ABSTRACT
Background: PD-1 plays a critical part in control of immune response to malignancy. Anti-PD-1 treatment is a hopeful strategy to improve the dismal prognosis of gliomas. To characterize the role of PD-1 in diffuse gliomas, we investigated its related biological process at transcriptome level and its clinical prognostic value.
Method and patients: Through Chinese Glioma Genome Atlas and TCGA datasets, we systematically reviewed a total of 994 cases with RNA-seq data and analyzed the functional annotation of PD-1 by Gene ontology (GO) analysis. Univariate and multivariate survival analysis were performed in 907 patients with survival data.
Results: We found PD-1 was significantly upregulated in glioblastoma and isocitrate dehydrogenase wild type tumors. According to TCGA transcriptional classification scheme, PD-1 expression was higher in tumors of mesenchymal subtype than other subtypes, and shown good predictive value to mesenchymal subtype. GO analysis revealed that genes significantly correlated with PD-1 were involved in essential functions associated with anti-tumor immune process. Through screening transcriptomic data, we found a strong correlation between PD-1 and immune cell populations especially for T cells. In addition, we investigated the association between PD-1 and genes related to its function, and found that PD-1 was significantly correlated with genes including TGFB1, IDO1, CD40, ICOS and SATB1, and other immune checkpoint molecules including CTLA4, LAG3, TIM3 and CD276. Survival analysis suggested that higher PD-1 expression was independently associated with worse prognosis of patients with diffuse gliomas.
Conclusion: Our results indicated that PD-1 was involved in key steps of anti-tumor immune process and independently predicted worse prognosis in diffuse gliomas. These findings may expend our understanding of potential anti-PD-1 treatments.
Introduction
Along with increasing understanding of tumor immune biology, recent breakthroughs in cancer immunotherapies have shed a new light on cancer therapy. A mass of data has elucidated the critical role of immune checkpoints in regulating immune response in malignancies. One prominent immune checkpoint is programmed cell death 1 (PD-1), encoded by the Pdcd1 gene. PD-1 interacts with programmed death ligand 1 (PD-L1), thus inhibits T cell activation and mediates an immunosuppressive effect in tumors.Citation1 Immunotherapy targeting PD-1 has shown unprecedented efficacy in many cancers including melanoma, non-small cell lung cancer and renal cancer, that were previously endowed with dismal prognosis.Citation2
Diffuse gliomas are the most common primary brain tumors in adults,Citation3 and frustratingly, almost incurable. Among diffuse gliomas, glioblastoma (GBM) is the most prevalent and invasive type. Despite treated with radical radio- and chemo-therapy, the median overall survival (OS) of patients with GBM remains to be only 14.6 months.Citation4 New treatment strategies are urgently needed to cure this fatal disease. Recently, it has been revealed that functional lymphatic vessels are lining the dural sinuses in central nervous system, which further justified the researches in neuroimmunology.Citation5,Citation6 Immunotherapy research in cancers has also made great progress in the past few years.Citation7-9 Currently, several clinical trials of anti-PD-1 therapies are being conducted in patients with GBM. Some latest results have shown durable disease control and well tolerance of patients. Anti-PD-1 therapies could be hopeful strategies to improve the prognosis of patients with gliomas.
Interactions between immunotherapy and cancer involve a complex network of molecules and pathways. Some biomarkers have been identified to predict responses to immune checkpoint blockade in several types of cancer.Citation10 Among them, the predictive role of PD-L1 have been intensively investigated. However, the results are contradicting. Some types of tumors have shown correlation of PD-L1 expression in tumors and response to anti-PD-1 therapy,Citation11,Citation12 while other tumors show response regardless of PD-L1 status.Citation13,Citation14 As the complexity of anti-tumor immune response, deeply investigating the biological process of PD-1-PD-L1 axis may help to guide anti-PD-1/PD-L1 therapy. This study evaluated PD-1 expression status and related biological process by analyzing RNA-seq data across two databases including a total of 994 glioma samples and to our best knowledge, this is the largest and comprehensive study characterizing PD-1 expression in whole grade glioma tumor masses.
Methods
Sample and data collection
Transcriptome data of patients diagnosed with diffuse glioma (WHO II-IV) were acquired from 2 independent databases: The Chinese Glioma Genome Atlas (CGGA) dataset (n = 325) (http://www.cgga.org.cn) and The Cancer Genome Atlas (TCGA) dataset (n = 669) (http://cancergenome.nih.gov/). Clinical specimen collection and data generating process in details of CGGA were described in our previous work.Citation15 All aspects of this study were approved by the Ethics Committee of Capital Medical University, Beijing, China. All participants provided informed consents for CGGA project.
IDH mutation detection
For the cohort of CGGA, IDH1/2 mutation status was examined by the method of pyrosequencing which has been described in our previous work.Citation16 Meanwhile, IDH mutation data for the TCGA cohort were obtained by whole exon sequencing or pyrosequencing.
Statistical analysis
In this study, statistical analysis was mainly performed by using R language (https://www.r-project.org/) with several publicly available packages. Survival curve was generated by Kaplan-Meier method based on log-rank test. Multivariate survival analysis was performed by using Cox proportional hazards model. A probability value (p) < 0.05 was considered to be significant in this study.
Results
PD-1 expression status
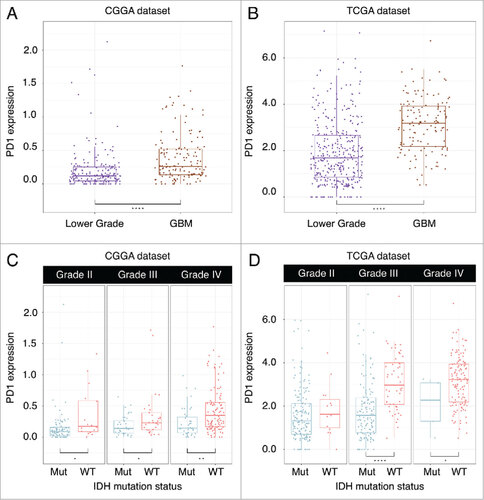
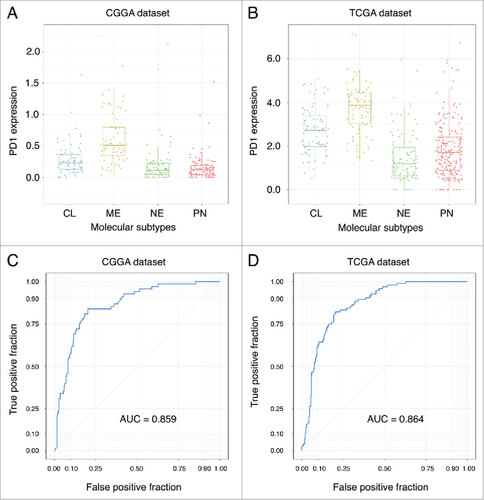
Higher PD-1 expression was found in GBM than lower grade gliomas in both CGGA dataset (Student-t test, p < 0.001) () and TCGA dataset (Student-t test, p < 0.001) (). When taking into account IDH mutation status, we found tumors possessing IDH mutation expressed lower PD-1 in grade II (Student-t test, p = 0.011), grade III (Student-t test, p = 0.024) and GBM (Student-t test, p = 0.001) in CGGA dataset (). For TCGA dataset, grade III (Student-t test, p < 0.001) glioma and GBM (Student-t test, p = 0.040) with IDH mutation expressed lower PD-1 (D). In both CGGA and TCGA dataset, PD-1 upregulating was seen in mesenchymal subtype than other subtypes (Student-t test, p < 0.001, respectively) (). In addition, we found PD-1 expression status can serve as a good predictor for mesenchymal subtype gliomas in both CGGA (AUC = 0.859) and TCGA (AUC = 0.864) datasets ().
PD-1 related biological process
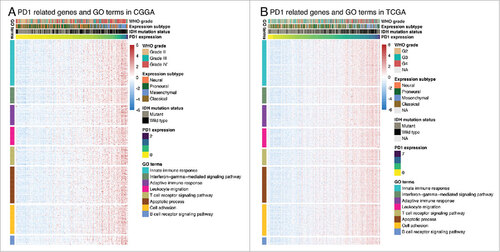
Genes significantly correlated with PD-1 expression (Pearson R > 0.4) were screened out in CGGA (361 genes) and TCGA (500 genes) datasets. These genes were further analyzed by Functional Annotation Tool online (DAVID, https://david.ncifcrf.gov/). We found that these genes were mainly involved in immune related biological process, including innate/adaptive immune response, T/B cell receptor signaling pathway, leukocyte migration and interferon-gamma-mediated signaling pathway ().
Association of PD-1expression and immune cell populations
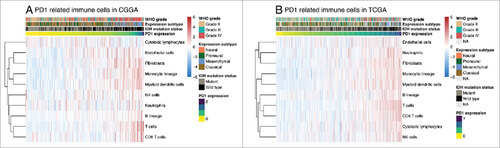
Through the Microenvironment Cell Populations-counter method described by Etienne Becht et all,Citation17 we evaluated the association between PD-1 and immune cell populations from transcriptomic data. A strong correlation between PD1 and T cells, Monocytic lineage and Myeloid dendritic cells was seen ().
Association of PD-1expression and immune system-related metagenes
To further investigate the role of PD-1 in anti-tumor immune response, we evaluated its association with seven immune related metagenes.Citation18 We found PD-1 expression was positively correlated with 6 of these metagenes except IgG (Supplementary Fig. S1).
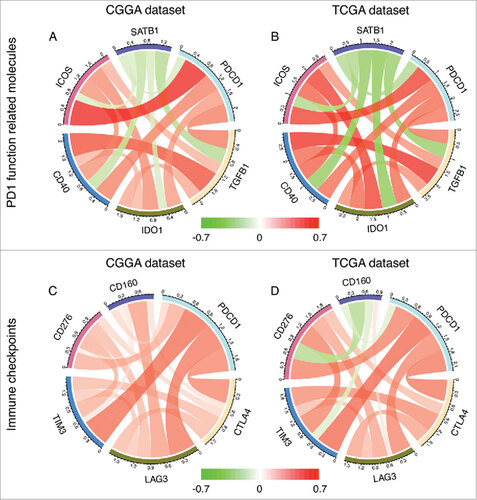
Correlation of PD-1 and immune related molecules
Through Pearson correlation, PD-1 was found to significantly correlated with molecules associated with its function including TGFB1 (CGGA, r = 0.503; TCGA, r = 0.534), IDO1 (CGGA, r = 0.403; TCGA, r = 0.567), CD40 (CGGA, r = 0.490; TCGA, r = 0.562), ICOS (CGGA, r = 0.693; TCGA, r = 0.667) and SATB1 (CGGA, r = -0.229; TCGA, r = -0.410) (), and other immune checkpoint molecules including CTLA4 (CGGA, r = 0.471; TCGA, r = 0.518), LAG3 (CGGA, r = 0.514; TCGA, r = 0.469), TIM3 (CGGA, r = 0.551; TCGA, r = 0.565) and CD276 (CGGA, r = 0.326; TCGA, r = 0.493) ().
Survival analysis of PD-1 expression status
As PD-1 expression was correlated with expression subtypes and WHO grades, we then explored the prognostic value of PD-1 in all grade gliomas as well as in GBM. Kaplan-Meier analysis demonstrated that higher PD-1 expression was associated with worse outcome in both whole grade gliomas patients and GBM patients in CGGA and TCGA datasets (). COX proportional hazard model was further performed, taking key clinical and molecular factors into account. In CGGA dataset, univariate and multivariate cox analysis showed that PD-1 was an independent prognosticator for glioma patients and this result was well validated in TCGA dataset ().
Figure 6. Survival differences of diffuse glioma and GBM patients with high and low PD-1 expression status in CGGA and TCGA datasets.
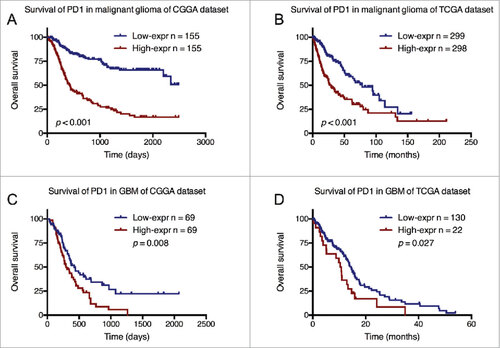
Table 1. Univariate and multivariate Cox analysis for factors potentially influence overall survival in CGGA and TCGA datasets.
Discussion
To date, this is the largest and most comprehensive study characterizing the expression pattern of PD-1 together with its related genetic features and prognostic values in diffuse gliomas. We revealed that PD-1 tended to express more in certain grades and molecular subtypes of glioma. Genes correlated with PD-1 expression were mainly involved in key roles of anti-tumor immune responses. To the clinical aspects, higher PD-1 expression was independently associated with worse prognosis of patients with gliomas.
Through two large datasets of different races, we depicted the PD-1 expression pattern at transcriptional level in diffuse glioma. Garber et alCitation19 investigated the expression of PD-1 protein across all glioma grades by using immunohistochemistry in 347 patients. Their work greatly increased our understanding of PD-1 expression in glioma. In this study, we asked the expression pattern of PD-1 at transcriptional level with nearly one thousand glioma samples in two large independent databases. We found that PD-1 expression in GBM was significantly higher than that in other grades of gliomas, which was in line with the previous finding of Garber et al mentioned above. In addition, tumors bearing IDH mutation were found to express lower level of PD-1, indicating the IDH status may be involved in the immune processes of glioma patients. Based on gene expression, GBM had been divided into four molecular subtypes: proneural, neural, classical, and mesenchymal.Citation20 The mesenchymal subtype is characterized of mesenchymal differentiation, and associated with worse outcome and treatment resistance.Citation21,Citation22 Interestingly, we noticed mesenchymal GBM subtype expressed significantly higher level of PD-1 than other subtypes, and PD-1 expression status may act as a good predictor for mesenchymal subtype gliomas. These findings were consistent with previous study that mesenchymal glioblastomas were more involved in anti-tumor inflammatory responses than other subtypes.Citation23
At present, most data on PD-1 related genetic functions have been mainly derived from melanomas. In this study, we investigated these biology process in diffuse gliomas, and revealed that the most correlated biological function with PD-1 were immune response, including T/B cell receptor signaling pathway and leukocyte migration and so on. In addition, interferon gamma (IFNγ) has been revealed to take part in upregulating PD-L1 on tumor cells.Citation24 According to our results, PD-1 expression was significantly associated with IFNγ mediated signaling pathway, which implied IFNγ involved in the PD-1-PD-L1 axis in gliomas.
In addition, through screening transcriptomic data, we evaluated the association between PD-1 and immune cell populations. This methodCitation17 had overcome some limitations of computational approaches, and could depict the immune infiltrates in tumors. We found a strong correlation between PD1 and T cells, Monocytic lineage and Myeloid dendritic cells in both datasets. Further, we investigated the association between PD-1 expression and 7 immune related metagenes. These clusters of metagenes could represent different immunological cell types. In our results, no significant correlation was seen between PD1 and the IgG metagene, which acting as a specific marker for B cells. The other 6 metagenes correlating with PD1 were mainly involved in the aspects of activities of T cells and antigen-presenting cells, which was in line with the results above, and interferon. As involving in a wide range of immune functions described above, PD-1 is believed to play a key role in anti-tumor immune responses.
Although the exact mechanisms of PD-1 expression regulation are not well understood, some molecules play important roles in this process. Transforming growth factor β (TGF-β) is a cytokine that involves in aspects of immunosuppression. Increased level of TGF-β is associated with higher PD-1 expression in tumor infiltrating T cells.Citation25 In this study, we found consistent result that PD-1 expression was significantly positively correlated with TGF-β. Special AT-rich binding protein 1 (Satb1) is a genomic organizing protein that plays key roles in restraining PD-1 expression.Citation26 In addition, Satb1 expression is down-regulated by TGF-β. Our findings showed the expression of Satb1 was significantly negatively correlated with PD-1 and TGF-β. These results suggest TGF-β and Satb1 may affect the efficacy of anti-PD-1 treatments, and could be targets for immunotherapy.
Moreover, targets for some immunological molecules may synergistically enhance anti-PD-1 treatments. Inducible T cell co-stimulator (ICOS) and CD40 are two immune-stimulatory agents involve in regulating T cell functions.Citation27,Citation28 The combined application of anti-PD-1 with anti-ICOS (NCT02904226) and anti-PD-1/L1 with anti-CD40 (NCT02304393, NCT02410512) are under evaluation by clinical trials. Meanwhile, indoleamine 2,3-dioxygenase 1 (IDO1), a metabolic modulator, could develop resistance to immunotherapy.Citation29 IDO 1 inhibiters have entered clinical evaluation in combination with PD-1 blockades for cancer therapy.Citation30 We found the above molecules are significantly positively correlated with PD-1 expression in gliomas, providing potential evidences for combination strategies to glioma treatments.
The combination of immune checkpoint blockades has shown potential benefits for cancer treatments. Besides PD-1, other immune checkpoint receptors including cytotoxic T lymphocyte antigen 4 (CTLA4), lymphocyte activation gene 3 protein (LAG3), T cell immunoglobulin domain and mucin domain 3 (TIM3), CD 276 and CD 160, have also been evaluated in preclinical or clinical research for cancer treatments.Citation31 Especially, the combination of anti-PD-1 plus anti-CTLA4 treatment has showed significantly higher response rates and improved survival in patients with metastatic melanoma.Citation14,Citation32 In addition, these studies indicated combination therapy had an acceptable safety profile. For other regimens, clinical trials of combing anti-PD-1 plus anti-LAG3 (NCT01968109), anti-PD-1 plus anti-TIM3 (NCT02817633) and anti-PD-1 plus anti-CD276 (NCT02475213) are carrying on. Since these approaches of combination therapy may serve effectively for glioma treatments, we examined the association of the expression status of PD-1 and other immune checkpoint receptors mentioned above. CTLA4, LAG3, TIM3 and CD276 all showed satisfying correlations (R > 3) with PD-1. These results suggest the combination of immune checkpoint blockades may be feasible for glioma treatments.
In addition, we found PD-1 expression status is a significantly independent prognosticator in diffuse glioma. Patients with higher PD-1 expressing tumors have significantly shorter PFS and OS. GBM and IDH-wildtype tumors, associated with worse prognosis in gliomas, were seen to express higher PD-1. These results indicate PD-1 may reflect the malignancy of gliomas.
In summary, we investigated the biological process and prognostic roles of PD-1 in nearly one thousand diffuse gliomas. These findings may help to expand our understanding to anti-tumor immunotherapy in gliomas.
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
2017ONCOIMM0580R-s02.docx
Download MS Word (2.7 MB)Funding
This study was supported by the Capital Medical Development Research Fund (2016-2-1073, 2016-1-1072), the National Key Research and Development Plan (No. 2016YFC0902500), the Beijing Postdoctoral Research Foundation (2017-ZZ-116), the National Natural Science Foundation of China (No. 81502495, 81402052, 81502495, 81702460, 81672479), Beijing Science and Technology Plan (Z141100000214009), Beijing Nova Program (No. xxjh2015B063, Z171100001117022), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201708).
References
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi:10.1126/scitranslmed.aad7118. PMID:26936508.
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. doi:10.1126/science.aaa8172.
- Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359:492-507. doi:10.1056/NEJMra0708126.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987-96. doi:10.1056/NEJMoa043330.
- Tambur AR, Roitberg B. Immunology of the central nervous system. Neurol Res. 2005;27:675-8. doi:10.1179/016164105X49544.
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337-341. doi:10.1038/nature14432. PMID:26030524
- Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1:1223-5. doi:10.4161/onci.21335.
- Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. Journal of hematology & oncology. 2013;6:74. doi:10.1186/1756-8722-6-74.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252-64. doi:10.1038/nrc3239.
- Lesterhuis WJ, Bosco A, Millward MJ, Small M, Nowak AK, Lake RA. Dynamic versus static biomarkers in cancer immune checkpoint blockade: unravelling complexity. Nat Rev Drug Discov. 2017;16:264-272. doi:10.1038/nrd.2016.233. PMID:28057932
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-65. doi:10.1056/NEJMoa1200694.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-28. doi:10.1056/NEJMoa1501824.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-33. doi:10.1056/NEJMoa1302369.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-17. doi:10.1056/NEJMoa1414428.
- Bao ZS, Chen HM, Yang MY, Zhang CB, Yu K, Ye WL, Hu BQ, Yan W, Zhang W, Akers J, et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome Res. 2014;24:1765-73. doi:10.1101/gr.165126.113.
- Yan W, Zhang W, You G, Bao Z, Wang Y, Liu Y, Kang C, You Y, Wang L, Jiang T. Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PLoS One. 2012;7:e30339. doi:10.1371/journal.pone.0030339. PMID:22291938.
- Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218. doi:10.1186/s13059-016-1070-5. PMID:27765066.
- Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11:R15. doi:10.1186/bcr2234. PMID:19272155.
- Garber ST, Hashimoto Y, Weathers S-P, Xiu J, Gatalica Z, Verhaak RGW, Zhou S, Fuller GN, Khasraw M, de Groot J, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro-Oncology. 2016;18:1357-66. doi:10.1093/neuonc/now132.
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98-110. doi:10.1016/j.ccr.2009.12.020.
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157-73. doi:10.1016/j.ccr.2006.02.019.
- Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331-46. doi:10.1016/j.ccr.2013.08.001.
- Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, Dziurzynski K, Gilbert M, Heimberger AB. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res. 2013;1:112-22. doi:10.1158/2326-6066.CIR-13-0028.
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi:10.1126/scitranslmed.3006504.
- Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA, Allison JP, Li MO. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35:123-34. doi:10.1016/j.immuni.2011.04.019.
- Stephen TL, Payne KK, Chaurio RA, Allegrezza MJ, Zhu H, Perez-Sanz J, Perales-Puchalt A, Nguyen JM, Vara-Ailor AE, Eruslanov EB, et al. SATB1 Expression Governs Epigenetic Repression of PD-1 in Tumor-Reactive T Cells. Immunity. 2017;46:51-64. doi:10.1016/j.immuni.2016.12.015.
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2-17.
- Jung K, Choi I. Emerging Co-signaling Networks in T Cell Immune Regulation. Immune Netw. 2013;13:184-93. doi:10.4110/in.2013.13.5.184.
- Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147-54. doi:10.1172/JCI31178.
- Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP, Platten M, Zitvogel L, Kroemer G, Galluzzi L, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3:e957994. doi:10.4161/21624011.2014.957994. PMID:25941578.
- Dempke WC, Fenchel K, Uciechowski P, Dale SP. Second-and third-generation drugs for immuno-oncology treatment—The more the better? European Journal of Cancer. 2017;74:55-72. doi:10.1016/j.ejca.2017.01.001.
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:1270-1. doi:10.1056/NEJMoa1504030.