ABSTRACT
Here, we report on a novel bispecific antibody-derivative, designated RTX-CD47, with unique capacity for CD20-directed inhibition of CD47-SIRPα “don't eat me” signaling. RTX-CD47 comprises a CD20-targeting scFv antibody fragment derived from rituximab fused in tandem to a CD47-blocking scFv. Single agent treatment with RTX-CD47 triggered significant phagocytic removal of CD20pos/CD47pos malignant B-cells, but not of CD20neg/CD47pos cells, and required no pro-phagocytic FcR-mediated signaling. Importantly, treatment with RTX-CD47 synergistically enhanced the phagocytic elimination of primary malignant B cells by autologous phagocytic effector cells as induced by therapeutic anticancer antibodies daratumumab (anti-CD38), alemtuzumab (anti-CD52) and obinutuzumab (anti-CD20). In conclusion, RTX-CD47 blocks CD47 “don't eat me” signaling by cancer cells in a CD20-directed manner with essentially no activity towards CD20neg/CD47pos cells and enhances the activity of therapeutic anticancer antibodies directed to B-cell malignancies.
Introduction
Solid and hematological malignancies exploit the inhibitory CD47/SIRPα pathway to evade elimination by the immune system.Citation1–3 Specifically, binding of tumor-overexpressed CD47 with phagocyte-expressed SIRPα inhibits phagocytic removal of cancer cells and reduces the immunogenic processing of tumor antigens by macrophages and dendritic cells.Citation4–6 Consequently, both innate and adaptive anticancer immunity is suppressed. Correspondingly, CD47 overexpression is associated with poor clinical prognosis in various malignancies.Citation3,7
Antibodies that block CD47/SIRPα interaction are of potential clinical interest and have yielded promising preclinical anti-tumor activity in various murine tumor models. CD47-blocking antibodies were shown to enhance the induction of antibody-dependent cellular phagocytosis (ADCP) of cancer cells upon treatment with therapeutically used anticancer antibodies. For instance, cotreatment of rituximab with the CD47-blocking murine antibody B6H12 synergized the phagocytic elimination of xenografted human CD20pos NHL cancer cells in various mouse tumor models in the absence of noticeable toxicity.Citation8 Correspondingly, humanized CD47-blocking antibodies Hu5F9-G4 and CC-90002 are currently being evaluated in Phase 1 clinical trials in patients with advanced solid and hematological malignancies (ClinicalTrials.gov identifier: NCT02216409 and NCT02367196).
However, the lack of CD47-related toxicity as observed in mouse models may not accurately reflect the impact of a generalized blockade of CD47 in humans, as the antibody B6H12 does not cross-react with mouse CD47.Citation9 CD47 is broadly expressed on normal cells, including mesenchymal stromal cells and blood cells, in particular erythrocytes and platelets.Citation9 Thus, a generalized blockade of CD47/SIRPα interaction may result in phagocytosis and immunological processing of normal healthy cells. Therefore, ubiquitous on-target/off-tumor inhibition of CD47/SIRPα interaction by conventional CD47-blocking antibodies in humans may associate with toxicity. Moreover, the abundant expression of CD47 throughout the human body is likely to form a massive “sink” that may limit tumor accretion of CD47-blocking antibodies.
Recently, two bispecific antibodies (bsAb) designed to enhance the selectivity of CD47-blocking activity towards CD20- and CD19-expressing cells, respectively.Citation10,11 The CD20-directed/CD47-blocking bsAb was of the so-called dual variable-domain immunoglobulin (DVD-Ig) format, whereas the CD19-directed/CD47-blocking bsAb was of the so-called κλ-body format. Both these bsAbs contained a functional IgG1 Fc effector domain which appeared to be required for their pro-phagocytic activity. However, the presence of functional Fc domains in these bsAbs may result in premature off-target activation of Fc-receptor (FcR)-expressing phagocytes which is associated with systemic toxicity.Citation12 Further, off-target Fc/FcR-binding may reduce the accretion of these bsAbs at the tumor cell surface.
Here, we report on an alternate bsAb format termed RTX-CD47 that consists of a CD47-blocking single chain fragment of variable regions (scFv) antibody fragment genetically fused in tandem to a CD20-targeting scFv derived from rituximab. This bispecific tandem scFv (bi-scFv) does not contain an Fc domain and was designed to have monovalent binding specificity for CD20 and CD47, respectively (for schematic representation see ). RTX-CD47 was constructed to promote CD20-directed blockade of CD47-SIRPα “don't eat me” signaling towards cancer cell types that express both CD20 and CD47, while preventing toxicity associated with untimely FcR cross-linking.
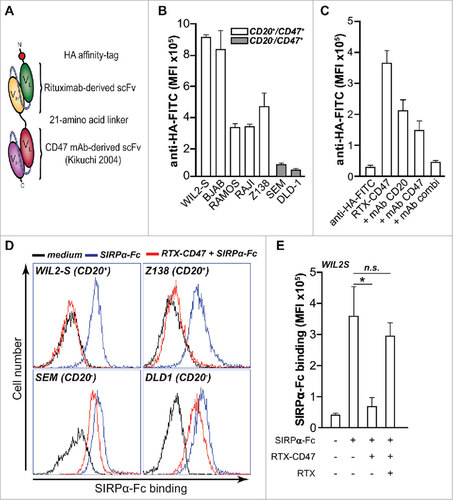
Figure 1. CD20-directed blocking of CD47-SIRPα interaction by RTX-CD47 (A) Schematic representation of RTX-CD47 comprising a CD20-targeting scFv derived from rituximab genetically fused to a CD47-blocking scFv and lacking an Fc domain. (B) RTX-CD47 selectively binds to CD20posCD47pos cell lines and not to CD20negCD47pos cell lines. Binding of RTX-CD47 to the cells was determined by flow cytometry using an HA tag antibody. (C) RTX-CD47 binding to Ramos CD20pos/CD47pos cells in the presence or absence of CD20-blocking antibody RTX (5 μg/mL) and/or CD47-blocking antibody B6H12 (5 μg/mL). Binding of RTX-CD47 could only be blocked by simultaneously adding excess amounts of CD20- and CD47-competing MAbs. (D) SIRPα-Fc binding to CD47 was blocked by RTX-CD47 on CD20/CD47 double positive cells (WIL2S and Z138) and not on CD20negCD47pos (SEM and DLD1). Binding of SIRPα-Fc to the cell surface of the cells was determined by flow cytometry using human recombinant SIRPα-Fc (5μg/ml) followed by staining with mouse anti-SIRPα and Alexa Fluor 488-conjugated goat anti-mouse IgG. (E) Bar graph displaying the MFI of the SIRPα-Fc binding as in (D), with the addition of a condition in which SIRPα-Fc binding was restored by an excess of the CD20-competing antibody rituximab (5 μg/ml) prior to RTX-CD47 binding on CD20pos/CD47pos WIL2S tumor cells.
Results and discussion
The bi-scFv RTX-CD47 construct was designed to promote CD20-directed blockade of CD47-SIRPα “don't eat me” signaling towards cancer cells that express both CD20 and CD47. In the bi-scFv, both scFvs retained functionality as evidenced by the binding of RTX-CD47 to CHO.CD47 cells but not parental CHO cells (Suppl. Fig 1A) and its binding to CEM.CD20 cells but not parental CEM cells (Suppl. Fig.1B). Next, the potential enhanced avidity due to simultaneous binding to CD20 and CD47 by RTX-CD47 was evaluated on a panel of cell lines. Significant binding of RTX-CD47 was only detected towards CD20pos/CD47pos malignant B-cell lines, with only minimal binding to CD20neg/CD47pos cancer cells (). Binding of RTX-CD47 to CD20pos/CD47pos tumor cells was partially reduced by competing CD20 or CD47 antibody, but was only abrogated in the presence of both CD20- and CD47-competing mAbs (). Thus, RTX-CD47 has enhanced avidity for CD20pos/CD47pos tumor cells. These findings are in concordance with previously published results with bsAbs, including those targeting CD47, showing an avidity effect upon concurrent binding to both antigens on the same cell.Citation10,13,14
Of note, bsAbs can cooperatively bind to the target cells, via a process in which binding to antigen 1 strongly promotes binding to antigen 2, even when the affinity for the latter antigen is low.Citation10,13,14 In this respect, our binding data suggests that binding of RTX-CD47 to CD20 is needed in order to achieve efficient CD47/SIRPα blocking. In line with this mode-of-action, incubation with RTX-CD47 completely blocked the binding of recombinant human SIRPα-Fc to CD20pos/CD47pos cancer cells, but did not inhibit binding of SIRPα-Fc to CD20neg/CD47pos cancer cells (). Furthermore, inhibition of CD20-specific binding of RTX-CD47 by rituximab abrogated the inhibitory effect of RTX-CD47 on SIRPα-Fc binding (). Taken together, RTX-CD47 appears to have CD20-restricted capacity for blocking CD47/SIRPα interaction towards CD20pos/CD47pos cells.
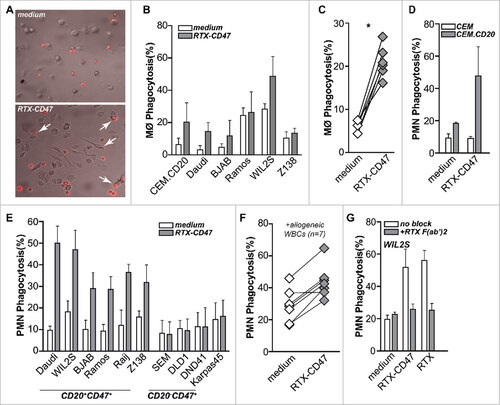
Next, we evaluated the capacity of RTX-CD47 to enhance ADCP activity of clinically used anticancer antibodies using mixed cultures of monocyte-derived macrophages and fluorescently-labeled cancer cells. Of note, macrophages are the key effector cells for ADCP induced by clinically used anticancer antibodies. Treatment with RTX-CD47 induced macrophage-mediated phagocytosis of 4 out of the 6 CD20pos malignant B cell lines (, ). Importantly, treatment with RTX-CD47 also significantly triggered phagocytosis of primary patient-derived malignant B cells by autologous macrophages (). Furthermore, RTX-CD47, significantly induced phagocytosis by granulocytes of CD20pos/CD47pos double-positive CEM cells, but not CD47 single-positive CEM (). Of note, granulocytes are the most prevalent phagocytic cell type in humans, albeit less capable of engulfing complete (or more than one) tumor cells than macrophages. In a panel of tumor cell lines, only CD20pos/CD47pos double-positive tumor cells were phagocytosed by granulocytes (). Importantly, single-agent treatment of a panel of primary patient-derived B malignant cells with RTX-CD47 also significantly triggered granulocyte-mediated phagocytosis compared to medium control (). Phagocytosis induced by RTX-CD47 was dependent on simultaneous binding to CD20 and CD47 as co-incubation with excess amounts of RTX-derived F(ab')2 antibody fragments inhibited phagocytosis ().
Figure 2. RTX-CD47 has single-agent pro-phagocytic activity towards CD20pos cancer cells. (A) Representative photos for macrophage phagocytosis assay with Daudi cells treated with RTX-CD47. RTX-CD47 induced macrophage-mediated phagocytosis of 4 out of the 6 CD20pos cancer cell lines. (B) RTX-CD47 triggered phagocytosis of primary patient-derived malignant B cells by autologous macrophages. (D) RTX-CD47 enhanced PMN-mediated phagocytosis of CEM.CD20 cells but not of parental CEM cells. (E) RTX-CD47 induced phagocytosis of all CD20/CD47 double positive cell lines in this cell panel and had no phagocytic activity on CD20negCD47pos cancer cell lines. (F) RTX-CD47 triggered granulocyte-mediated phagocytosis of primary patient-derived B malignant cells. (G) Phagocytosis induced by RTX-CD47 on WIL2 S cells is blocked by the addition of RTX F(ab')2 (5 µg/ml).
Thus, RTX-CD47 has prominent single-agent pro-phagocytic activity that involves blocking CD47 ‘don't eat me’ signaling in a CD20-restricted manner. This CD20-restricted pro-phagocytic activity of RTX-CD47 is fully independent of FcR signaling and only due to blocking of CD47/SIRPalpha interaction. Such CD47-selective pro-phagocytic activity is in line with the effect of ‘Fc-less’ F(ab')2 versions of CD47 mAbs.Citation8,15 Notably, the pro-phagocytic activity of the above-mentioned DVD-Ig and κλ-body bsAb formats partly relies on FcR signaling. Indeed, the pro-phagocytic activity of the CD20-CD47 DVD-Ig bsAb was completely inhibited by pre-incubation with a cocktail of FcR-blocking agents.Citation10
The CD20-restricted blocking of CD47 “don't eat me” signaling by RTX-CD47 may be of particular clinical use as an adjuvant approach to promote phagocytosis induction by therapeutic anticancer antibodies. Here, a therapeutic anticancer antibody that targets malignant B-cells recruit appropriate immune effector cells, while RTX-CD47 potentiates the ADCP effect in a CD20-restricted manner. To explore the feasibility of this approach, mixed cultures of allogeneic macrophages and (CD20pos/CD38pos) Daudi cells were treated with a suboptimal dose of RTX or an anti-CD38 antibody (both containing a human IgG1 Fc domain) and RTX-CD47. For both RTX and the anti-CD38 mAb, co-treatment with RTX-CD47 significantly augmented macrophage-mediated phagocytosis of Daudi lymphoma cells compared to single-agent treatment (). Further, co-treatment of CEM.CD20 cells, but not CEM cells, with a suboptimal and non-saturating dose of RTX and RTX-CD47 significantly enhanced macrophage-mediated phagocytosis (). In contrast, co-treatment of CEM.CD20 with the anti-CD38 antibody and RTX-CD47did not potentiate phagocytosis, as CEM.CD20 cells lack expression of CD38 (Fig.3B and Suppl. Fig. 1C). Thus, at non-saturating RTX doses the combination with RTX-CD47 is able to augment CD20-restricted phagocytosis of cancer cells. Interestingly, the subset of macrophages that were found to phagocytose target cells also engulfed significantly more tumor cells per macrophage upon combination treatment of RTX-CD47 with rituximab or anti-CD38 antibody (, ). Thus, adjuvant treatment with RTX-CD47 indeed appears to augment macrophage-mediated phagocytosis/ADCP as induced by therapeutic anticancer antibodies.
Figure 3. RTX-CD47 enhances ADCP by therapeutic anticancer antibodies. (A) RTX-CD47 synergized with therapeutic mAb in mediating phagocytosis of Daudi cells by macrophages. (B) RTX-CD47 enhanced (RTX mediated) phagocytosis of CEM.CD20 cells but not of parental CEM cells. (C) Representative photos of single macrophages engulfing V450-labeled target cells upon combined treatment with RTX-CD47 and RTX. (D) Quantification of phagocytic index from (A) showing average number of engulfed tumor cell per macrophage. (E) Combined treatment with RTX-CD47 and therapeutic antibodies (cetuximab, daratumumab, alemtuzumab (5 ng/ml) and rituximab (10 ng/ml)) significantly augmented phagocytosis levels BJAB cells. (F) Combined treatment with RTX-CD47 and alemtuzumab (1 µg/ml) significantly augmented phagocytosis levels of primary b-cell malignancies. (G) RTX-CD47 treatment potentiated macrophage-mediated phagocytosis of CD20pos cancer cell lines by obinutuzumab. (H) RTX-CD47 potentiated obinutuzumab mediated phagocytosis of primary patient-derived MCL cells by autologous macrophages. (I) RTX-CD47 treatment potentiated PMN- mediated phagocytosis of CD20pos cancer cell lines by obinutuzumab.
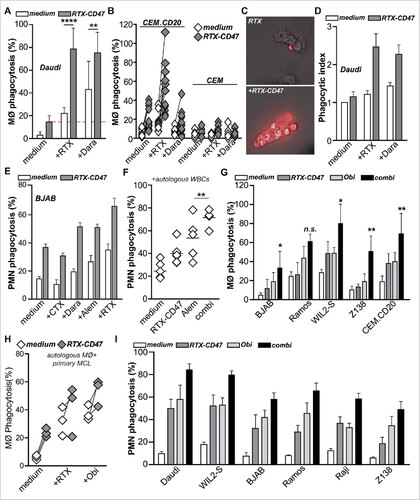
In analogous experiments with granulocytes, co-treatment of BJAB Burkitt lymphoma cells with therapeutic antibodies and RTX-CD47 also significantly augmented phagocytosis compared to single- agent treatment with low suboptimal concentrations of anti-CD38 antibody, alemtuzumab and rituximab (). In contrast, co-treatment of BJAB cells with control EGFR-targeted antibody cetuximab and RTX-CD47 did not induce phagocytosis (). To further validate the relevance of this adjuvant activity, phagocytosis of primary patient–derived B-cell malignancies, expressing CD47, CD20 and CD52 (data not shown), by autologous patient-derived granulocytes was evaluated. Co- treatment of mixed cultures with alemtuzumab and RTX-CD47 strongly induced phagocytosis in 5 out of 5 of these primary malignant B-cell samples, an effect significantly stronger than treatment with either alemtuzumab or RTX-CD47 alone (). Similar results were obtained when allogeneic granulocytes from healthy donors were used, with single treatment with RTX-CD47 significantly increasing granulocyte-mediated phagocytosis of 6 out of 7 primary malignant B-cell samples (Suppl. Fig. 1D).
As detailed above, combination of RTX-CD47 with low and non-saturating dose of RTX is capable of augmenting ADCP activity. However, the RTX concentration applied in patients may inhibit RTX-CD47 binding due to direct competition for the binding to the CD20 epitope. To circumvent this potential problem, we evaluated the adjuvant activity of RTX-CD47 in combination with obinutuzumab, a humanized anti-CD20 antibody that is known to target a non-overlapping epitope on the extracellular loop of CD20.Citation16 In a CD20 binding competition assay, no inhibitory effect of RTX-CD47 on CD20 binding by FITC-labeled obinutuzumab was detected (Suppl. Fig. 1E). In contrast, RTX-CD47 did partially block CD20 binding of FITC-labeled rituximab (Suppl. Fig. 1F). Rituximab and obinutuzumab did compete for CD20 binding with each other (Suppl. Fig. 1E, F), suggesting the presence of steric hindrance in the full IgG antibody format that does not occur when combining obinutuzumab with the bi-scFv RTX-CD47 format. Of note, obinutuzumab is glycoengineered to have superior pro-phagocytic activity compared to rituximabCitation17 and was recently FDA-approved for the treatment of CLL. In an NHL cell line panel, RTX-CD47 treatment indeed significantly potentiated macrophage-mediated phagocytosis by obinutuzumab in 3 out 4 B-cell leukemia cell lines and in CEM.CD20 cells (), whereas combination treatment with RTX enhanced phagocytosis only in 1 out of 4 NHL cell lines (Suppl. Fig. 1G). Importantly, combination of RTX-CD47 with obinutuzumab also augmented phagocytosis of primary malignant B cells by autologous primary patient-derived macrophages with significantly increased macrophage-mediated phagocytosis in comparison to RTX co- treatment (). Similar results were obtained in granulocyte phagocytosis assays, with RTX-CD47 potently enhancing the phagocytic activity by obinutuzumab in 6 out of 6 CD20posCD47pos cell lines (). Thus, combination of RTX-CD47 with obinutuzumab is feasible and may augment its therapeutic anticancer activity.
In conclusion, RTX-CD47 blocks CD47/SIRPα “don't eat me” signaling in a CD20-restricted manner, resulting in the selective phagocytic removal of cancer cells by human phagocytes and enhances phagocytosis in combination with various therapeutic anticancer antibodies. RTX-CD47 requires binding to CD20 in order to block CD47/SIRPα interaction due to its monovalent binding affinity for each antigen. This feature reduces the risk of toxicity that would occur upon off-target phagocytosis with ubiquitous CD47 blocking. Further, the absence of a functional IgG1 Fc-domain has the inherent advantage of being devoid of toxicity issues related to premature on-target/off-tumor triggering of FcR-mediated Antibody-Dependent Cellular Cytotoxicity (ADCC) and antibody- dependent phagocytosis (ADCP) or Complement Dependent Cytotoxicity (CDC). Thus, RTX-CD47 represents a prototype tumor-selective pro-phagocytic therapeutic protein that may be particularly useful to safely augment the tumoricidal activity of anticancer monoclonal antibodies.
Materials and methods
Antibodies
Fluorescent-conjugated murine mAbs directed against human CD16 (clone 3G8), CD20 (clone HI47), CD52 (clone HI186), CD7 (clone MEM-186) where purchased from Immunotools, anti- human CD47 (clone B6H12) from Abcam and anti-HA tag from GenScript. Fluorescently labeled secondary antibodies; mouse anti-SIRPα (Abcam) and goat anti-mouse IgG (Life technologies). Cetuximab (CTX, human IgG1- chimeric anti-EGFR), alemtuzumab (IgG1 humanized anti-CD52) and rituximab (RTX, human IgG1 chimeric anti-CD20) were from Merck KGaA, Genzyme and Roche, respectively. A human IgG1 chimeric daratumumab-analogue (anti-human CD38) was produced in- house using published VH and VL data and standard antibody engineering technology. F(ab')2 fragments of rituximab were generated using the Pierce ™ F(ab')2 Micro Preparation Kit (Thermo Scientific) according to manufacturer's instructions.
Construction of CD47-blocking tandem bs-scFv
RTX-CD47 was constructed by inserting DNA fragments encoding the indicated scFv antibody fragments in either of the two multiple cloning sites (MCS) of plasmid pEE14-bs-scFv. This plasmid is equipped with a kappa light chain leader sequence for protein excretion into the culture supernatant and adds an in-frame N-terminal HA detection tag to the protein. MCS#1 and MCS#2 are separated by DNA sequences encoding a 25 amino acid flexible linker sequence. In short: a 749 bps DNA fragment encoding a CD20-directed scFv antibody fragment (scFvRTX) derived from rituximab was directionally inserted into the SfiI and NotI restriction sites of MCS#1. Subsequently, a 750 bps DNA fragment encoding a CD47-blocking scFv derived from MAb MABLCitation18,19 was inserted in-frame using the XhoI and XbaI restriction sites of MCS#2, yielding end product pEE14-bs-scFvRTX-CD47. The full sequence of the L-HA-scFvRTX-scFvCD47 construct (with genbank accession nr. MF990309) is ATGGAGACAGACACACTCCTGCTATGGGTACTGCTGCTCTGGGTTC CAGGTTCCACTGGTGACTATCCATATGATGTTCCAGATTATGCTGGGGCCCAGCCGGCCATGGCCCAGGTACAACTGCAGCAGCCTGGGGCTGAGCTGGTGAAGCCTGGGGCCTCAGTGAAGATGTCCTGCAAGGCTTCTGGCTACACATTTACCAGTTACAATATGCACTGGGTAAAACAGACACCTGGTCGGGGCCTGGAATGGATTGGAGCTATTTATCCCGGAAATGGTGATACTTCCTACAATCAGAAGTTCAAAGGCAAGGCCACATTGACTGCAGACAAATCCTCCAGCACAGCCTACATGCAGCTCAGCAGCCTGACATCTGAGGACTCTGCGGTCTATTACTGTGCAAGATCGACTTACTACGGCGGTGACTGGTACTTCAATGTCTGGGGCGCAGGGACCACGGTCACCGTCTCTTCAGGAGGAGGCGGATCCGGCGGAGGCGGAAGCGGTGGCGGAGGCTCTCAAATTGTTCTCTCCCAGTCTCCAGCAATCCTGTCTGCATCTCCAGGGGAGAAGGTCACAATGACTTGCAGGGCCAGCTCAAGTGTAAGTTACATCCACTGGTTCCAGCAGAAGCCAGGATCCTCCCCCAAACCCTGGATTTATGCCACATCCAACCTGGCTTCTGGAGTCCCTGTTCGCTTCAGTGGCAGTGGGTCTGGGACTTCTTACTCTCTCACAATCAGCAGAGTGGAGGCTGAAGATGCTGCCACTTATTACTGCCAGCAGTGGACTAGTAACCCACCCACGTTCGGAGGGGGGACCAAGCTGGAAATCAAACGTGCGGCCGCGGAATTCGCCAAAACAACAGCCCCATCGGTCTATCCACTGGCCCCTGTGCTCGAGCAGGTGCAGCTGGTGCAGTCTGGGGCTGAGGTGAAGAAGCCTGGGGCCTCAGTGCAGGTTTCCTGTAAGGCATCTGGATACACCTTCACCAACCATGTTATTCACTGGCTGCGACAGGCCCCCGGGCAATGCCTTGAGTGGATGGGATATATTTATCCTTACAATGATGGTACTAAGTATAATGAGAAGTTCAAGGACAGAGTCACGATGACCTCAGACACGTCCATCAGCACAGCCTACATGGAGTTGAGCAGTCTCAGATCTGACGACACGGCCGTCTATTATTGTGCTAGAGGGGGTTACTATACTTACGACGACTGGGGCCAAGCAACCCTGGTCACAGTCAGTTCGGGTGGCGGTGGATCCGGTGGTGGCGGCAGCGGTGGTGGCGGCAGCGATATTGTGATGACTCAGTCTCCACTCTCCCTGCCCGTCACCCCTGGAGAGCCGGCCTCCATCTCCTGCAGATCAAGTCAGAGCCTTGTGCACAGTAATGGAAAGACCTATTTACATTGGTATCTGCAGAAGCCAGGCCAGTCTCCAAGACTCCTGATCTACAAAGTTTCCAACCGATTTTCTGGTGTCCCAGACAGATTCAGCGGCAGTGGGTCAGGCACTGATTTCACACTGAAAATCAGCAGGGTGGAGGCTGATGATGTTGGAATTTATTACTGCTCTCAAAGTACACATGTTCCGTACACGTTTGGCTGCGGGACCAAGTTGGAGATCAAACGTGCCGCTTAA. These sequence data have been submitted to the GenBank databases under accession number. The tandem bs-scFv antibody was produced by transfecting HEK293 production cells (ATCC; Manassas, VA) with plasmid pEE14-scFvCD47:scFvRTX using the Fugene-HD reagent (Promega). Supernatants of transfected HEK293 cells were harvested, cleared by centrifugation and stored at −20°C.
Cell lines and patient-derived malignant cells
All cell lines used in this study were obtained from the American Type Culture Collection (Manassas, VA). CD20pos malignant B-cell lines used are: WIL2 S (hereditary spherocytosis), Z138 (Mantle cell lymphoma) and Burkitt lymphoma cell lines Ramos, Raji, BJAB and CD20 transfected CEM cells. CD20neg cell lines used are: CEM (T-ALL), SEM (B-ALL), DND41 (T-ALL), Karpas 45 (T-ALL) and DLD1 (colorectal adenocarcinoma). Cells were cultured in RPMI culture medium (Lonza) supplemented with 10% fetal calf serum (FCS, Thermo Scientific) at 37°C in humidified 5% CO2 containing atmosphere. Expression levels of CD20 and CD47 were evaluated by flow cytometry using appropriate fluorescently labeled antibodies and an Accuri C6 flow cytometer (BD Biosciences). Cell line CEM.CD20 ectopically expressing human CD20 was kindly provided by Department of pathology, UMCG. Of note, ectopic expression of CD20 by CEM.CD20 cells did not alter the endogenous levels of CD47 expression (data not shown). Peripheral blood mononuclear cells (PBMCs) from patient blood were isolated by using gradient centrifugation and then phenotyped with CD3, CD19, CD20, CD38 and CD52. Primary human leukocytes were obtained from healthy volunteers using the ammonium chloride lysis method. PBMCs were obtained from healthy volunteers after informed consent using density gradient centrifugation. Monocytes were isolated from PBMCs using anti-CD14-coated magnetic beads (Miltenyi Biotec, USA). For generation of immature macrophages, monocytes (3 × 106/ml) were cultured in the presence of 50 U/ml GM-CSF and 50 U/ml M-CSF for 7 d, and then primed with LPS and IFN-γ. This study was carried out in The Netherlands in accordance with the applicable rules concerning the review of Research Ethics Committees and informed consent.
Binding assays
CD20-specific binding by RTX-CD47 was evaluated on a cell line panel of CD20posCD47pos and CD20negCD47pos tumor cell types using a secondary FITC-conjugated anti-HA antibody (GenScript). Simultaneous binding of RTX-CD47 to CD20 and CD47 was evaluated by incubating CD20posCD47pos Ramos cells in the presence (or absence) of excess amounts (5 μg/mL) of either rituximab (anti-CD20) or MAb B6H12 (anti-CD47) or both. CD20-specific binding activity of RTX-CD47 was further evaluated by comparing its binding characteristics to parental CEM and CEM.CD20 cells. Blocking of CD47-SIRPα interaction by bs-scFv RTX-CD47 was assessed by evaluating its ability to prevent binding of recombinant human SIRPα-Fc (5μg/ml, R&D systems) to CD47pos cancer cells (5.0 × 105 cells) by flow cytometry using appropriate fluorescently labeled secondary antibodies. All incubations were for 1 h at 4°C and followed by 2 washes with PBS.
Granulocyte-mediated phagocytosis assay
Tumor-directed inhibition of CD47/SIRP-alpha “don't eat signaling” by bs-scFv RTX-CD47 was evaluated by its capacity to induce specific phagocytosis of DiD- fluorescently labeled tumor cells by IFN-γ activated granulocytes. In short, human granulocytes were pre-activated for 1 h with IFN-γ (50 ng/ml) at 37°C and then co-cultured with cancer cells at an effector to target cell ratio of 1 to 1.Phagocytosis cancer cell lines: mixed cultures were incubated for 2 h at 37°C in the presence (or not) of anticancer antibodies cetuximab (5 ng/ml), daratumumab (5 ng/ml), alemtuzumab (5 ng/ml), rituximab (10 ng/ml)) and (or not) bs-scFv RTX-CD47, bs-scFv CD7- CD47 and indicated combinations thereof. Phagocytosis of primary patient–derived B-cell malignancies: mixed cultures were incubated over night at 37°C in the presence of bs-scFv RTX- CD47. To assess the capacity of bs-scFv RTX-CD47 to enhance the efficacy of alemtuzumab, mixed cultures were incubated 4 h at 37°C in the presence of alemtuzumab (1 µg/ml) and/or bs-scFv RTX- CD47. Afterwards cells were washed and stained with CD16-FITC antibody. Phagocytosis of cancer cells by the granulocytes under the various conditions indicated was evaluated by determining the percentage of CD16pos/DiDpos granulocytes using flow cytometry.
Macrophage-mediated phagocytosis assay
Cell proliferation reagent V450-stained tumor cells were mixed with pre-seeded macrophages at E:T ratio 5:1 in 96-well plates. Mixed cells were treated either with control medium or medium contained RTX-CD47 in the presence or absence of therapeutic mAbs (RTX, 2.5 µg/ml,daratumumab, 0.5 µg/ml, obinutuzumab, 2.5 µg/ml) for 3 h. After gently washing tumor cells away, adherent macrophages were left in the well for further analysis with fluorescence microscopy. The phagocytic index was calculated using the following formula: phagocytic index = (total number of phagocytosed tumor cells)/(number of macrophages that have phagocytosed tumor cells).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Financial support
This work was supported by Dutch Cancer Society grants RUG 2009–4355, RUG2011-5206, RUG2012-5541, RUG2013-6209, RUG2014-6986.
2017ONCOIMM0599R-f04-z-4c.eps
Download EPS Image (1.4 MB)Acknowledgments
We would like to thank dr. Jeannette H.W. Leusen of the University Medical Center Utrecht, The Netherlands, for kindly providing the CEM and CEM.CD20 cells. We would like to thank Roche and dr. Christian Klein for provision of the anti-CD20 antibody obinutuzumab.
References
- Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71(4):1374–84. doi:10.1158/0008-5472.CAN-10-2238. PMID:21177380
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–85. doi:10.1016/j.cell.2009.05.046. PMID:19632178
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–7. doi:10.1073/pnas.1121623109. PMID:22451913
- Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110(27):11103–8. doi:10.1073/pnas.1305569110. PMID:23690610
- Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209–15. doi:10.1038/nm.3931. PMID:26322579
- Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113(19):E2646–54. doi:10.1073/pnas.1604268113. PMID:27091975
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–99. doi:10.1016/j.cell.2009.05.045. PMID:19632179
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell. 2010;142(5):699–713. doi:10.1016/j.cell.2010.07.044. PMID:20813259
- Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood. 2006;107(6):2548–56. doi:10.1182/blood-2005-04-1463. PMID:16291597
- Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, Wang L, Weiskopf K, Majeti R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7(5):946–56. doi:10.1080/19420862.2015.1062192. PMID:26083076
- Dheilly E, Moine V, Broyer L, Salgado-Pires S, Johnson Z, Papaioannou A, Cons L, Calloud S, Majocchi S, Nelson R, et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol Ther. 2017;25(2):523–33. doi:10.1016/j.ymthe.2016.11.006. PMID:28153099
- Weiner GJ, Kostelny SA, Hillstrom JR, Cole MS, Link BK, Wang SL, Tso JY. The role of T cell activation in anti-CD3 x antitumor bispecific antibody therapy. J Immunol. 1994;152(5):2385–92. PMID:8133049
- Mazor Y, Hansen A, Yang C, Chowdhury PS, Wang J, Stephens G, Wu H, Dall'Acqua WF. Insights into the molecular basis of a bispecific antibody's target selectivity. MAbs 2015;7(3):461–9. doi:10.1080/19420862.2015.1022695. PMID:25730144
- Mazor Y, Sachsenmeier KF, Yang C, Hansen A, Filderman J, Mulgrew K, Wu H, Dall'Acqua WF. Enhanced tumor-targeting selectivity by modulating bispecific antibody binding affinity and format valence. Sci Rep. 2017;7:40098. doi:10.1038/srep40098. PMID:28067257
- Chen J, Zhong M, Guo H, Davidson D, Mishel S, Lu Y, Rhee I, Pérez-Quintero L, Zhang S, Cruz-Munoz M, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via mac-1 integrin. Nature. 2017;544(7651):493–7. doi:10.1038/nature22076. PMID:28424516
- Sachdeva M, Dhingra S. Obinutuzumab: A FDA approved monoclonal antibody in the treatment of untreated chronic lymphocytic leukemia. Int J Applied Basic Medical Res. 2014;5(1):54–7.
- Herter S, Umana P, Bacac M. Obinutuzumab (GA101) more potently engages phagocytic-lineage cells resulting in enhanced monocyte and macrophage activity when compared to rituximab and ofatumumab. Blood Am Society Hematol. 2013;122(21):5136
- Kikuchi Y, Uno S, Yoshimura Y, Otabe K, Iida S, Oheda M, Fukushima N, Tsuchiya M. A bivalent single-chain fv fragment against CD47 induces apoptosis for leukemic cells. Biochem Biophys Res Commun. 2004;315(4):912–8. doi:10.1016/j.bbrc.2004.01.128. PMID:14985099
- Wiersma VR, He Y, Samplonius DF, van Ginkel RJ, Gerssen J, Eggleton P, Zhou J, Bremer E, Helfrich W. A CD47-blocking TRAIL fusion protein with dual pro-phagocytic and pro-apoptotic anticancer activity. Br J Haematol. 2014;164(2):304–7. doi:10.1111/bjh.12617. PMID:24164421
