ABSTRACT
We have previously identified a panel of autoantibodies (AABs), including p53, GAGE7, PGP9.5, CAGE, MAGEA1, SOX2 and GBU4-5, that was helpful in the early diagnosis of lung cancer. This large-scale, multicenter study was undertaken to validate the clinical value of this 7-AABs panel for early detection of lung cancer in a Chinese population. Two independent sets of plasma samples from 2308 participants were available for the assay of AABs (training set = 300; validation set = 2008). The concentrations of AABs were quantitated by enzyme-linked immunosorbent assay (ELISA), and the optimal cutoff value for each AAB was determined in the training set and then applied in the validation set. The value of the 7-AABs panel for the early detection of lung cancer was assessed in 540 patients who presented with ground-glass nodules (GGNs) and/or solid nodules. In the validation set, the sensitivity and specificity of the 7-AABs panel were 61% and 90%, respectively. For stage I and stage II non-small cell lung cancer (NSCLC), the sensitivity of the 7-AABs panel was 62% and 59%, respectively, and for limited stage small cell lung cancer (SCLC) it was 59%; these sensitivity values were considerably higher than for traditional biomarkers (including CEA, NSE and CYFRA21-1). Importantly, the combination of the 7-AABs panel and low-dose computed tomography (CT) scanning significantly improved the diagnostic yield in patients presenting with GGNs and/or solid nodules. In conclusion, our 7-AABs panel has clinical value for early detection of lung cancer, including early-stage lung cancer presenting as GGNs.
Introduction
Lung cancer is one of the most common cancers, as well as the leading cause of cancer-related deaths worldwide.Citation1,2 The average 5-year survival rate of lung cancer is 17.4%, but this decreases dramatically as the cancer becomes more advanced, e.g. to only 4.2% for metastasized lung cancer. Although the 10-year survival rate of stage Ia lung cancer could reach as high as 92% with optimized treatment,Citation3 approximately 85% of patients with lung cancer are diagnosed at more advanced stages, making detection of early stage lung cancer crucially important to improve the overall survival. Low-dose computed tomography (LDCT) screening is recommended in US for the early detection of lung cancer in patients at high risk,Citation4,5 and this procedure has demonstrated a sensitivity of 93.8% and a reduction in mortality of 20%.Citation6 However, LDCT lacks the accuracy to distinguish benign nodules from early stage malignant cancer, which leads to high rate of false-positive results (96.4%).Citation6,7 In addition, the cumulative radiation with repeated CT scanning raises the concern of an increased risk of developing radiation-related cancer.
Tumor-associated antigens (TAAs) produced by tumor cells during their development include tumor/testis-specific antigens, aberrantly-overexpressed or ectopically-expressed antigens, and stem cell transcriptional factors, etc.Citation8,9 Based on the immunoediting theory, TAAs are captured by the immune system and lead to the formation of autoantibodies (AABs) via humoral immune responses. Interestingly, AABs have been found to be present before the disease becomes symptomatic.Citation10-14 Therefore, they could be valuable for the early detection of lung cancer.Citation11,15,16 A previous study found that a panel consisting of 4 AABs (NOLC1, HMMR, MALAT1 and SMOX) was associated with early stage lung cancer in Chinese patients, although the specificity was relatively low (only 60%).Citation14 Given the heterogeneity of human lung cancers, researchers have tried to include more AABs to achieve higher sensitivity. In a study that used 7 AABs (p53, c-myc, HER2, NY-ESO-1, CAGE, MUC1, and GBU4-5) in European patients with lung cancer (n = 104), a sensitivity of 76% and specificity of 92% were observedCitation17; and an audit study of EarlyCDT®-Lung (6-AABs or 7-AABs) in 1600 patients also showed high specificity of 83% or 91%.Citation15 Since there are noticeable differences in the genetic makeup of European and Asian lung cancer patients, this panel of AABs may not be ideal for the Chinese population, and a similar study needs to be performed in Chinese patients to confirm these results.
In this large-scale clinical study, we validated the clinical value of a 7-AABs panel in the early detection of lung cancer in a Chinese population. We compared the sensitivity and specificity of this AAB panel with traditional tumor biomarkers, including carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and cytokeratin 19 fragment 21-1 (CYFRA21-1), in lung cancer patients with different disease stages. In addition, we analyzed the utility of the 7-AABs panel in combination with a LDCT scan to achieve higher diagnostic accuracy for patients presenting with ground-glass nodules (GGNs) and/or solid nodules.
Results
Study population
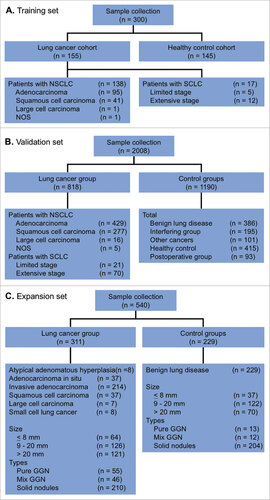
A total of 2308 participants were enrolled into the study They comprised a training set (n = 300; ) and a validation set (n = 2008; ). The clinical characteristics of the study population are summarized in . A total of 155 patients with pathologically-confirmed lung cancer (including 137 with non-small cell lung cancer [NSCLC] and 17 with small cell lung cancer [SCLC]) and 145 healthy controls were included in the training set. The validation set comprised 818 patients with lung cancer (including 722 with NSCLC and 90 with SCLC), 386 with benign lung disease, 101 with other cancers, 93 post-thoracic surgery patients, 181 interfering group patients (with different diseases), and 415 healthy controls. Most were males (63.8%) and non-smokers (66.5%).
Table 1. Baseline characteristics of all included participants.
Another 540 patients who presented with GGNs and/or nodules were identified to test the utility of the 7-AABs panel in the early detection of lung cancer (). The major clinical characteristics of this population are summarized in .
Table 2. Baseline characteristics of the patients with lung nodules/GGNs.
Determination of the reactivity of the 7 AABs in the training set
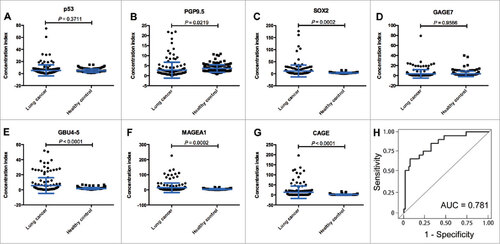
To determine the reactivity of the AABs panel, 7 antigens (p53, PGP9.5, SOX2, GAGE7, GBU4-5, MAGEA1 and CAGE) were identified from 43 cancer-related antigens, and the average concentrations of the AABs targeting these antigens were quantitated using ELISA in 300 independent serum samples (lung cancer, n = 155; healthy controls, n = 145). The results showed that the serum AAB concentrations of PGP9.5, SOX2, GBU4-5, MAGEA1 and CAGE in lung cancer patients were significantly higher than in healthy controls (P = 0.022, P < 0.001, P < 0.001, P < 0.001, and P < 0.001; respectively), but the serum concentrations of p53 and GAGE7 in lung cancers were similar to those in healthy controls (P = 0.371 and P = 0.957; respectively) []. Almost all of the AABs except PGP9.5 demonstrated good discriminative ability between lung cancer and healthy controls based on the ROC curves shown in Supplementary Fig. S1. The combined AUC for all 7 AABs in lung cancer sera versus healthy controls was 0.781 ().
Evaluation of the diagnostic performance of the 7-AABs panel in the validation Set
To further assess the diagnostic value of the 7-AABs panel in distinguishing lung cancer from benign lung diseases, other cancers, different diseases (i.e. the interfering group), postoperative patients, and healthy controls, we measured the serum concentrations of the 7 AABs in the 2008 participants included in the validation set. With the exception of PGP9.5, which did not show a significant difference between lung cancer and other cancers, virtually all of the AABs were significantly increased in patients with lung cancer as compared with benign lung diseases, other cancers, the interfering and postoperative groups, and healthy controls (Supplementary Table S1). The difference in GAGE7 concentrations reached marginal statistical significance for lung cancer versus benign lung disease (P = 0.065) and for lung cancer versus other cancers (P = 0.060).
To exclude a possible effect of surgical resection on AAB serum concentrations, we collected serum samples from 93 patients before and after surgery. The results showed that the serum concentrations of each AAB did not change significantly after surgery (Supplementary Table S2). Immunoassays of the 7 AABs in combination enhanced cancer detection and achieved a panel sensitivity of 61% and panel specificity of 90% ( and ).
Figure 3. Diagnostic performance of the 7 autoantibodies (AABs) panel in the validation set. (A) sensitivity (patients with lung cancer); (B) specificity (control groups); (C) Venn diagram for patients who received the 7-AABs panel and/or CT scanning; (D) positive predictive value (PPV) of the 7-AABs panel combined with CT scanning in lung cancer patients. AID, autoimmune disease; BLD, benign lung disease; HC, healthy controls; OC, other cancers.
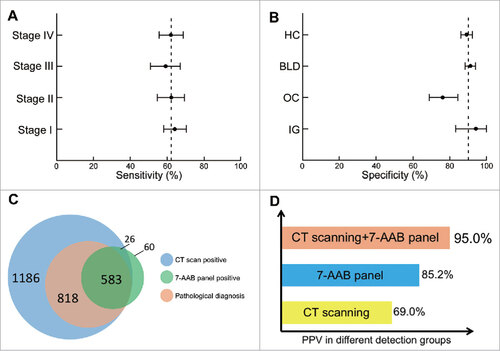
We also conducted subgroup analyses to investigate the diagnostic value of the 7-AABs panel in patients with different disease stages and histological types and in control groups. The sensitivities ranged from 59% to 64% in NSCLC patients, with a sensitivity of 62% (95% CI 55%-69%) in stage I disease, 59% (95% CI 51%-67%) in stage II disease, 62% (95% CI 55%-70%) in stage III disease, and 64% (95% CI 58%-70%) in stage IV disease. The specificities ranged from 76% to 94% in control groups, with a specificity of 91% (95% CI 88%-94%) in benign lung diseases, 76% (95% CI 69%-84%) in other cancers, 94% (95% CI 83%-100%) in autoimmune diseases, and 89% (95% CI 86%-92%) in healthy controls ( and ). Interestingly, when we compared the sensitivity values for traditional tumor markers, the7-AABs panel showed a higher sensitivity in the early stages of lung cancer (stage I + II, 60%; limited-stage SCLC, 59%) than CEA, NSE, and CYFRA 21-1 (Supplementary Fig. S3).
Diagnostic Value of the 7-AABs panel in combination with CT scanning in lung cancer patients
In the validation set, 1186 patients were found having radiological nodules and among them 818 were pathologically diagnosed with lung cancer. A total of 583 patients had positive 7-AAB assay results. Among these, 523 (included in the 1186 patients) were found to have positive radiological nodules and positive 7-AABs assay results, and 497 of the 523 patients were finally diagnosed with lung cancer by pathological examination (). The positive predictive values (PPVs) of CT scanning and the 7-AABs panel were therefore 69.0% (818/1186) and 85.2% (497/583), respectively. Of note, the 7-AABs panel in combination with CT scanning significantly improved the PPV when compared with AABs panel alone (95.0% vs 85.2%; P < 0.001) or with CT scanning alone (95.0% vs 69.0%; P < 0.001) [].
Effectiveness of the 7-AABs panel in early diagnosis of lung cancer
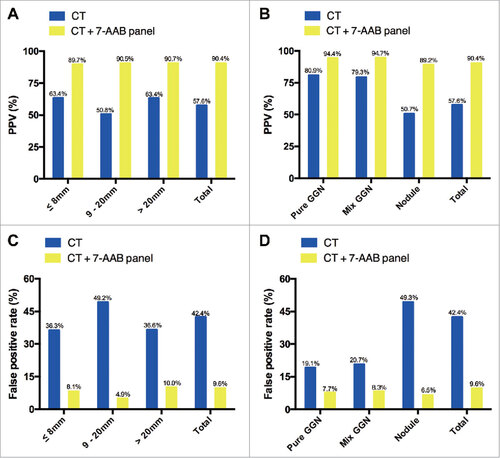
To further investigate the value of the 7-AABs panel in early diagnosis of lung cancer, we identified 540 patients who presented with radiological GGNs and/or nodules. Among these patients, 311 (57.6%) were confirmed as having malignant lesions by pathological examination. In comparison with CT scanning alone, a combination of CT scanning and the 7-AABs panel significantly increased the diagnostic accuracy of malignant lesions from a PPV of 57.6% (311/540) to 90.4% (151/167) [P < 0.001; ]. For 110 patients with radiological GGNs and/or nodules ≤8 mm in size, 64 were pathologically confirmed. In these patients, the PPV was improved from 63.4% to 89.7% (26/29; ) when we combined CT scanning and the 7-AABs panel (P = 0.013). Similarly, for patients with GGNs and/or nodules between 8 and 20 mm and >20 mm in size, the diagnostic accuracy was increased from 50.8% to 90.5% (P < 0.001) and from 63.4% to 90.7% (P < 0.001), respectively, when CT scanning and the 7-AABs assay were combined (). The combination of CT scanning and the 7-AABs assay also improved the diagnostic accuracy in patients with pure and mixed GGNs from 80.9% (55/68) and 79.3% (46/58) to 94.4% (17/18) and 94.7% (18/19), respectively (). In patients with just nodules, a similar trend was observed: the diagnostic accuracy was only 50.0% with CT scanning alone but was increased to 89.1% with the combination (P < 0.001; ). Moreover, CT scanning plus the 7-AABs assay significantly decreased the false positive rate in patients with distinct size and pathological types GGNs and/or nodules ( and ).
Figure 4. Effectiveness of the 7 autoantibodies (AABs) panel in patients with radiological ground-glass nodules (GGNs) and/or nodules. (A) sub-analysis of PPV according to size; (B) sub-analysis of PPV according to pathological type; (C) sub-analysis of the false-positive rate according to size; (D) sub-analysis of the false-positive rate according to pathological type.
Discussion
Early diagnosis of lung cancer in asymptomatic patients remains challenging. In this study, we identified a panel of 7 AABs and subsequently performed the first prospective large-scale study investigation of 2008 subjects to validate its diagnostic value in a Chinese population. Our results showed that this 7-AABs panel has clinical value to aid the diagnosis of lung cancer, including early-stage disease. We also validated its value in patients presenting with GGNs or solid nodules, and showed that use of the 7-AABs panel in combination with CT scanning significantly increased the PPV in comparison with CT alone. Taken together, these findings suggest that the7-AABs panel can be incorporated into protocols for early detection of lung cancer in Chinese patients.
Although LDCT screening is by far the only approach to reduce the mortality of lung cancer in US, it does have drawbacks, including a high false-positive rate and potential radiation toxicity. In addition, its real applicability and value in mortality reduction has been challenged by randomized trials from regions outside the US, e.g. the DANTE study performed in Europe.Citation18,19 Thus, there is an urgent need to find approaches that can be used as an alternative to or together with LDCT screening such as the use of biomarkers. Various biomarkers using circulating tumor cells (CTCs), microRNAs, the DNA methylation status, and autoantibody panels have been developed as noninvasive methods to detect lung cancer in asymptomatic patients.Citation17,20-22 Among these, the autoantibody panel EarlyCDT®-Lung from Oncimmune Inc.Citation15,23,24 and PAULA's (Protein Assay Using Lung Cancer Analytes) test from 20/20 Genesystems have both demonstrated promising results.Citation16 PAULA's test, which uses a 4-biomarker panel including 3 tumor antigens (CEA, CA-125, and CYFRA 21–1) and 1 autoantibody marker (NY-ESO-1) to discriminate NSCLC patients from controls, was found to have a sensitivity of 77% and specificity of 80% in a validation set of 150 patients.Citation25 EarlyCDT®-Lung, which uses a panel of 6 (p53, NY-ESO-1,CAGE, GBU4-5, Annexin I, and SOX2) or 7 (p53,NY-ESO-1, CAGE, GBU4-5, SOX2, HuD, and MAGE A4) AABs, was found to have a specificity/sensitivity of 83%/46% for the 6 AABs panel and 91%/37% for the 7 AABs panel.Citation15 Interestingly, several other studies have also reported the specificity of AABs to be generally high.Citation13,25-27 Consistent with these observations, we found a specificity of around 90% with our 7-AABs panel in this multicenter prospective study involving 2008 participants irrespective of the histological types and clinical stages, suggesting that this AAB panel is highly specific, even in the early stages of lung cancer.
In comparison with the 6-AAB and the 7-AAB panels of the EarlyCDT®-Lung test, our AABs panel achieved higher sensitivity. This is probably due to a combination of various factors: (1) Firstly, we used different AABs. Three of the 7-AABs panel, i.e. GAGE7, MAGE-A1 and PGP9.5, are not included in the EarlyCDT®-Lung test. Interestingly, GAGE7 and MAGE-A1 are cancer/testis antigens and PGP9.5 is an ectopically expressed protein. (2) Secondly, the ethnic groups were different. It is theoretically possible that the concentrations of AABs and the predominance of certain AABs could be different in Chinese populations in comparison with European populations, reflecting the variation of the mutation spectrum between Asian and European populations. (3) Thirdly, the approaches to quantitate the serum concentrations of AABs were different. Autoantibodies in human blood are mixed polyclonal antibodies, and variations among individuals are predicted to be substantial. Thus, choosing the appropriate calibrator is another hurdle to commercialize autoantibody detection kits. Taken together, the sensitivity of our 7-AABs panel was as high as 61% in this study, and it had a significantly higher sensitivity than traditional antigens such as CEA, NSE, and CYFRA 21-1.
Because of the utility of our 7-AABs panel, we also tested its combination with CT imaging and found it could improve the PPV of CT imaging up to 95% for the early diagnosis of lung cancer. As ELISA of AABs is relatively low cost, non-invasive, and easy-to-perform, detection kits based on this AABs panel hold promise for early detection of lung cancers, especially when combined with CT imaging. Indeed, the EarlyCDT®-Lung test is currently being evaluated in a large-scale screening study in patients at high risk of lung cancer in Scotland, UK. We also validated the diagnostic value of our 7-AABs panel in patients with early stage lung cancer presenting with GGNs or solid nodules. Although recent advances in diagnostic imaging modalities and the widespread use of chest CT screening for high-risk individuals have increased the detection of pure or mixed GGNs, we found that combination of CT scanning and our 7-AABs panel could significantly increase the diagnostic accuracy of lung malignancies to 90.4% as compared with 57.6% with CT scanning alone (P < 0.001). In addition, a significant increase in diagnostic accuracy was also observed for different sizes and types of radiological GGNs and/or nodules, suggesting that our 7-AABs panel is helpful in distinguishing malignant GGNs from benign GGNs and thus in making appropriate therapeutic decisions.
Besides AABs, several other circulating biomarkers also showed promising initial results for the noninvasive diagnosis of lung cancer such as CTCs, ctDNA, microRNAs, the DNA methylation status.Citation17,20-22 Among them, a circulating marker of compliment activation (c4d) and two circulating miRNA signatures have reached the prospective testing stage.Citation28-30 Just like the biomarker in the current study, we hope more and more validated effective biomarkers to aid the early diagnosis of lung cancer in the screening setting. These biomarkers will be able to reduce screening costs, reduce the incidence of over-diagnosed lung cancers, and eliminate the number of false positives on surgery of screening-detected nodules.
In summary, this study, which is the first large-scale investigation to validate the diagnostic value of a 7-AABs panel in lung cancer, achieved a sensitivity of 61% and a specificity of 90%. This 7-AABs panel proved to be better than traditional tumor markers such as CEA, NSE, and CYFRA 21-1 to aid early diagnosis, and its value was consistent across different stages and pathological types of lung cancer, including early stage lesions presenting as GGNs or solid nodules. In addition, the 7-AABs panel improved PPV and reduced the false-positivity rate with LDCT screening. Taken together, our 7-AABs panel showed robust potential for the early diagnosis of lung cancer, suggesting that it should be further implemented in clinical practice.
Materials and methods
Patients and blood samples
Blood samples were collected from 2308 participants (), including patients with histopathologically-confirmed lung cancer, benign pulmonary disease, and other cancers, an interfering patient group (with different diseases), and healthy controls at 6 centers in China, including Shanghai Pulmonary Hospital, Beijing Chest Hospital, Henan Provincial Cancer Hospital, Zhejiang Provincial Cancer Hospital, Hubei General Hospital, and Nanjing Drum Tower Hospital. Approval to use the serum samples was obtained from an Institutional Review Board, and signed consent forms were obtained from each participant. The protocol for the study was reviewed and approved by the ethical committees of each hospital prior to sample collection.
The participants' major clinicopathological characteristics including demographic information, smoking history, and lung cancer histology (WHO classification) were collected.Citation31 A ‘never smoker’ was defined as a person who had smoked less than 100 cigarettes during his/her lifetime.
Quantitation of autoantibodies in serum samples
The serum concentrations of autoantibodies were quantitated by enzyme-linked immunosorbent assay (ELISA). Briefly, TAAs were expressed in E. coli and purified via multiple steps including affinity chromatography and size exclusion chromatography. Each TAA protein contains a streptavidin tag for immobilization into microwells, which were pre-absorbed with bovine serum albumin (BSA)-biotin in an immobilization buffer. Serum samples were diluted with phosphate-buffered saline (PBS) [1:109] and added to the microwells (50 μL/well) for binding of the autoantibodies to their respective TAAs. After washing off the extra proteins with washing buffer, horseradish peroxidase (HRP)-conjugated anti-human IgG was added to each well to bind autoantibodies. ELISA substrate TMB (3,3’,5,5’- tetramethylbenzidine) was added for color development followed by the addition of stopping solution (1N HCl), and the absorbance was read at optical density (OD) 450 nm on a spectrometer.
The serum concentrations of autoantibodies were quantitated by using a standard curve of serial dilutions of a positive antibody. All incubations were carried out with shaking at room temperature and plates were washed three times with PBS containing Tween 20 (0.1% v/v; Sigma, Poole, UK) between each step.
Statistical analysis
The optimal cutoff values for the 7 AABs were defined as an OD value greater than either the mean plus 2 standard deviations (SDs) or the mean plus 3 SDs of the normal cohort in the training set.Citation32 The cutoff values were optimized using a Monte Carlo direct search method to find a set of antigen-specific cutoffs yielding the maximum sensitivity for a fixed specificity of 90%.Citation33 These cutoff values were used for evaluating the diagnostic power in the validation set. The more stringent cut-off point (3 SDs) was applied to the PGP9.5, SOX2, p53, GAGE7 and CAGE autoantibody assays, incorporating, on average, 99% of the distribution of the data. An OD value greater than the mean plus 2 SDs of the normal population was applied to the GBU4-5 and MAGEA1 autoantibody assays. In all autoantibody assays in normal plasma, values that were greater than the mean plus 5 SDs were removed to produce cut-off values, but were included in the analysis of specificity. All assays were performed in triplicate.
To approve the sensitivity and specificity results, the area-under-the-curve (AUC) and standard error (SE) for the respective receiver operating characteristic (ROC) curves were calculated.Citation32,33 The ROC curves were constructed by calculating the sensitivity/specificity of the test for a succession of deviations from the original cut-offs, with the same deviation for each antigen in the panel.
Mean and SD values were calculated to describe the study population. The number and proportion of positive samples were reported with 95% exact confidence intervals (95% CI) for binomial proportions. χ2-tests were applied to flag where the proportion of positive results showed a significant difference between the lung cancer patients and healthy controls. Differences between 2 groups were compared using the Mann-Whitney U-test, and for 3 or more groups, the Kruskal-Wallis test was used. A 2-sided P-value < 0.05 was considered statistically significant. All analyses were performed using SPSS® 20.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA).
Disclosure of potential conflicts of interest
Jing Zhou, Xiaopeng Liu, and Xuejun Hu are employees of Hangzhou Cancer Probe Biotechnology Company. The other authors declare no potential conflicts of interest.
Author's contributions
Shengxiang Ren and Caicun Zhou designed this study. Shengxiang Ren, Tao Jiang, Shucai Zhang, Yayi He, Zhiyong Ma, Hourong Cai, Yan Li, and Xiaohong Xu collected the blood samples and clinical data. Jing Zhou, Xiaopeng Liu, and Xuejun Hu performed the panel assays, and Tao Jiang and Shengxiang Ren performed the statistical analyses. Tao Jiang, Jun Zhang, Shengxaing Ren, and Caicun Zhou drafted the manuscript. Caicun Zhou, Jun Zhang, Hui Yu, and Fred R. Hirsch provided critical comments and suggestions. All authors read and approved the final version of the manuscript.
Acknowledgments
The Autoantibodies (AABs) Kit including the 7-AABs panel used in this study was approved for early diagnosis and to aid CT diagnosis of lung cancer by the China Food and Drug Administration (CFDA) based on the prospective data reported in this manuscript.
Funding
This study was sponsored by the Hangzhou Cancer Probe Biotechnology Company, Hangzhou, China, and supported in part by grants from the National Natural Science Foundation of China (No. 81772467, 81672286 and 81372392), an IASLC Fellowship award (S. Ren and Y. He), the Chronic Diseases Program of Shanghai Shen Kang Pharmaceutical Development Co. Ltd (No. SHDC 12015314), the Outstanding Young Doctor Program of the Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097), and “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 16SG18).
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-32. doi:10.3322/caac.21338. PMID:26808342
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-89. doi:10.3322/caac.21349.
- International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763-71. doi:10.1056/NEJMoa060476. PMID:17065637
- Screening. GoLC. Chicago (IL): American Lung Association; 2015.
- Statement. SfLCUPSTFR. Rockville (MD): US Preventive Services Task Force; 2013.
- National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873. PMID:21714641
- Boiselle PM. Computed tomography screening for lung cancer. JAMA. 2013;309(11):1163-70. doi:10.1001/jama.2012.216988. PMID:23512063
- Chen YT S, Obata Y, Old LJ. Identification of human tumor antigens by serological expression cloning. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2000. p. 557-70.
- Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. Database of T cell-defined human tumor antigens: the 2013 update. Cancer Immun. 2013;13:15. PMID:23882160
- Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12(2):136-43. PMID:12582023
- Li Y, Karjalainen A, Koskinen H, Hemminki K, Vainio H, Shnaidman M, Ying Z, Pukkala E, Brandt-Rauf PW. p53 autoantibodies predict subsequent development of cancer. Int J Cancer. 2005;114(1):157-60. doi:10.1002/ijc.20715. PMID:15523685
- Storr SJ, Chakrabarti J, Barnes A, Murray A, Chapman CJ, Robertson JF. Use of autoantibodies in breast cancer screening and diagnosis. Expert Rev Anticancer Ther. 2006;6(8):1215-23. doi:10.1586/14737140.6.8.1215. PMID:16925487
- Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1(6):513-9. doi:10.1016/S1556-0864(15)30352-X. PMID:17409910
- Yao Y, Fan Y, Wu J, Wan H, Wang J, Lam S, Lam WL, Girard L, Gazdar AF, Wu Z, et al. Potential application of non-small cell lung cancer-associated autoantibodies to early cancer diagnosis. Biochem Biophys Res Commun. 2012;423(3):613-9. doi:10.1016/j.bbrc.2012.06.050. PMID:22713465
- Jett JR, Peek LJ, Fredericks L, Jewell W, Pingleton WW, Robertson JF. Audit of the autoantibody test, EarlyCDT(R)-lung, in 1600 patients: an evaluation of its performance in routine clinical practice. Lung Cancer. 2014;83(1):51-5. doi:10.1016/j.lungcan.2013.10.008. PMID:24268382
- Doseeva V, Colpitts T, Gao G, Woodcock J, Knezevic V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med. 2015;13:55. doi:10.1186/s12967-015-0419-y. PMID:25880432
- Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Tureci O, Wiewrodt R, Barnes AC, Robertson JF. Autoantibodies in lung cancer: Possibilities for early detection and subsequent cure. Thorax. 2008;63(3):228-33. doi:10.1136/thx.2007.083592. PMID:17932110
- Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, Passera E, Angeli E, Chiarenza M, Aranzulla G, et al. A randomized study of lung cancer screening with spiral computed tomography: Three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180(5):445-53. doi:10.1164/rccm.200901-0076OC. PMID:19520905
- Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, Brambilla G, Angeli E, Aranzulla G, Chiti A, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191(10):1166-75. doi:10.1164/rccm.201408-1475OC. PMID:25760561
- Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, Zhao C, Deng Q, Li W, Gao G, et al. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol. 2015;10(8):1163-71. doi:10.1097/JTO.0000000000000606. PMID:26200270
- Jiang T, Ren S, Zhou C. Role of circulating-tumor DNA analysis in non-small cell lung cancer. Lung Cancer. 2015;90(2):128-34. doi:10.1016/j.lungcan.2015.09.013. PMID:26415994
- Powrozek T, Krawczyk P, Kucharczyk T, Milanowski J. Septin 9 promoter region methylation in free circulating DNA-potential role in noninvasive diagnosis of lung cancer: Preliminary report. Med Oncol. 2014;31(4):917. doi:10.1007/s12032-014-0917-4. PMID:24633736
- Chapman CJ, Healey GF, Murray A, Boyle P, Robertson C, Peek LJ, Allen J, Thorpe AJ, Hamilton-Fairley G, Parsy-Kowalska CB, et al. EarlyCDT(R)-Lung test: Improved clinical utility through additional autoantibody assays. Tumour Biol. 2012;33(5):1319-26. doi:10.1007/s13277-012-0379-2. PMID:22492236
- Lam S, Boyle P, Healey GF, Maddison P, Peek L, Murray A, Chapman CJ, Allen J, Wood WC, Sewell HF, et al. EarlyCDT-Lung: An immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila). 2011;4(7):1126-34. doi:10.1158/1940-6207.CAPR-10-0328. PMID:21733826
- Qiu J, Choi G, Li L, Wang H, Pitteri SJ, Pereira-Faca SR, Krasnoselsky AL, Randolph TW, Omenn GS, Edelstein C, et al. Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol. 2008;26(31):5060-6. doi:10.1200/JCO.2008.16.2388. PMID:18794547
- Ma L, Yue W, Teng Y, Zhang L, Gu M, Wang Y. Serum anti-CCNY autoantibody is an independent prognosis indicator for postoperative patients with early-stage nonsmall-cell lung carcinoma. Dis Markers. 2013;35(5):317-25. doi:10.1155/2013/935943. PMID:24167380
- Ma L, Yue W, Zhang L, Wang Y, Zhang C, Yang X. Clinical significance and diagnostic value of Survivin autoantibody in non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2010;13(7):706-12. PMID:20673487
- Ajona D, Pajares MJ, Corrales L, Perez-Gracia JL, Agorreta J, Lozano MD, Torre W, Massion PP, de-Torres JP, Jantus-Lewintre E, et al. Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer. J Natl Cancer Inst. 2013;105(18):1385-93. doi:10.1093/jnci/djt205. PMID:23940286
- Bianchi F, Nicassio F, Marzi M, Belloni E, Dall'olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G, Di Fiore PP. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol Med. 2011;3(8):495-503. doi:10.1002/emmm.201100154. PMID:21744498
- Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, Calabro E, Croce CM, Pastorino U, Sozzi G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108(9):3713-8. doi:10.1073/pnas.1100048108. PMID:21300873
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244-85. doi:10.1097/JTO.0b013e318206a221. PMID:21252716
- Sharma B, Jain R. Right choice of a method for determination of cut-off values: A statistical tool for a diagnostic test. Asian J Med Sci. 2014;5(3):30-34. doi:10.3126/ajms.v5i3.9296
- Boyle P, Chapman CJ, Holdenrieder S, Murray A, Robertson C, Wood WC, Maddison P, Healey G, Fairley GH, Barnes AC, et al. Clinical validation of an autoantibody test for lung cancer. Ann Oncol. 2011;22(2):383-9. doi:10.1093/annonc/mdq361. PMID:20675559