-2-Positive Cervical Cancer Cells in Patient-Derived Mouse Models. PLoS One. 2015;10:e0120358. doi:10.1371/journal.pone.0120358. PMID:26047477
- Uchugonova A, Zhang Y, Salz R, Liu F, Suetsugu A, Zhang L, Koenig K, Hoffman RM, Zhao M. Imaging the different mechanisms of prostate cancer cell-killing by tumor-targeting Salmonella typhimurium A1-R. Anticancer Res. 2015;35:5225-30.
- Mercado-Lubo R, Zhang Y, Zhao L, Rossi K, Wu X, Zou Y, Castillo A, Leonard J, Bortell R, Greiner DL, et al. A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat Commun. 2016;7:12225. doi:10.1038/ncomms12225.
- Mandell D, Bennett JE. Principles and Practice of Infectious Diseases. 7th Edition. Cambridge, UK: Elsevier. 2010.
- Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209-14. doi:10.1055/s-2008-1027702.
- Zschüttig A, Auerbach C, Meltke S, Eichhorn C, Brandt M, Blom J, Goesmann A, Jarek M, Scharfe M, Zimmermann K, et al. Complete Sequence of Probiotic Symbioflor 2 Escherichia coli Strain G3/10 and Draft Sequences of Symbioflor 2 E. coli Strains G1/2, G4/9, G5, G6/7, and G8. Genome Announc. 2015;3. doi:10.1128/genomeA.01330-14.
- Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. Modulating the innate immune response by combinatorial engineering of endotoxin. PNAS. 2013;110:1469-9. doi:10.1073/pnas.1218080110.
- Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191-5. doi:10.1038/nature07830.
- Felgner S, Frahm M, Kocijancic D, Rohde M, Eckweiler D, Bielecka A, Bueno E, Cava F, Abraham W-R, Curtiss R, et al. aroA-Deficient Salmonella enterica Serovar Typhimurium Is More Than a Metabolically Attenuated Mutant. mBio. 2016;7:e01220-16. doi:10.1128/mBio.01220-16.
- Takaya A, Erhardt M, Karata K, Winterberg K, Yamamoto T, Hughes KT. YdiV: a dual function protein that targets FlhDC for ClpXP-dependent degradation by promoting release of DNA-bound FlhDC complex. Mol Microbiol. 2012;83:1268-84. doi:10.1111/j.1365-2958.2012.08007.x.
- Erhardt M, Namba K, Hughes KT. Bacterial nanomachines: the flagellum and type III injectisome. Cold Spring Harb. Perspect. Biol. 2010;2. doi:10.1101/cshperspect.a000299. PMID:20926516
- Kocijancic D, Felgner S, Schauer T, Frahm M, Heise U, Zimmermann K, Erhardt M, Weiss S. Local application of bacteria improves safety of Salmonella -mediated tumor therapy and retains advantages of systemic infection. Oncotarget. 2017;8:49988-50001. PMID:28637010
- Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952-60. doi:10.1158/0008-5472.CAN-07-2984.
- Kocijancic D, Leschner S, Felgner S, Komoll R-M, Frahm M, Pawar V, Weiss S. Therapeutic benefit of Salmonella attributed to LPS and TNF-alpha is exhaustible and dictated by tumor susceptibility. Oncotarget. 2017;8:36492-508.
- Crull K, Bumann D, Weiss S. Influence of infection route and virulence factors on colonization of solid tumors by Salmonella enterica serovar Typhimurium. FEMS Immunol Med Microbiol. 2011;62:75-83. doi:10.1111/j.1574-695X.2011.00790.x.
- Felgner S, Kocijancic D, Pawar V, Weiss S. Biomimetic Salmonella: A Next-Generation Therapeutic Vector? Trends Microbiol. 2016;24:850-2. doi:10.1016/j.tim.2016.08.007.
- Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996-2002. doi:10.1086/315497.
- Zhang Y, Zhang N, Zhao M, Hoffman RM. Comparison of the selective targeting efficacy of Salmonella typhimurium A1-R and VNP20009 on the Lewis lung carcinoma in nude mice. Oncotarget. 2015;6:14625-31.
- WHO. Typhoid vaccines: WHO position papers. Wkly Epidemiol Rec. 2008;83:49-60.
- Felgner S, Kocijancic D, Frahm M, Curtiss R, Erhardt M, Weiss S. Optimizing Salmonella enterica Serovar Typhimurium for Bacteria-mediated Tumor Therapy. Gut Microbes. 2016;7:171-7. doi:10.1080/19490976.2016.1155021.
- Zheng JH, Nguyen VH, Jiang S-N, Park S-H, Tan W, Hong SH, Shin MG, Chung I-J, Hong Y, Bom H-S, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9:eaak9537 doi:10.1126/scitranslmed.aak9537. PMID:28179508
- Kim J-E, Phan TX, Nguyen VH, Dinh-Vu H-V, Zheng JH, Yun M, Park S-G, Hong Y, Choy HE, Szardenings M, et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics. 2015;5:1328-42. doi:10.7150/thno.11432.
- Phan TX, Nguyen VH, Duong MTQ, Hong Y, Choy HE, Min JJ. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol. 2015;59:664-75. doi:10.1111/1348-0421.12333.
- Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MHG, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, et al. Efficacy of Salmonella typhimurium A1-R Versus Chemotherapy on a Pancreatic Cancer Patient-Derived Orthotopic Xenograft (PDOX). J Cell Biochem. 2014;115:1254-61. doi:10.1002/jcb.24769.
- Hiroshima Y, Zhang Y, Murakami T, Maawy A, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346-57. doi:10.18632/oncotarget.2641.
- Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, et al. Tumor-Targeting Salmonella typhimurium A1-R Arrests a Chemo-Resistant Patient Soft-Tissue Sarcoma in Nude Mice. PLoS One. 2015;10:e0134324. doi:10.1371/journal.pone.0134324. PMID:26237416
- Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:12783-90. doi:10.18632/oncotarget.7226.
- Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882. doi:10.1371/journal.pone.0160882. PMID:27500926
- Kawaguchi K, Igarashi K, Murakami T, Chmielowski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929-36.
- Murakami T, Igarashi K, Kawaguchi K, Kiyuna T, Zhang Y, Zhao M, Hiroshima Y, Nelson SD, Dry SM, Li Y, et al. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget. 2017;8:8035-42.
- Nguyen VH, Kim H-S, Ha J-M, Hong Y, Choy HE, Min J-J. Genetically Engineered Salmonella typhimurium as an Imageable Therapeutic Probe for Cancer. Cancer Res [Internet]. 2010;70:18 LP-23.
- Eom JS, Seok Kim J, Im Jang J, Kim B-H, Young Yoo S, Hyeon Choi J, Bang I-S, Lee IS, Keun Park Y. Enhancement of host immune responses by oral vaccination to Salmonella enterica serovar Typhimurium harboring both FliC and FljB flagella. PLoS One. 2013;8:e74850. doi:10.1371/journal.pone.0074850. PMID:24069357
- Olsen JE, Hoegh-Andersen KH, Casadesús J, Rosenkranzt J, Chadfield MS, Thomsen LE. The role of flagella and chemotaxis genes in host pathogen interaction of the host adapted Salmonella enterica serovar Dublin compared to the broad host range serovar S. Typhimurium. BMC Microbiol. 2013;67. doi:10.1186/1471-2180-13-67.
- Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, Von Köckritz-Blickwede M, Schilling B, Brandau S, Weiss S, Jablonska J. Type i IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982-93. doi:10.1002/ijc.29945.
- Dean P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev. 2011;1100-25.
- Xu X, Hegazy WAH, Guo L, Gao X, Courtney AN, Kurbanov S, Liu D, Tian G, Manuel ER, Diamond DJ, et al. Development of an Effective Cancer Vaccine Using Attenuated Salmonella and Type III Secretion System to Deliver Recombinant Tumor-Associated Antigens. Cancer Res. 2014;74:6260-70.
- Jeong J-H, Kim K, Lim D, Jeong K, Hong Y, Nguyen VH, Kim T-H, Ryu S, Lim J-A, Kim J Il, et al. Anti-Tumoral Effect of the Mitochondrial Target Domain of Noxa Delivered by an Engineered Salmonella typhimurium. PLoS One. 2014;9:e80050. doi:10.1371/journal.pone.0080050. PMID:24416126
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154-8.
- Johnston BJ, Novales ET. Clinical effect of Coley's toxin. A seven-year study. Cancer Chemother reports. 1962;21:43.
- Moest TP, Méresse S. Salmonella T3SSs: successful mission of the secret(ion) agents. Curr Opin Microbiol. 2013;16:38-44. doi:10.1016/j.mib.2012.11.006.
