ABSTRACT
Background: Immunotherapy may be a rational strategy in leiomyosarcoma (LMS), a tumor known for its genomic complexity. As a prerequisite for therapeutic applications, we characterized the immune microenvironment in LMS, as well as its prognostic value. Methods: CD163+ macrophages, CD3+ T-cells, PD-L1/PD-L2 and HLA class I expression (HCA2, HC10 and β2m) were evaluated using immunohistochemistry in primary tumors (n = 75), local relapses (n = 6) and metastases (n = 19) of 87 LMS patients, as well as in benign leiomyomas (n = 7). Correlation with clinicopathological parameters and survival analyses were assessed. Effect of LMS cells on macrophage differentiation was investigated using coculture of CD14+ monocytes with LMS cell lines or their conditioned media (CM). Results: 58% and 52% of the tumors were highly infiltrated with CD163+ macrophages and T-cells, respectively, with HLA class I expression observed in almost all tumors and PD-L1 expression in 30%. PD-L2 expression was also detected in some PD-L1+ tumors. All these immune markers correlated with high tumor grade but only CD163 associated with overall survival (p = 0.003) and disease-specific survival (p = 0.041). In vitro, CD163 was upregulated in the presence of LMS cells producing M-CSF, suggesting that this tumor drives macrophages towards the M2 phenotype. Conclusion: The clinical significance of M2 macrophages, possibly induced by LMS cell-secreted factors, suggests that 2/3 of high-grade LMS patients might benefit from macrophage-targeting agents. Furthermore, PD-L1 expression together with high T-cell infiltrate and HLA class I expression in around 30% of high grade LMS reflects an active immune microenvironment potentially responsive to immune checkpoint inhibitors.
Introduction
Leiomyosarcomas constitute a group of malignant mesenchymal neoplasms that originate from smooth-muscle lineage.Citation1 These tumors usually occur in middle-aged or older adults in any soft-tissue or visceral site of the body. Histological grade according to the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC), tumor size and tumor depth are the three major clinicopathological factors related to survival. Wide surgical excision is the cornerstone of leiomyosarcoma treatment. The additional value of radiotherapy largely depends on location and grade of the tumor.Citation2 In case of advanced disease, some systemic agents have been considered active, historically doxorubicin and ifosfamide, and more recently gemcitabine-based combination, trabectedin, pazopanib and eribulin.Citation3 Nevertheless, overall survival for patients treated with any first-line chemotherapy is around 12–15 months, emphasizing an unmet medical need for new therapeutic targets.Citation4
In this regard, the recent success of immunotherapy in many other tumor types raises the question whether such therapies, and which ones, would be applicable in sarcomas. The prognostic significance of tumor-associated macrophages (TAMs) has been extensively investigated in epithelial malignancies, but only few studies focused on sarcomas.Citation5–8 TAMs constitute a heterogeneous population of myeloid cells in tumor microenvironment and the concept of classical M1- and alternative M2-polarization represents two extremes of a spectrum of functional states.Citation9 To date, some agents targeting TAMs have already been successfully tested in a clinical setting, notably a small-molecule CCR2 inhibitor in pancreatic cancers and CSF-1R blockade in tenosynovial giant-cell tumors.Citation10–13
Another recent concept is to link genomics to immunotherapeutic approaches.Citation14 Leiomyosarcoma is known for its genomic complexity, therefore with a supposed high mutational burden. In epithelial malignancies, this high mutational load, as well as the presence of a spontaneous anti-tumor immune reaction prior to treatment, have been associated with predicting better response to immune checkpoint inhibitors.Citation15,Citation16 So far, pre-clinical studies investigating the prevalence and the predictive value of immune markers such as T-cell infiltrate or PD-1/PD-L1 expression in soft tissue sarcoma were performed including several histological subtypes, which makes it difficult to draw firm conclusions about one specific histological subtype.Citation17–20
In this study, we evaluated the macrophage population, the HLA class I status, the PD-L1/PD-L2 expression and the degree of tumor-infiltrating lymphocytes (TILs) on a large series of leiomyosarcomas by using immunohistochemistry. Association of these immune markers with clinicopathological factors was assessed, as well as the effect of leiomyosarcoma cells on monocyte-to-macrophage differentiation in vitro.
Results
Leiomyosarcoma-infiltrating macrophages exhibit M2-phenotype and correlate with decreased patient survival
First we used a selected subset of 21 leiomyosarcomas, including different tumor grade and location, to characterize the macrophage population. By using a combination of immunofluorescent stainings (CD14-HLA-DRα and CD14-CD163-CD40), CD163-positive macrophages were shown to be the major population in leiomyosarcoma as almost all of the CD14-positive cells also exhibited CD163 positivity, a marker for alternatively activated and immunosuppressive M2 macrophages (Supplementary Figure 1). Based on this finding, CD163 immunohistochemistry was performed in a cohort of 100 LMS tumors from 87 patients and 7 benign uterine leiomyomas. Patient demographics and relevant clinicopathological characteristics are described in . Overall, 61 of 100 leiomyosarcomas were highly infiltrated (>20%) with CD163-positive cells (), and this proportion was found quite similar between primary tumors (48 of 75) and relapses or metastases (13 of 25) (p = 0.29). For nine patients, material from primary tumor and the corresponding relapse/metastasis was available. Globally, the same pattern of CD163 infiltrate was observed between the associated lesions (). Spatial distribution of the macrophages was found homogeneous within the tumors. Infiltration of CD163-positive macrophages was significantly higher in tumors with higher FNCLCC histological grade ( and ; p < 0.0001). Notably, the seven leiomyomas included in our series were all poorly infiltrated with CD163-positive cells.
Figure 1. CD163 infiltrate in leiomyosarcoma. Representative images of primary leiomyosarcoma with low CD163 infiltrate (≤ 20%) (A) and high CD163 infiltrate (>20%) (B) using immunohistochemistry (scale bars 50 μm). The same pattern of CD163 infiltration was observed in the primary tumor and in the associated relapse/metastasis (C). Overall, 60% of leiomyosarcomas were highly infiltrated with CD163-positive cells, which strongly correlated with tumor grade (D). CD14-positive cells were differentiated for 6 days with GM-CSF or M-CSF as controls for M1 and M2 phenotype, respectively, and with leiomyosarcoma cells (LMS04, LMS05) using transwell or their conditioned media (CM). Expression of the surface marker CD163 (M2) on differentiated CD14-positive cells was analyzed by flow cytometry (E). Bars indicate relative geometric mean fluorescence intensity (MFI) ± standard error of mean (SEM) of three independent healthy donors, normalized to the M-CSF condition. Nonparametric Mann-Whitney test or the Kruskal-Wallis test followed by Dunn's post-test were used to compare differences between conditions. #p < 0.05. M-CSF measurement using a Luminex assay on LMS04 and LMS05 conditioned media (F). Results are expressed in pg/ml (n = 2).
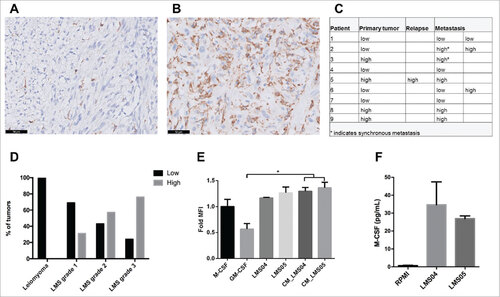
Table 1. Demographic and clinicopathological characteristics.
Table 2. Clinicopathological characteristics based on CD163, CD3 and PD-L1 expression.
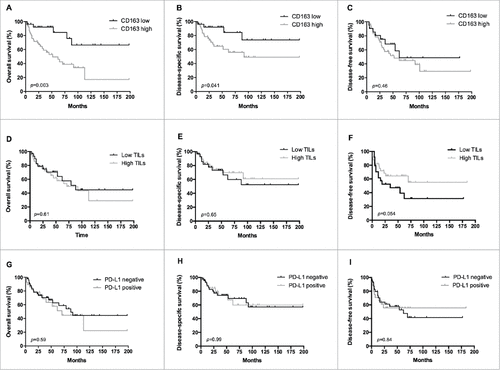
To evaluate the clinical relevance of these M2 tumor-associated macrophages in leiomyosarcoma, we performed Kaplan-Meier survival analyses of patients with primary tumors (n = 75). CD163-infiltrate significantly correlated with overall patient survival and disease-specific survival (log rank; p = 0.003 and 0.041 respectively) but not to disease-free survival (log rank; p = 0.46) (). In a multivariate Cox regression model including age, gender and histological grade, CD163-infiltrate was confirmed to be an independent prognostic factor for overall survival (HR = 2,85 (1.03–7.93) p = 0,045).
Figure 2. Prognostic significance of CD163, CD3 and PD-L1 in leiomyosarcoma. Kaplan-Meier survival curves for overall survival (A, D, G), disease-specific survival (B, E, H) and disease-free survival (C, F, I) according to CD163 infiltration (low n = 27; high n = 48), CD3 infiltration (low n = 30; high n = 43) and PD-L1 expression (negative n = 46; positive n = 28) in primary leiomyosarcomas. A high CD163 infiltrate (> 20%) is associated with poor overall and disease specific survival. P-value obtained by log-rank test.
Factors secreted by leiomyosarcoma cells drive macrophages toward the M2-polarization status
To explore the effect of leiomyosarcoma cells on monocyte-to-macrophage differentiation, we cultured freshly isolated monocytes from healthy donors (n = 3) with two leiomyosarcoma cell lines LMS04 and LMS05 (0.4 µm transwell coculture) or with their conditioned media. Macrophage markers were analyzed by flow cytometry after six days and compared to GM-CSF and M-CSF-differentiated macrophages used as control for M1 and M2 phenotype, respectively. In the presence of either leiomyosarcoma cell lines or their conditioned media, the M2 macrophage marker CD163 was upregulated and comparable to the phenotype seen on M-CSF-differentiated macrophages (). In addition, CD86 was upregulated by M-CSF in comparison with GM-CSF and was also upregulated by the LMS cells lines (Supplementary Figure 2). We also observed a strong expression of the activation marker CD40 on GM-CSF-differentiated macrophages but not with supernatant of the LMS cell lines. Expression of CD14 and HLA-DR were strongly induced by the LMS cells (Supplementary Figure 2). Together, this indicates that LMS cells induce a M2 phenotype, as rather than M1. To assess potential soluble factors capable of inducing M2 differentiation, Luminex assay was used to evaluate the possible secretion of h-M-CSF and h-IL-34 by LMS cells. M-CSF was detected between 22 and 47 pg/ml on LMS conditioned media () while IL-34 was not detected.
HLA class I is upregulated in leiomyosarcoma
As surface HLA class I expression is an important determinant for CD8 T-cell recognition, we assessed its expression by immunohistochemistry on the total cohort of 100 leiomyosarcomas and seven leiomyomas, using the antibodies HCA2 (HLA-A), HC10 (HLA-B/C) and β2-microglobulin. Staining patterns on smooth muscle cells from blood vessels within the tumor tissue blocks and on uterus were also assessed and representative images of these stainings are shown in . We observed a completely negative or very weak expression of HLA class I on normal smooth muscle cells from blood vessels and only HLA-B/C expression was detected on uterine smooth muscle cells (not shown). In the seven benign leiomyomas, the staining pattern was quite similar with heterogeneous expression of β2-microglobulin and HLA-B/C, associated with absent HLA-A. Interestingly, on 98 evaluable leiomyosarcomas, positive (n = 35) or heterogeneous (n = 57) expression of β2-microglobulin was found in almost all tumors, with absent or weak expression in only six patients, including two primary tumors, two local relapses and two metastases of different patients. Positive and diffuse expression (n = 40) or heterogeneous expression (n = 47) of HLA-A was detected in 97 evaluable leiomyosarcomas. The same pattern was observed with HLA-B/C expression, which was strongly positive (n = 50) or heterogeneous (n = 41) in 95 evaluable tumors. Notably, in tumours with heterogeneous HLA expression, we observed a frequent spatial colocalization between HLA expression on tumor cells and presence of T cells. Low expression of both HLA-A and HLA-B/C markers was found in only three leiomyosarcomas, of which two primary tumors and one relapse, and associated with low or heterogeneous expression of β2-microglobulin.
Figure 3. HLA class I expression in leiomyosarcoma. Representative staining patterns of β2-microglobulin, HLA-A (HCA2 antibody) and HLA-B/C (HC10 antibody) expression in smooth muscle cells from blood vessel, leiomyoma and leiomyosarcoma using immunohistochemistry. In general, basal low HLA class I expression was found on normal smooth muscle cells while frequently heterogeneous or strongly positive expression was seen in leiomyosarcomas, suggesting that HLA class I is upregulated in the majority of these tumors. Scale bars, 50 μm.
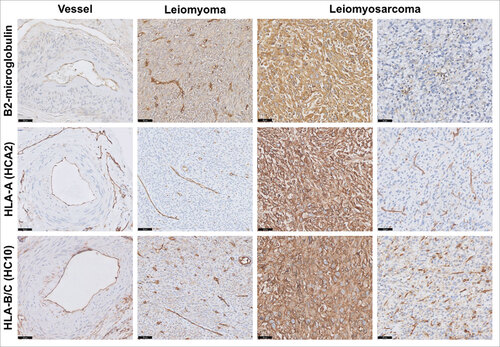
Altogether, considering the basal low HLA class I expression on normal smooth muscle cells and the frequently heterogeneous or strongly positive expression on leiomyosarcomas, this suggests that HLA class I is upregulated in the majority of these tumors.
Increased PD-L1 expression and T-cell infiltration in high-grade leiomyosarcoma
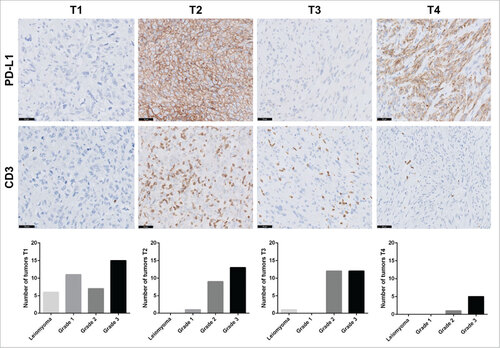
PD-L1 expression and the presence of tumor-infiltrating T cells were evaluated using immunohistochemistry on the entire tumor series using whole sections. Representative images of these stainings are shown in . Overall, 32/106 evaluable tumors (30%) had positive membranous PD-L1 expression, observed on ≥1% of tumor cells and/or immune cells. Interestingly, PD-L1 expression was specifically detected in grade 2 (n = 10, 34%) and grade 3 (n = 19, 42%) leiomyosarcomas, as only one grade 1 tumor (8%) was found positive and all leiomyomas were PD-L1 negative (p = 0.005) (). High T-cell infiltration (>5 CD3+cells/HPF) was observed in 55/105 tumors (52%), mainly grade 2 (72%) and grade 3 (56%) leiomyosarcomas (p = 0.036). Only one grade 1 leiomyosarcoma (8%) and one leiomyoma (14%) were infiltrated by T cells. PD-L1 positivity and high T-cell infiltration were strongly correlated (p < 0.0001). Neither PD-L1 expression nor T-cell infiltrate correlated with age, gender, tumor size, tumor type (primary, relapse, metastasis) or patient survival, as detailed in and . Tumors were categorized according to the Tumor Immunity in MicroEnvironment (TIME) classification, as it helps to characterize the tumor immune response and to predict response to anti PD-1 therapy.Citation21 When combining both immune markers in the TIME classification, we identified 39 tumors with the T1 subtype (PD-L1−, TIL−), 23 with the T2 subtype (PD-L1+, TIL+), 25 with the T3 subtype (PD-L1−, TIL+) and six tumors with the T4 subtype (PD-L1+, TIL−). This classification also correlated with tumor grade (p = 0.004), as shown in .
Figure 4. Tumor-infiltrating lymphocytes and PD-L1 expression in leiomyosarcoma categorized with the TIME classification. Representative images for PD-L1 and CD3 immunostaining in leiomyosarcoma patients. Scale bars, 50 μm. According to the TIME classification, 39 tumors exhibited the T1 subtype (TILs−, PD-L1−), 23 the T2 subtype (PD-L1+, TIL+), 25 the T3 subtype (PD-L1−, TIL+) and 6 the T4 subtype (PD-L1+, TIL−). Distribution of tumor grades within each subtype is represented by a bar chart.
Frequent PD-L1 expression was noticed after neoadjuvant treatment, mainly radiotherapy, (6/10 evaluable tumors; 60%) compared to patients who had surgery first (26/96 tumors; 27%, p = 0,031). In order to better characterize the changes in the immune microenvironment after neoadjuvant radiotherapy, PD-L1 as well as CD3 and CD163 expression, were assessed by IHC on an independent cohort of seven leiomyosarcoma patients with pre- (biopsies) and post-radiation (resection) material collected. Eleven of the 13 tumors available were highly infiltrated by CD163-positive cells (five biopsies and six resections). CD3+ TILs were observed in four resection samples but in only 1 biopsy. There was no significant change regarding PD-L1 expression between biopsies (one PD-L1 positive) and post-radiation resections (two PD-L1 positive) (Supplementary Table 2).
PD-L2 is co-expressed in some PD-L1 positive leiomyosarcomas
As PD-L2 expression was reported on some tumor cells and immune cells, notably macrophages, we assessed its expression in 35 tumors from this series, selected according to their PD-L1 status and macrophage infiltrate. PD-L2 positivity was found on 10 of these 35 tumors, on both tumor cells and immune cells (8/9 tumors PD-L1+CD163+, 1/5 tumor PD-L1+CD163−, 1/8 tumor PD-L1−CD163+ and none of the 13 tumors PD-L1−CD163−). Surprisingly, nine tumors with PD-L2 expression were also PD-L1 positive, with a frequent colocalization (Supplementary Figure 3A,B). This co-expression PD-L1/PD-L2 was also observed on alveolar macrophages surrounding lung metastatic lesions (Supplementary Figure 3C ,D).
Discussion
Comprehensive assessment of the immune microenvironment for each sarcoma subtype is an important prerequisite for developing tumor type specific immunotherapeutic strategies. Macrophages are among the most abundant immune cells recruited in the tumor microenvironment and are present at all stages of tumor progression.Citation22 Using a combination of different macrophage markers by immunofluorescence, CD163-positive cells were identified as the major population in leiomyosarcomas. As CD163 is a well-recognized marker for M2-type macrophages, it reflects the predominance of tumor-promoting macrophages in these tumors, indicative of an immunosuppressive microenvironment.Citation23 Overall, around 60% of leiomyosarcomas were highly infiltrated with CD163 positive TAMs, which was strongly correlated with increased histological grade and was found to be an independent poor prognostic factor for overall survival. Using gene expression profiling follow by immunohistochemistry on tissue microarrays, the prognostic significance of CD68- and CD163-positive TAMs has been previously reported in nongynecologic leiomyosarcoma patients, which was independent of the histological grade.Citation8 Therefore, the current series, for which we used immunofluorescent staining for combined macrophage markers in a pilot series followed by CD163 immunohistochemistry on whole tumor sections, can be seen as an independent validation of the previous published results. While TAMs have been associated with increased metastatic potential, there was no correlation between TAMs and disease-free survival in both cohorts.Citation24 As opposed to carcinomas, we have previously shown a favorable prognostic effect of TAMs in osteosarcoma, suggesting that tumor immunology may be different amongst the different sarcoma types.Citation6,Citation25
The phenotype of macrophages observed in vitro after coculture with leiomyosarcoma cells appeared more similar to that of M-CSF-generated macrophages (M2) than GM-CSF-generated macrophages (M1), which is in line with the immunohistochemical findings in leiomyosarcoma patients. The same M-CSF-like phenotype was observed with conditioned media from tumor cells, and is therefore most likely related to leiomyosarcoma cell-secreted factors. M-CSF receptor (also known as CSF1-R) is expressed on macrophages and has been shown to polarize macrophages toward the alternative and immunosuppressive M2 phenotype.Citation26 Notably, a paracrine loop involving M-CSF secreted by tumor cells and TAMs has been described in mammary tumors.Citation27 Recently, IL-34 has been shown to induce the differentiation of human monocytes into immunosuppressive macrophages, also via M-CSF receptor.Citation28,Citation29 In this study, M-CSF was detected in LMS conditioned media and might be partly responsible for the macrophage polarisation in leiomyosarcoma. IL-34 was not detected but its expression is usually very low and therefore our assay might be not sensitive enough. Preclinical data already identified an M-CSF signature in a subset of leiomyosarcomas, both gynecologic and nongynecologic, associated with poor prognosis.Citation30 Moreover, M-CSF blockade strategy successfully translated into clinical objective responses in tenosynovial giant-cell tumors and is currently being tested in various solid malignancies.Citation12,Citation13 Based on our study, 57% of the grade 2 and 76% of the grade 3 LMS might benefit from such macrophage-targeted agents.
Another successful antitumor approach in epithelial malignancies is the T cell-based immunotherapy, notably with immune checkpoint inhibitors, which has transformed the prognosis of patients with selected advanced cancers.Citation31 The search for potential application in sarcoma patients with advanced disease is currently very active considering the often poor responsiveness to radio- and chemotherapy. HLA-A and HLA-B both are antigen-presenting molecules and required for T-cell based immunotherapies. It is known that HLA class I expression is low or negative on normal smooth muscle cells and upregulated in inflammatory conditions such as myositis but so far, there was no data regarding HLA expression in LMS.Citation32 Our study demonstrates for the first time that HLA class I molecules are heterogeneously or strongly upregulated in LMS compared to normal smooth muscle cells and leiomyomas. While loss or down-regulation of HLA molecules is a common mechanism deployed by tumour cells to escape immune surveillance, our data indicate that this mechanism is not present in LMS and that T cells can globally recognize LMS cells. Furthermore, half of the tumors, especially those of high histological grade, were highly infiltrated with T cells, reflecting an active host immune response. However, PD-L1 expression, reflecting an immune escape mechanism, was observed in 30% of tumors, which also significantly correlated with higher histological grade and high T-cell infiltration. Previous studies have reported PD-L1 expression in soft-tissue sarcoma series, including few LMS with a variable PD-L1 expression (between 11 and 70% of positive cases).Citation17–20 Such discrepancies are explained by the small numbers of cases, the use of different PD-L1 antibodies and different scoring systems. In addition, some groups worked on tissue-microarrays and others on whole sections. Efforts to standardize the PD-L1 immunostaining have been done in epithelial malignancies and are also essential for sarcoma. While PD-L1 was more frequently expressed in our cohort after neoadjuvant treatment, this observation was not confirmed in an independent cohort, which highlighted an increased number of TILs after radiation. Larger patient series are needed before any conclusions on the effect of neoadjuvant therapy in immune microenvironment can be drawn in leiomyosarcoma. Prevalence of TILs or PD-L1 expression have been associated with survival outcome in several malignancies but were not significant in our LMS series, in accordance with a previous study in soft-tissue sarcomas.Citation18,Citation33,Citation34 In the literature, this anti-tumor immune reaction prior to treatment has been associated with better response to immune checkpoint inhibitors and clearly warrants further investigation as predictive biomarker in sarcoma, as recently highlighted by Budczies et al in high-grade soft-tissue sarcomas.Citation16,Citation35
Recent data from the multicenter phase II study of pembrolizumab in patients with advanced soft-tissue and bone sarcomas (SARC028, NCT02301039), were disappointing.Citation36 However, data from translational research conducted in this clinical trial suggested the predictive value of PD-L1 expression, which correlated with T-cell infiltrate. While nivolumab also failed to demonstrated antitumor activity in metastatic uterine LMS (NCT02428192), a mixed partial response/stable disease was obtained in three metastatic LMS with the same anti PD-1 agent.Citation37,Citation38 Furthermore, a successful treatment of refractory and metastatic PD-L1 positive LMS has been recently reported.Citation39 It highlights the potential efficacy of such agents in selected advanced LMS and the challenge of identifying predictive biomarkers. Among possible biomarkers, the immune profile of the tumor or immunoscore, i.e. the recent TIME classification, seems to be a good candidate as tumors with a T2 subtype (PD-L1+, TILs+) are likely to be sensitive to immunotherapeutic strategies. In this series, around 25% of tumors exhibited the T2 subtype, which represents an important proportion of patients predicted to respond to immune checkpoint inhibitors. We have learnt from melanoma studies that combination of CTLA-4 and PD-1 inhibitors was more efficacious than the respective monotherapies.Citation40 Actually, two clinical trials assessing efficacy and safety of combined immune checkpoint inhibitors in sarcoma are ongoing (NCT03138161 and NCT02428192). Of note, the T2 phenotype was observed in high grade LMS (>95%) but most of these tumors (88%) were also highly infiltrated with immunosuppressive TAMs, which may limit the anti PD-1 efficacy.Citation41 Therefore, based on our findings and the current literature, we can speculate that the combined or sequential use of macrophage-targeting agents may be an effective therapeutic approach to reduce the immunosuppressive microenvironment and to enhance the efficacy of PD-1 inhibition in patients with high-grade LMS with a T2 subtype. For the other TIME subtypes, other combination strategies have to be developed in order to generate an inflammatory microenvironment (T1 and T4 subtypes) or to break an existing T-cell tolerance (T3 subtype).Citation21
Conclusion
The presence and clinical significance of M2 macrophages, possibly induced by LMS cell-secreted factors, suggest that two-thirds of high-grade LMS patients might benefit from macrophage-targeting agents. Furthermore, PD-L1 expression together with high T-cell infiltrate and HLA class I expression in around 30% of high grade LMS reflects an active immune microenvironment potentially responsive to immune checkpoint inhibitors. Selection of sarcoma patients according to their tumor immune microenvironment seems of major interest to develop immunotherapeutic strategies, as single agents or in combination.
Materials and methods
Patient material
Formalin-fixed, paraffin-embedded material from the archives of the Pathology department of the Leiden University Medical Center (LUMC) was collected for this study. Primary tumors (n = 75), local relapses (n = 6) and metastases (n = 19) of 87 leiomyosarcoma patients were included, as well as benign uterine leiomyomas (n = 7). For nine leiomyosarcoma patients, material from both primary tumor and matched local relapse or metastasis was available.
We used whole sections to assess density, location and type of immune infiltrate. Diagnoses and histological grade according to FNCLCC were confirmed by an experienced bone and soft-tissue tumor pathologist (J.V.M.G.B.) according to the 2013 World Health Organization classification.Citation42 All the specimens were coded and handled according to the ethical guidelines described in the Code for Proper Secondary Use of Human Tissue in the Netherlands of the Dutch Federation of Medical Scientific Societies as reviewed and approved by the LUMC ethical board (B17.018).
A separate small independent sample set consisting of material from pre- and post-radiation treatment of seven leiomyosarcoma patients treated at the Netherlands Cancer Institute was also collected in order to assess the effect of radiation on immune microenvironment (reviewed and approved by the NKI Institutional Review Board (CFMPB470)).
Immunohistochemical analyses
Four μm sections were deparaffinized with xylene and rehydrated in graded concentrations of ethanol. Endogenous peroxidase was blocked in 0.3% H2O2 solution (except for immunofluorescent procedure) and microwave antigen retrieval was performed in Tris-EDTA pH 9.0 or Citrate pH 6.0 according to the antibody, as described in Supplementary Table 1. To define macrophage polarization, we used a CD163 staining, a well-known marker of alternatively activated and immunosuppressive M2 macrophages.Citation43 According to standard laboratory methods, primary antibodies were diluted in PBS/bovine serum albumin 1% and incubated overnight at 4°C. Anonymized tonsils were used as positive controls and primary antibodies were omitted for the negative controls. Stainings of normal smooth muscle cells were assessed on blood vessels from the same tumor tissue blocks and on anonymized uterus material.
For immunohistochemistry, antibodies were detected using poly-HRP (ImmunoLogic), visualized with a DAB+ substrate chromogen system (Dako) and slides were counterstained with hematoxylin, dehydrated and mounted using CV Mount (Leica Microsystems). Whole sections were evaluated independently by two observers (M.K. and A.C. or J.V.M.G.B.) blinded to clinicopathological data, and tumors were categorized with low or high macrophage-infiltrate using the cut-off of >20% CD163-positive cells, and low or high T-cell infiltrate using the cut-off of >5 CD3+cells/HPF with the average of five randomly chosen HPFs.Citation44,Citation45 HLA class I expression (HCA2, HC10 and β2-microglobulin staining) was categorized as negative/focal weak, heterogeneous or positive, as previously detailed.Citation46 PD-L1 and PD-L2 positivity was defined as ≥1% of tumor cells or immune cells showing a membranous PD-L1 staining of any intensity.Citation47
For immunofluorescence, isotype-specific secondary antibodies labelled with Alexa fluorochromes (Life Technologies) were added during 1 h at room temperature and the slides were mounted using Vectashield mounting medium containing DAPI (Vector Laboratories). Five randomly selected representative images were then acquired at 250x magnification using a confocal scanning microscope (LMS700, Zeiss) in a multitrack setting with a 25x/0.80 Plan-Neofluoar objective.
In vitro cell culture
The leiomyosarcoma cell lines LMS04 and LMS05 (kindly provided by J.A. Fletcher, Brigham and Women's Hospital, Harvard Medical School, Boston, USA) were cultured in RPMI-1640 (Gibco, Invitrogen Life Technologies) supplemented with 10% heat-inactived Fetal Calf Serum (Gibco, Invitrogen Life Technologies) at 37°C in a humidified incubator (5% CO2). Mycoplasma test were performed on a regular basis and authentication of cell lines was confirmed using short tandem repeat (STR) typing (GenePrint 10 system, Promega). For experiments with conditioned media, cell culture medium was changed to serum-free medium at 90% confluence and tumor cells were kept in culture for 24 h. Conditioned medium was collected, filtered through a 0.2 µm syringe (GE Healthcare Life Sciences) and used directly at a 20% concentration in cell culture medium or frozen for cytokine analysis.
Peripheral blood mononuclear cells (PBMCs) from three different blood donor buffy coats (Sanquin Blood bank, Region Southwest, Rotterdam, the Netherlands) were obtained by centrifugation over Ficoll gradient. CD14-positive monocytes were isolated from PBMCs by positive selection using anti-CD14 MicroBeads on a LS MACS column (Miltenyi Biotech). Monocyte-to-macrophage differentiation was induced by culturing monocytes (1 × 106cells/mL) for 6 days in RPMI-1640 supplemented with 10% FCS and 80 ng/mL GM-CSF (Peprotech) to polarize macrophages towards the M1 phenotype, or 20 ng/mL M-CSF (R&D Systems) to polarize macrophages towards the M2 phenotype, as previously reported.Citation48 To assess the effect of leiomyosarcoma cells on monocyte-to-macrophage differentiation, 1 × 106 freshly isolated monocytes were cocultured for 6 days in 24-well plates with either 1 × 104 LMS04 or 2,5 × 104 LMS05 cells in 0.4 µm Falcon® cell culture inserts (Corning Life Sciences) or with their conditioned media.
Flow cytometry
After 6 days, macrophages were detached by incubation in Accutase (Sigma-Aldrich) at 37°C during 30 minutes and stained for cell surface markers. Antibodies used were anti-CD1 a-APC, CD45-PE-Cy5.5, CD14-PE-Cy7, CD163-PE, HLA-DR-V500, CD86-FITC and CD40-APC-H7 (Supplementary Table 1). Data were acquired on a LSR II flow cytometer and analyzed using Kaluza FACS software (Beckman Coulter Life Sciences).
Cytokine analysis
Conditioned media from LMS cell lines LMS04 and LMS05 were used for quantification of h-M-CSF and h-IL-34 by Luminex according to the manufacturer's instructions (Bio-Rad Laboratories).
Statistics
Statistical analyses were performed using SPSS software version 23.0 (IBM Corporation) and graphs were constructed using GraphPad Prism software version 6. Correlation between immunohistochemical data and clinicopathological variables was analyzed using Spearman's rank correlation coefficient. A log-rank test was used for assessing CD163, CD3 and PD-L1 from primary tumors in relation to patient survival, then a multivariate Cox regression model including age, gender, grade and CD163-infiltrate. Nonparametric Mann-Whitney test or the Kruskal-Wallis test followed by Dunn's post-test were used to compare differences between in vitro conditions. P-value < 0.05 was considered to be statistically significant.
Financial support
Marie Kostine was supported in part by a grant from the Université de Bordeaux / Centre Hospitalier Universitaire de Bordeaux, France.
Disclosure/conflict of interest
The authors declare that they have no conflict of interest.
2017ONCOIMM0639R-s01.docx
Download MS Word (16.5 MB)Acknowledgements
The authors would like to thank Dr. Jonathan A. Fletcher, Brigham and Women's Hospital, Boston, for providing LMS04 and LMS05 cell lines, René Zwartbol for expert technical assistance in preparing the paraffin sections, Ekaterina S. Jordanova and Brendy E. van den Akker for help with the immunofluorescent procedures, as well as Noel F.C.C. de Miranda for providing the HLA antibodies and useful discussion.
References
- Lazar A, Evans H, Shipley J. Leiomyosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PC, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC Press, 2013; p. 111–13.
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii102–12. doi:10.1093/annonc/mdu254. PMID:25210080
- Duffaud F, Ray-Coquard I, Salas S, Pautier P. Recent advances in understanding and managing leiomyosarcomas. F1000prime Rep. 2015;7:55. doi:10.12703/P7-55. PMID:26097728
- Penel N, Italiano A, Isambert N, Bompas E, Bousquet G, Duffaud F, French Sarcoma Group. (Groupe Sarcome Français/Groupe d'Etude des Tumeurs Osseuses). Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Ann Oncol. 2010;21(6):1361–5. doi:10.1093/annonc/mdp485. PMID:19880438
- Nabeshima A, Matsumoto Y, Fukushi J, Iura K, Matsunobu T, Endo M, Fujiwara T, Iida K, Fujiwara Y, Hatano M, et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3 K/Akt pathways. Br J Cancer. 2015;112(3):547–55. doi:10.1038/bjc.2014.637. PMID:25562433
- Buddingh EP, Kuijjer ML, Duim RAJ, Bürger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PCW, Lankester AC, et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin Cancer Res. 2011;17(8):2110–9. doi:10.1158/1078-0432.CCR-10-2047. PMID:21372215
- van Dongen M, Savage NDL, Jordanova ES, Briaire-de Bruijn IH, Walburg KV, Ottenhoff THM, Hogendoorn PCW, van der Burg SH, Gelderblom H, van Hall T. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J Cancer. 2010;127(4):899–909. PMID:20013807
- Lee C-H, Espinosa I, Vrijaldenhoven S, Subramanian S, Montgomery KD, Zhu S, Marinelli RJ, Peterse JL, Poulin N, Nielsen TO, et al. Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin Cancer Res. 2008;14(5):1423–30. doi:10.1158/1078-0432.CCR-07-1712. PMID:18316565
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35(5):585–600. doi:10.1007/s00281-013-0367-7. PMID:23657835
- Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17(5):651–62. doi:10.1016/S1470-2045(16)00078-4. PMID:27055731
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59. doi:10.1016/j.ccr.2014.05.016. PMID:24898549
- Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI, et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med. 2015;373(5):428–37. doi:10.1056/NEJMoa1411366. PMID:26222558
- Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Müller C, Jegg A-M, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16(8):949–56. doi:10.1016/S1470-2045(15)00132-1. PMID:26179200
- Lim J, Poulin NM, Nielsen TO. New Strategies in Sarcoma: Linking Genomic and Immunotherapy Approaches to Molecular Subtype. Clin Cancer Res. 2015;21(21):4753–9. doi:10.1158/1078-0432.CCR-15-0831. PMID:26330427
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi:10.1126/science.aaa1348. PMID:25765070
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi:10.1038/nature13954. PMID:25428505
- Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin K-H, Hu H, Kim KS, Choi YD, Kim S, et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16:434. doi:10.1186/s12885-016-2451-6. PMID:27393385
- D'Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin L-X, Carvajal RD, Dickson MA, Gounder M, Keohan ML, Schwartz GK, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46(3):357–65. doi:10.1016/j.humpath.2014.11.001. PMID:25540867
- Paydas S, Bagir EK, Deveci MA, Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol. 2016;33(8):93. doi:10.1007/s12032-016-0807-z. PMID:27421997
- Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PloS One. 2013;8(12):e82870. doi:10.1371/journal.pone.0082870. PMID:24349382
- Zhang Y, Chen L. Classification of advanced human cancers based on tumor immunity in the microEnvironment (TIME) for cancer immunotherapy. JAMA Oncol. 2016;2(11):1403–4. doi:10.1001/jamaoncol.2016.2450. PMID:27490017
- Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41(1):49–61. doi:10.1016/j.immuni.2014.06.010. PMID:25035953
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96. doi:10.1038/ni.1937. PMID:20856220
- Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi:10.1016/j.cell.2010.03.014. PMID:20371344
- Cleton-Jansen A-M, Buddingh EP, Lankester AC. Immunotherapy: Is it different for sarcomas? Oncoimmunology. 2012;1(2):255–7. doi:10.4161/onci.1.2.18345. PMID:22720262
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi:10.1016/j.immuni.2014.06.008. PMID:25035950
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64(19):7022–9. doi:10.1158/0008-5472.CAN-04-1449. PMID:15466195
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320(5877):807–11. doi:10.1126/science.1154370. PMID:18467591
- Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. Antagonistic effects of GM-CSF and IFNγ. PloS One. 2013;8(2):e56045. doi:10.1371/journal.pone.0056045. PMID:23409120
- Espinosa I, Beck AH, Lee C-H, Zhu S, Montgomery KD, Marinelli RJ, Ganjoo KN, Nielsen TO, Gilks CB, West RB, et al. Coordinate expression of colony-stimulating factor-1 and colony-stimulating factor-1-related proteins is associated with poor prognosis in gynecological and nongynecological leiomyosarcoma. Am J Pathol. 2009;174(6):2347–56. doi:10.2353/ajpath.2009.081037. PMID:19443701
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi:10.1038/nrc3239. PMID:22437870
- Appleyard ST, Dunn MJ, Dubowitz V, Rose ML. Increased expression of HLA ABC class I antigens by muscle fibres in duchenne muscular dystrophy, inflammatory myopathy, and other neuromuscular disorders. Lancet. 1985;1(8425):361–3. doi:10.1016/S0140-6736(85)91384-4. PMID:2857418
- Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi:10.1038/bjc.2011.189. PMID:21629244
- Zhang Y, Kang S, Shen J, He J, Jiang L, Wang W, Guo Z, Peng G, Chen G, He J, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine. 2015;94(6):e515. doi:10.1097/MD.0000000000000515. PMID:25674748
- Budczies J, Mechtersheimer G, Denkert C, Klauschen F, Mughal SS, Chudasama P, Bockmayr M, Jöhrens K, Endris V, Lier A, et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology. 2017;6(3):e1279777. doi:10.1080/2162402X.2017.1279777. PMID:28405504
- Burgess M, Bolejack V, Van Tine B, Schuetze S, Hu J, D'Angelo S, Attia S, Priebat D, Okuno S, Riedel RF, et al. Multicenter phase II study of pembrolizumab (P) in advanced soft tissue (STS) and bone sarcomas (BS): Final results of SARC028 and biomarker analyses. J Clin Oncol. 2017;35 No 15_suppl:11008–11008.
- Ben-Ami E, Barysauskas CM, Solomon S, Tahlil K, Malley R, Hohos M, Polson K, Loucks M, Severgnini M, Patel T, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer. 2017;123(17):3285–3290. doi:10.1002/cncr.30738.
- Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, Rosen G. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6:24. doi:10.1186/s13569-016-0064-0. PMID:28042471
- Heine A, Kristiansen G, Schild HH, Brossart P. Successful treatment of refractory leiomyosarcoma with the PD-1 inhibitor nivolumab. Ann Oncol. 2016;27(9):1813–4. doi:10.1093/annonc/mdw243. PMID:27329248
- Wolchock JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall Survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi:10.1056/NEJMoa1709684.
- Toulmonde M, Penel N, Adam J, Chevreau C, Blay J-Y, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S, Grellety T, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: A phase 2 clinical trial. JAMA Oncol. 2017; doi:10.1001/jamaoncol.2017.1617. PMID:28662235
- Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults; Study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42. doi:10.1002/ijc.2910330108. PMID:6693192
- Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi:10.1186/1479-5876-9-216. PMID:22176642
- Zhang Q, Liu L, Gong C, Shi H, Zeng Y, Wang X, Zhao Y, Wei Y. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PloS One. 2012;7(12):e50946. doi:10.1371/journal.pone.0050946. PMID:23284651
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi:10.1056/NEJMoa020177. PMID:12529460
- Kostine M, Cleven AH, de Miranda NF, Italiano A, Cleton-Jansen AM, Bovée JV. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod Pathol. 2016;29(9):1028–37.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. doi:10.1056/NEJMoa1501824. PMID:27312065.
- de Vrij J, Maas SLN, Kwappenberg KMC, Schnoor R, Kleijn A, Dekker L, Luider TM, de Witte LD, Litjens M, van Strien ME, et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 2015;137(7):1630–42. doi:10.1002/ijc.29521. PMID:25802036
