ABSTRACT
Curbing PD-1 immunosuppressive signaling represents an effective immune awakening or immune-reactivation approach for tumor eradication for many cancers. Yet, the potential involvement of this critical PD-1 immunosuppressive signaling in de novo malignant transformation of epithelial cells to pre-cancerous or cancerous lesions is largely unknown. In this study, we demonstrate that PD-1 signaling is critically involved in de novo malignant transformation of oral mucosa upon carcinogen exposure in vivo. Our findings revealed that 4NQO-treated mice had almost double the numbers of PD-1-positive CD4+ cells and PD-1-positive CD8+ cells in peripheral blood lymphocytes as well as elevated PD-1 expression in tumor infiltrating lymphocytes (when compared to that of control-treated mice), strongly supportive of a general immune-suppression induced by carcinogen challenges in vivo. Importantly, inhibition of PD-1 signaling during the carcinogenesis process (immediately after 4NQO challenge) significantly reduced and delayed de novo formation of both pre-cancerous and cancerous lesions in vivo, in conjunction with effective PD-1 down-modulation in the tumor infiltrating leukocyte and peripheral lymph organs. Lastly, reduction of carcinogen-induced lesions upon PD-1 mAb treatment in vivo was accompanied by reduction of potent immunosuppressive myeloid-derived suppressor cells (MDSCs), and increase in “activated” T cell accumulations in the lesion-microenvironment (127% increase) and peripheral lymph nodes (25% increase). These data support PD-1 blockade as a new approach to enhance the efficacy of T-cell immunotherapy and reduce canceration rate in premalignant lesions.
| Abbreviations | ||
| 4NQO | = | 4-nitroquinoline-1 oxide |
| CTLA-4 | = | cytotoxic T-lymphocyte-associated protein 4 |
| DMBA | = | dimethylolbutanoic acid |
| FACS | = | flow cytometry |
| GITR | = | glucocorticoid-induced tumor necrosis factor receptor |
| HIV | = | human Immunodeficiency Virus |
| HNSCCs | = | head and neck squamous cell carcinomas |
| IHC | = | immunohistochemistry |
| IL-2 | = | interleukin-2 |
| IFN-γ | = | interferon-γ |
| MDSCs | = | myeloid-derived suppressor cells |
| MFI | = | median fluorescence intensity |
| OX-40 | = | tumor necrosis factor receptor superfamily, member 4 (TNFRSF4) |
| PBMC | = | peripheral blood mononuclear cell |
| PD-1 | = | programmed death receptor 1 |
| PD-L1 | = | programmed death ligand 1 |
| ROS | = | reactive oxygen species |
| SCCs | = | squamous cell carcinomas |
| TAM | = | tumor-associated macrophage |
| TILs | = | tumor-infiltrating lymphocytes |
Introduction
The introduction of immune checkpoint inhibitors for cancer therapy has revolutionized cancer treatment in recent years. It is now understood that T cells do harbor two kinds of immunomodulatory signals, co-stimulatory and co-inhibitory signals. Both are needed to orchestrate an optimal antigen-specific immune response in our body. A myriad of both activating receptors such as OX-40, GITR, or CD 28 and inhibitory receptors (also called immune checkpoints) such as programmed death receptor 1 (PD-1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) can impact the functions of T cells during tumorigenesis.Citation1 Activation of these immune checkpoints results in T cell de-activation.
Programmed death-1 or PD-1 is a checkpoint molecule expressed on T cells with a vital role in regulating peripheral tolerance.Citation2 Direct interaction between PD-1 and its two ligands, PD-L1 and PD-L2, can result in a state of “exhaustion” or anergy of antigen-specific T cells in the tumor microenvironment. This “exhausted” state can be, at least, partially reversed by PD-1 blockade.Citation3,4 To date, PD-1/PD-L1 blockade represents a new approach to enhance the anti-tumor efficacy of T-cells and provides significant benefits for patients with advanced stage cancer,Citation5 including advanced melanoma,Citation6 non-small cell lung cancer,Citation7 renal-cell cancer,Citation8 as well as head and neck cancer.Citation9 These findings establish an important role of this immunosuppressive PD-1 signaling in cancer growth and, likely disease progression. However, it remains unclear if this key PD-1 immunosuppressive signaling plays any role in the initial process of carcinogenesis or pre-malignant transformation of cancer in vivo.
Oral-specific carcinogenesis can be induced in mice by administration of a water-soluble chemical carcinogen, 4-nitroquinoline-1 oxide (4NQO), which mimics the tobacco-mediated oral carcinogenesis in human.Citation10 4NQO is known to impose intracellular oxidative stress, which causes mutations and DNA strand breaks. In vivo exposure to 4NQO can result in manifestations of all stages of oral epithelial transformation into pre-cancerous as well as cancerous lesions in mice with similar histologies as in human.Citation11 Studies found that low doses of 4NQO in drinking water would induce the formation of dysplastic oral lesions,Citation12–14 while prolonged exposure to higher doses of 4NQO in drinking water alone was sufficient to cause dysplastic lesions as well as squamous cell carcinomas (SCCs) of the tongue, oral mucosa, and esophagus in C57 BL/6 and CBA mice.Citation14 The known progressive appearance of premalignant and malignant lesions in the tongue and oral mucosa of this mouse model makes it highly useful for mechanistic investigation of step-wise oral carcinogenesis in vivo. Here, we employed this 4NQO-induced carcinogenesis model in immunocompetent C57 BL/6 mice to study the potential role of PD-1 immunosuppressive signaling in carcinogen-induced pre-malignant transformation of oral epithelium in vivo.
Results
4NQO treatment results in PD-1 overexpressing in precancerous and cancerous oral lesions in vivo
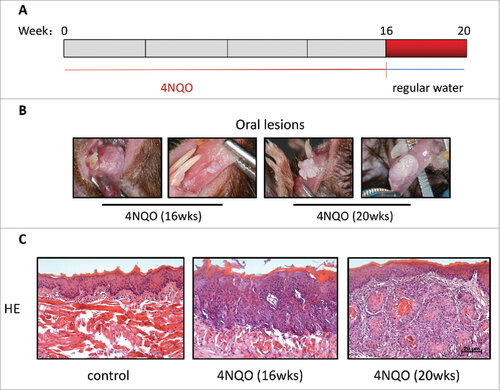
To examine the potential role of the immunosuppressive PD-1 signaling in oral carcinogenesis, we first establish the 4NQO-induced carcinogenesis model in immunocompetent C57 BL/6 mice. Mice were given drinking water containing 50ug/ml of 4NQO (or water in the absence of 4NQO as control), one time a week for 16 consecutive weeks, followed by sterile water for 4 weeks (). At week 16, 80% of mice (8/10 mice) were found to have pre-cancerous whitish thickened patches on their tongues. Histological examination with H&E staining revealed evident loss of organization of epithelium, pronounced rete pegs, and cellular atypia, demonstrative of successful establishment of precancerous tongue lesions upon 4NQO treatment ( & ). The number and size of these lesions continued to grow even after 4NQO withdrawal (during 4NQO withdrawal, mice were only given water). At week 20, all 4NQO-expsoured mice (100%, 10/10 mice) developed at least one or more oral tumors with squamous cell carcinoma (SCC) histologies ( & ). Detailed examination of these SCC lesions showed invasion of neoplastic epithelial cells into the sub-epithelial tissue. No pre-cancerous nor tumors were observed in the water-treated control group at week 20.
Figure 1. Tumor formation induced by 4NQO. (A) 4NQO was administered 1 time a week for 16 consecutive weeks and then were given regular water until week 20. (B) Representative oral lesions in 4NQO-treated mice. (C) Representative Hematoxylineosin (HE) of 4NQO-treated mice and control mice. Most mice had one or more dysplastic lesions per tongue at week 16. All mice developed one or more large SCC per tongue at week 20.
To examine if PD-1 signaling was involved in this carcinogen-induced oral carcinogenesis model in immunocompetent mice, we first examined the expression of PD-1 protein in the tumor-infiltrating lymphocytes of these tongue lesions by IHC. As shown in , all 4NQO-induced tongue lesions expressed elevated levels of PD-1 protein in tumor-infiltrating lymphocytes when compared to that of normal tongue tissues from control mice (; p = 0.032). Unexpectedly, flow cytometry analysis of peripheral blood lymphocytes from 4NQO-treated mice also revealed PD-1 upregulation. We found that the number of PD-1+CD4+ T cells from 4NQO-treated mice were ∼2 fold higher (20.65% vs. 9.432%) than that of the control mice (; p = 0.0005). Similarly, we found that the number of PD-1+CD8+ T cells from 4NQO-treated mice were ∼2 fold higher (10.21% vs. 5.064%) than that of the control mice (; p = 0.0410). These findings suggested a compromised immune status in both local oral lesions as well as the peripheral immune system. Furthermore, we found that PD-1 expression was higher in week 20 mice that developed more cancerous lesions (SCCs), as compared to week 16 pre-cancerous oral lesions (data not show).
Figure 2. Upregulation of PD-1 in tumor-infiltrating lymphocytes in precancerous and cancerous oral lesions in 4NQO-treated mice at week 16. Representative tissue sections from the tongue of 4NQO-treated mice and control mice were analyzed for their expression of PD-1 by IHC. And blood was sampled from 4NQO treated-mice and control mice tails, then analyzed by flow cytometry. (A) Representative images show high expression of PD-1 in the 4NQO-treated mice group. (B) Representative results of flow cytometric staining of PD-1 expression on CD4+ T cells in peripheral blood from 4NQO-treated mice and control mice at 16 weeks revealed high expression of PD-1 on CD4+ T cells in the 4NQO-treated mice group. (C) Representative results of flow cytometric staining of PD-1 expression on CD8+ T cells in peripheral blood from 4NQO-treated and control mice at 16 weeks revealed high expression of PD-1 on CD8+ T cells in the 4NQO-treated mice group. All data represent average ± SD. Statistical significance was determined by Student t test, #p < 0.05, ##p < 0.01, ###p < 0.001.
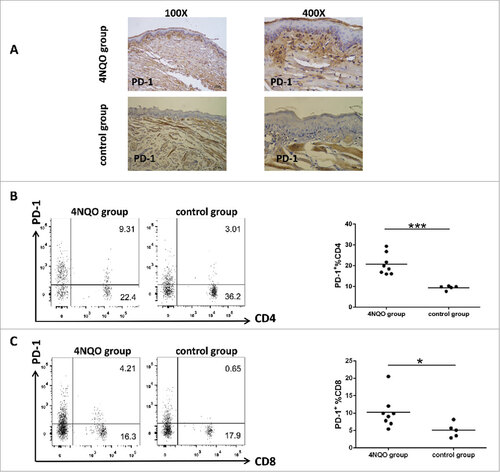
Table 1. Immunohistochemical analysis of the expression of PD-1 in tissue sections at week 16.
Specific inhibition of PD-1 signaling inhibits tongue musoca lesion formation, and delays oral carcinogenesis
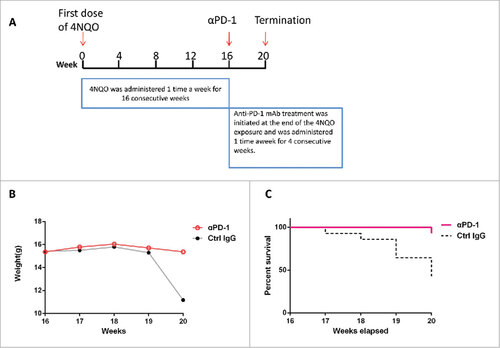
The observed PD-1 overexpression in the carcinogen-induced lesions and peripheral immune system implicates a likely involvement of PD-1-mediated immunosuppression in de novo oral carcinogenesis in immunocompetent mice. Thus, we sought to determine the role of PD-1 in de novo pre-cancerous oral lesion formation by targeted inhibition of PD-1 using anti-PD-1 mAb. Briefly, C57 BL/6 mice were exposed to 4NQO challenges as previously for 16 weeks, and then treated with anti-PD-1 mAb or control IgG, one time a week for 4 consecutive weeks (), then followed by tumor incidences and survival monitoring. As shown in & , anti-PD-1 mAb treatment (vs. control IgG-treated group) significantly increased the weight (p = 0.0107) and overall survival (p = 0.0041) of 4NQO-treated mice.
Figure 3. Anti-PD-1 mAbs treatment extends mice survival. (A) 4NQO was administered 1 time a week for 16 consecutive weeks. Anti-PD-1 mAb treatment was initiated at the end of the 4NQO exposure and was administered 1 time a week for 4 consecutive weeks. (B) Anti- PD-1 mAbs treated group and IgG control group were weighed and documented every 1 weeks. Body weights were significantly reduced at week 20 in IgG control group. The Student t test was performed for the significance determined, p = 0.0107. (C) Survival of IgG control group and anti-PD-1 mAbs treated group were monitored and showed. The survival curves were estimated using the Kaplan-Meier method. The log-rank test was performed for the significance determined, p = 0.0041.
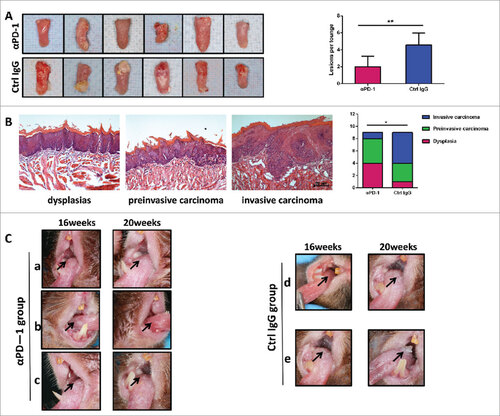
Furthermore, anti-PD-1 mAb treatment was able to reduce the number of 4NQO-induced lesions (), accompanied by less malignant phenotypes in the lesions when compared with control IgG-treated group. The average number of lesions was 2 in the anti-PD-1 mAb treatment group, vs 4.5 in the control IgG-treated group (p = 0.0015) (). Thus, specific inhibition of PD-1 signal was sufficient to reduce oral lesions by >50%. More importantly, histological evaluation showed that anti-PD-1 mAb-treated mice had less invasive carcinoma lesions but more of the dysplasia (early lesions) in their tongues (vs. control mice; ). Only one case of invasive carcinoma, four cases of dysplasia, and four cases of pre-invasive carcinoma (carcinoma in situ) were identified in the anti-PD-1 mAb-treated mice (). Whilst five cases of invasive carcinoma, three cases of pre-invasive carcinoma (carcinoma in situ), and only one case of dysplasia were noted in the control IgG-treated mice (). The differences of the tumor progression between these two treatment groups were statistically significant (, p = 0.037). showed representative lesions in the anti-PD-1 mAb treated group, with noticeable reduction of tumor size comparing to the sizes prior to treatment ( a and b), or no increase of tumor size or morphology indicating attenuation of disease progression (). Meanwhile in the control group, lesions appearing as leukoplakic lesion with smooth surface changed to rough, granular surface () or exogenous verrucous surface () with increased area of damage. Our findings clearly demonstrated a less transformative phenotype/activities of the 4NQO-carcinogen when PD-1 was blocked immediately after carcinogen challenge. This is consistent with the observed longer survival of anti-PD-1 mAb treated group.
Figure 4. Anti-PD-1 mAb treatment inhibits tongue musoca lesions growth and delays carcinogenesis. (A) Photos of representative tongues of control IgG-treated and anti-PD-1 mAbs-treated mice. Anti-PD-1 mAbs-treated mice had decreased tongue musoca lesions. Data represent average ± SD. Statistical significance was determined by Student t test, ##p = 0.0015. (B) Representative Hematoxylineosin (HE) of dysplasias, preinvasive carcinoma (carcinoma in situ) and invasive carcinoma. Statistical significance was determined by Mann-Whitney U test, #p < 0.05. (C) The contrast of macroscopic observation of lingual mucosal lesion with anti-PD-1 antibodies or Hamster IgG antibodies. a and b, Reduction of lingual mucosal lesion with anti-PD-1 antibodies. c, No change of lingual mucosal lesion with anti-PD-1antibodies. Lesions appearing as leukoplakic lesion with smooth surface changed to rough, granular surface (d) or exogenous verrucous surface (e).
PD-1 antibody treatment suppressed PD-1 expression on TILs and T cells in peripheral lymph tissues but promoted CD4+ cells and CD8+ cell populations
In order to examine if PD-1 blockade could also affect local or systemic immunity during the formation of pre-cancerous or cancerous lesions in this immunocompetent model, we examined carefully the effects of PD-1 mAb treatment in the locoregional as well as systemic immunity in vivo. Representative tissue sections from the tongues of control IgG-treated mice and anti-PD-1 mAb-treated mice were analyzed for PD-1 expression as well as the accumulation of CD3+ T cells by IHC. As shown in , specific PD-1 blockade did result in down-regulation of PD-1 expression in local lesions, in conjunction with CD3+ T cell accumulation (;vs. control IgG-treated group). As summarized in , 66.66% (6/9 samples) and 33.33% (3/9 samples) of the tongue lesions from the control IgG-treated group showed either high and moderate PD-1 expression. In contrast, only 1 (11.11%) and 3 samples (33.33%) of the lesions of the anti-PD-1 mAb-treated group had high and moderate PD-1 expression, whereas the remaining majority of lesions (5/9 samples, 55.55%) had only low levels of PD-1 expression in situ. Notably, as many as 56% of samples (5 out of the 9 samples) showed high levels of CD3 expression, and the remaining 33% of samples (4/9 samples) showed moderate CD3 expression and none with low CD3 expression in the lesions of the anti-PD-1 mAb-treated group (). An opposite pattern was observed in the control IgG-treated group () that only 1 sample (11.11%) showed high CD3 expression, and the remaining 8 samples showed predominately moderate (4 samples; 44%) and low (4 samples; 44%) CD3 expression in the lesions. The results indicate that PD-1 blockade might result in immune activation in local tumor microenviroment.
Figure 5. PD-1 antibody-treatment downregulates PD-1 expression but increase the accumulation of CD4+ T cells, CD8+ T cells. (A) Representative tissue sections from the tongue of anti-PD-1 mAb-treated mice and control mice were analyzed for their expression of PD-1 by immunohistochemical staining, which indicates descreased PD-1 expression in the lesions of anti-PD-1 mAb -treated group. (B) Representative tissue sections from the tongue of anti-PD-1mAb-treated and control mice were analyzed for CD3+ T cells expression by immunohistochemical staining, which shows increased infiltration of CD3+ T cells in the lesions of anti-PD-1 mAb -treated group. (C) MFI for PD-1 of lymphocytes in spleen, lymph node and blood are shown. (D) Representative flow cytometric analysis of PD-1 expression on CD4+ T cells in spleen, lymph node and blood of both anti-PD-1 mAb-treated and control group. The percentages of PD-1 expression on CD4+ T cells are shown. (E) Representative flow cytometric analysis of PD-1 expression on CD8+ T cells in spleen, lymph node and blood of both anti-PD-1 mAb-treated and control group. The percentages of PD-1 expression on CD8+ T cells are shown. (F, G) The percentages of CD4+ and CD8+ T cells in spleen, lymph node and periphery blood in anti-PD-1 mAb-treated and control IgG-treated mice are shown. All data represent average ± SD. Statistical significance was determined by Student t test, #p < 0.05, ##p < 0.01. MFI, mean fluorescence intensity.
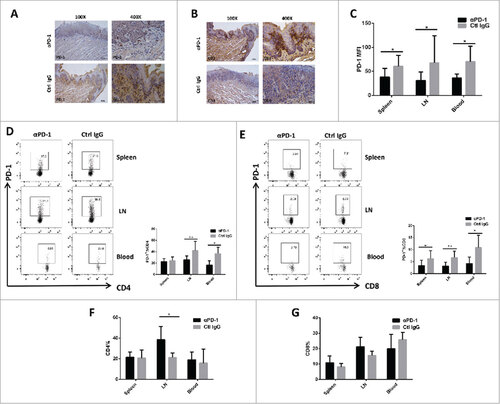
Table 2. Immunohistochemical analysis of the expression of PD-1 and CD3 in tissue sections.
To further investigate the potential effects of PD-1 blockade on peripheral lymph tissues, cells from spleen, blood and lymph nodes of all mice were analyzed by flow cytometry. We noted a marked reduction of PD-1+ T cells in spleen, blood as well as lymph nodes of the anti-PD-1 mAb-treated mice vs. control IgG-treated mice (). Among anti-PD-1 mAb-treated mice, the MFI values for PD-1 staining were 36.08 (p = 0.0355), 31.05 (p = 0.0466) and 38.19 (p = 0.0219) for spleen, blood and lymph nodes respectively, indicative of a low PD-1 expression in the peripheral immune system upon PD-1 inhibition. Whereas in control mice, the MFI values for PD-1 staining were notably higher: 70.02 (p = 0.0355), 67.73 (p = 0.0466) and 60.71 (p = 0.0219) for spleen, blood and lymph nodes, respectively. Furthermore, PD-1 blockade significantly reduced the percentage of the PD-1+CD4+ T cells () in lymph nodes (mean value for anti-PD-1 mAb-treated group vs. control IgG-treated group: 25.76% vs. 42.33%; p = 0.0058) and blood (16.66% vs. 36.67%; p = 0.0142). PD-1 inhibition also resulted in a significant decrease in the percentage of PD-1+CD8+ T cells () in spleen (mean value for anti-PD-1 mAb-treated group vs. control IgG-treated group: 3.398% vs. 6.282%; p = 0.0412), blood (4.26% vs. 10.94%; p = 0.0311) as well as lymph nodes (3.175% vs. 6.72%; p = 0.0037).
In addition, we also found that PD-1 blockade remarkably increased the percentage of CD4+ T and CD8+ T cell populations in spleen, lymph node, as well as in the blood when compared with that of the IgG-treated control mice( and ). Importantly, we even observed a significantly increase of CD4+ T cell populations in lymph node of anti-PD-1 mAb-treated mice (∼38.5% of the lymphocytes in anti-PD-1 mAb-treated group vs. 21.2% in control IgG-treated group) (; p = 0.0174). Our data first demonstrated that specific PD-1 inhibition administered immediately after carcinogen challenge not only could effectively reduce the expression of the PD-1 immunosuppressive molecules on TIL and T cells of peripheral tissues, but it was also capable of effectively upregulating T cell populations in the peripheral tissues in vivo, indicative of both the loco-regional and systemic immune activation in mice with carcinogen-induced early lesions.
PD-1 blockade reduced MDSCs accumulation and promoted activation of CD4+ T cells and CD8+ T cells in vivo
Myeloid-derived suppressor cells (MDSC) are key regulators of the immunosuppressive network which suppress NK cells, CD4+ and CD8+ T cells. The important role of MDSCs in cancer development has been noticed for many years.Citation15 The prevailing view supports that MDSC-mediated immunosuppression may have unanticipated implications for cancer immunity.Citation15 Therefore, to determine whether PD-1 blockade would alter MDSC population in vivo, we examined potential changes of MDSC numbers in the spleen and the neck draining lymph nodes (loco-regional lymph nodes) by flow cytometry. Our finding revealed that the CD11b+Gr1+ MDSCs of 4NQO group in spleen were higher than that of control group that have not received 4NQO at 16 week (supplementary Fig.1; p = 0.0277). PD-1 blockade remarkably reduced CD11b+Gr1+ MDSCs in the spleen (, mean value for anti-PD-1 mAb-treated group, IgG-treated control group: 19.48%, 31.95%; p = 0.0345). Thus, PD-1 blockade may possibly confer an anti-oncogenic effects by suppressing the key immunosuppressive regulator, MDSC, in the peripheral immune organs.
Figure 6. Blocking of the PD-1/PD-L1 pathway results in decreased levels of MDSCs and enhanced IL-2 and IFN-γ secretion by CD4+ T cells and CD8+ T cells in anti-PD-1 mAb -treated group. (A) Representative flow cytometric analysis and the percentages of MDSCs in spleen, lymph node are shown. (B) Representative flow cytometric analysis and the percentages of CD4+ and CD8+ T cells secreting IFN-γin spleen, lymph node are shown. (C) Representative flow cytometric analysis and the percentages of CD4+ and CD8+ T cells expressing IL-2 in spleen, lymph node are shown. All data represent average ± SD. Statistical significance was determined by Student t test, #p < 0.05, ##p < 0.01, ###p < 0.001,####p < 0.0001.
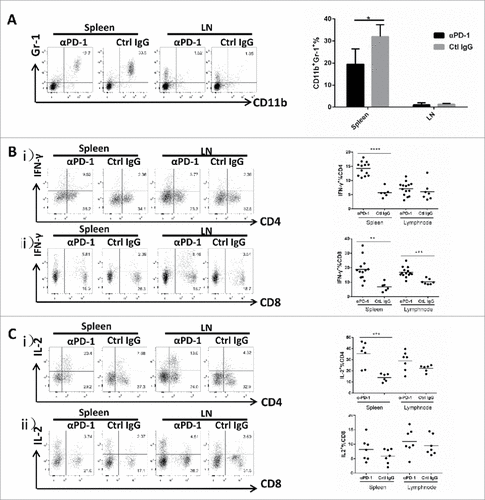
To further investigate the potential anti-oncogenic effect of PD-1 blockade along the T cell activation axis, cells from spleen and lymph nodes were also analyzed by flow cytometry. Our results demonstrated that CD4+ and CD8+ T cells present in the spleen of anti-PD-1 mAb-treated mice were able to produce IFN-γrather than in IgG -treated controls ( & ; CD4+ T cells: 14.26% vs. 5.74%, p<0.0001; CD8+ T cells: 18.81% vs. 6.783%, p = 0.0016). The IFN-γ produced by CD8+ T cells in lymph nodes was also higher in anti-PD-1mAb-treated group than that in control group (, 16.91% vs. 10.32%, p = 0.0002), whereas the IFN-γ produced by CD4+ T cells in lymph nodes presented no significant difference between the anti-PD-1 mAb-treated group and the control group (, 7.117% vs. 6.077%, p = 0.4154). Moreover, PD-1 blockade also increased IL-2 secreting from CD4+ T cell population in spleens of the anti-PD-1 mAb-treated group when compared with IgG-treated controls (; mean value for anti-PD-1 vs. control group: 35.37% vs. 14.02%; p = 0.0007) but not in lymph nodes and nor from CD8+ T cell population (). Similarly, Our findings strongly indicate that PD-1 blockade promoted a general T-cell activation, which may be involved in the prevention of formation of oral pre-cancerous and/or cancerous lesions induced upon carcinogen challenge.
Discussion
In the study, we used C57 BL/6 mice provided 4-Nitroquinoline-1-oxide in the drinking water to establish an immunocompetent animal model for oral carcinogenesis, for the investigation of the potential involvement of the PD-1 immunosuppressive signaling in premalignant lesions and early stage of oral cancer. PD-1 blockade has been shown to result in very good antitumor activity in various preclinical cancer models.Citation16 It has been recently approved for the treatment of various advanced malignancies, including head and neck squamous cell carcinoma (HNSCC).Citation9
Though PD-1 signaling seems to support growth and progression of cancers, however, its potential involvement in early carcinogenesis or even pre-cancerous lesions have not been clearly investigated. In the current investigation, we demonstrate that PD-1 blockade (by anti-PD-1 mAb) significantly hampered the formation of early pre-cancerous or cancerous lesions in immunocompetent C57 BL/6 mice upon carcinogen challenge (4NQO), and resulted in increased overall survival of the mice post-4NQO exposure. Furthermore, PD-1 blockade effectively reduced the development of invasive carcinoma. Our finding revealed the active involvement of PD-1 signaling in the carcinogenesis of oral cancer upon carcinogen challenge.
Various animal models have been developed for the study of carcinogenesis. The sequential stages of carcinogenesis like hyperplasia, dysplasia, severe dysplasia, in situ carcinoma and SCC induced by 4NQO was found to be a better carcinogen for the establishment of oral cancer as compared to DMBA in rat model.Citation17 We found that most mice exposed to 4NQO had developed dysplastic lesions on tongue at the end of week 16, and all mice (100%) developed one or more oral cancers at the end of week 20, which agrees with previous reports.Citation18,19 This suggests that 4NQO model may be an effective way to lead to premalignant lesions and contribute to the dynamic studies of the process of carcinogenesis.
We found that PD-1 expression on the lymphocyte were increased both in the tumor microenviroment and the peripheral blood of C57 BL/6 mice exposed to 4NQO at the end of week 16. PD-1 signaling represents a major immune resistance mechanism within the tumour microenvironment. On effector T cells, expression of PD-1 is thought to be both a marker for and contributor to “exhaustion”, and increases the susceptibility of these cells to PD-L1 mediated death signals.Citation3 Therefore, the expression of PD-1 on T cells circulating in PBMCs is increasingly recognized as a marker of disease progression.Citation20-22 For example, increased PD-1+CD8+ T cell numbers in PBMCs of patients with HIV infection correlate positively with impaired immune effector functions, disease progression, and declining CD4+ T cell counts.Citation23,24 Our results not only indicated a close relationship between PD-1 expression and oral precancerous and/or cancerous lesions progression, but it proved at the same time that the immune response of mice with oral precancerous lesions might be compromised via PD-1 signaling. Further, PD-1 blockade can effectively hamper the development of precancerous and cancerous oral lesions.
However, PD-L1 expression was not detected on those hyperplasia or dysplasia cells of mice by immunohistochemistry in our study. Actually, taking PD-L1 expression in tumor tissue as the first and obvious potential biomarker for PD-1 antibody therapy might be inaccurate. The proportion of tumor cells that are PD-L1 positive may be quite low in many tumors due to the heterogeneity and variability of immunohistochemical examination, and thus pose inherent limitations on its potential use as a predictive marker for the efficacy of PD-1/PD-L1 blockade.Citation25-27 Moreover, the observation that also patients, who do not show PD-L1 expression, might respond to checkpoint blockade is well documented.Citation28 Thus, further investigation leading to uniform methods of PD-L1 evaluation and more detailed analysis of specific cells and their functional responses to PD-1 ligation may be helpful to decrease the discrepancies observed.
PD-1 has been shown to play a more prominent role in modulating T cell activity in peripheral tissues via interaction with its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC).Citation29,30 In order to explore the mechanism of PD-1 antibody treatment, we tested the numbers of T cells and MDSCs in spleen, lymph nodes and blood and the production of cytokine in spleen and lymph nodes. We found that it was enhanced immune responses which inhibited the transformation of precancerous lesions into cancer in the mice provided with PD-1 antibody. Our results, on one hand, demonstrated that blockade of PD-1/PD-L1 pathway not only induced the accumulation of T cells in local lesions and peripheral tissues but the activation of T cells in spleen and lymph nodes which came from the evidence of enhanced IL-2 and IFN-γ expression. Many other surveys in advanced cancer have produced results essentially in agreement with our figures which are found for the first time in premalignant lesions.Citation31-33 On the other hand, decreased the accumulation of PD-1+CD4+, PD-1+CD8+ T cells and CD11b+Gr1+ MDSCs in blood, spleen as well as lymph nodes also suggested attenuated immune inhibitory axis. This result is also basically consistent with previous observation that a dramatic decrease in tumor microenviroment of the percentage of CD8+ T cells expressing PD-1 and myeloid-derived suppressor cells (MDSC) in animal modelCitation34-36 and the patients with metastatic melanomaCitation37 head and neck squamous cell carcinoma,Citation38 and hepatocellular carcinoma.Citation31 The tumor-associated PD-1 expressing effector cells are usually dysfunctional,Citation39,40 whereas the reactivities of PD-L1-deficient CD4+ and CD8+ T cells were strikingly augmented in vitro and in vivo as compared with those of wild-type T cells,Citation41 PD-1 or PD-L1 blocking antibodies can partially recover the biological activity of T cells,Citation42 thus permit T cells to maintain their tumor cell-killing function and inhibit the initiation, progression and metastasis of tumors.
MDSCs could rapidly expand and accumulate to suppress tumor-reactive T cells to promote tumor progression in human cancer patients and tumor-bearing mice.Citation43,44 PD-L1 expression also has been observed in MDSCs in tumor-bearing mice, and its expression has been linked to hypoxia and HIFa.Citation45,46 Under hypoxia, MDSCs could inhibit T cell function by decreasing the percentage of IFN-γ+ CD8+ and CD4+ T cells; whereas blockade of PD-L1 signifcantly increased the percentage of IFN-γ+ CD8+ T and IFN-γ+ CD4+ T cells in spleen and thus abrogated the suppressive activity of MDSCs.Citation45 Finke et al.Citation15 also suggested that persistence of intratumor MDSCs was paralleled by depressed intratumor T cell IFN-γ response. These results are coincide with our observation that PD-1 blockade resulted in a significant increase of the production of IFN-γ induced by CD4+ and CD8+ T cells in spleen and lymph nodes and decrease of proportion CD11b+Gr1+ MDSCs in spleen of mice. However, Lu et al.Citation44 found that PD-L1+ MDSCs were observed in the tumor microenvironment and IFN-γ neutralization significantly decreased PD-L1+ MDSCs in the tumor microenvironment in vivo. That means the role of MDSCs might be different in immune suppression in cancer depending on their location. Corzo et al.Citation46 showed that MDSCs from the tumor site and spleen of the same mouse differ profoundly in the ability to suppress T cell function. For example, spleen MDSC contain a high level of ROS but tumor MDSC had no increase in ROS but a very high level of NO and arginase I.Citation47 ROS has been proved to play an crucial role in antigen-specific T cell tolerance mediated by MDSC.Citation48,49 Moreover, MDSC transferred into tumor site seemed to differentiate to TAM much more rapidly than those in spleen.Citation46 We also tried to test the frequency of CD11b+Gr1+ MDSCs in premalignant lesions of mice, but hardly did we found any of them in the lesions. These biochemical disparities in different microenviroment translated into fundamental differences in MDSC ability to suppress T cells, so further precise studies are needed to clarify these issues.
In conclusion, we found that PD-1 is directly involved in the formation of carcinogen-induced precancerous and cancerous oral lesions in immunocompetent models. Further, PD-1 blockade can activate the immune system in vivo, resulting in reduction of early precancerous and invasive cancer oral lesions.
Materials and methods
Mice
Six-week-old female C57 BL/6 mice were purchased from Guangzhou University of Chinese Medicine. All animals were maintained in a pathogen-free facility at Sun Yat-Sen University. All animal procedures were conducted under institutional guidelines that comply with national laws and policies and the study protocol was approved by the institutional Animal Care and Use Committee.
4NQO-Induced Tumorigenesis
4NQO was obtained from Sigma-Aldrich. The carcinogen 4NQO was dissolved in propylene glycol (Sigma-Aldrich) as stock solution (4 mg/mL), stored at 4°C The 4NQO stock solution was diluted to a concentration of 50 ug/ml in the drinking water. Female C57BL/6 mice 6-week-old were given either water (control) or 4NQO for 16 weeks, and the water was replaced once a week. After the 16-week carcinogen treatment, the drinking water was reverted to regular water. Mice were analyzed for oral precancerous and cancerous lesions at different times for up to 16 or 20 weeks.
Antibody treatment
The anti mPD-1 antibody was a kind gift from Lieping Chen. Female C57 BL/6 mice 6-week-old were given either water (control) or 4NQO for 16 weeks, and the water was replaced once a week. After the 16-week carcinogen treatment, the drinking water was reverted to regular water. According to the difference of tongue musoca lesions, the mice were randomly divided into control IgG groups and αPD-1 treated group at 16 week. 200ug of anti mPD-1 antibody were administered intraperitoneally once a week for four weeks.The mice were euthanized at the end of the studies.
Pathological analysis and immunohistochemistry
Oral leisons were identified and photographed. Mice were killed by CO2 asphyxiation. Oral lesions were harversted, fixed in 10% formalin, embedded in paraffin, sectioned into 4-um sections. Hematoxylin and eosin staining was performed on tissue sections. The lesions observed were classified as dysplasia, in situ carcinoma or invasive carcinoma. For routine histological analysis, the results were examined under a light microscope (Olympus Optical) and reviewed by 2 certified pathologists. Anti-mouse CD3 monoclonal antibody (Clone 17A2;Biolegend) and anti-mouse PD-1 monoclonal antibody Clone RMP1-14;Biolegend) were used for immunohistochemical analysis of paraffin sections. The antibodies were used in the following concentrations: CD3 rat monoclonal antibody, 1:100; PD-1 rat monoclonal antibody, 1:50. Tissue samples were deparaffinized, and underwent high-pressure heating antigen retrieval with EDTA buffer (PH 8.0). Endogenous peroxidase activity was quenched by treatment with 3% H2O2 for 10 min and then incubated in the blocking solution (5% bovine serum albumin in PBS) for 1 hour. The section was then incubated with monoclonal antibody overnight at 4°C. The immunostaining was visualized with an PV kit (ZSGB-Bio, China) using a peroxidase and diaminobenzidine substrate. The sections were counterstained with Mayer's hematoxylin and examined using a light microscope (Olympus Optical) and reviewed by 2 certified pathologists. Scoring of immune stained positive TILs was done independently by two pathologists. CD3+, PD-1+ were counted in five randomly selected high power fields at 40X magnification and the counts were averaged. Initially TIL count was recorded as: +(1-25 cells), ++ (26-50 cells), +++ (≥51 cells) in the lesions.
Flow cytometry and analysis
PBMCs were isolated from leukapheresis products using Ficoll-Paque Plus density gradient centrifugation (GE Healthcare Life Sciences, 17-1440-03). Antibodies against CD4, CD8, PD-1, CD11, Gr-1, IL-2 and IFN suspensions from spleens, lymph node were prepared according to a standardized protocol. After PBMCs were blocked by anti-mouse FcR mAb for 30min, the cells were further stained with the specific antibodies in PBS with FBS for 30min at 4°C.
Intracellular staining of IFN-γ, IL-2 was performed as follows: Cells were stimulated with PMA (eBioscience) and ionomysin (eBioscience) for 5h at 37°C with 5% CO2. GolgiPlug (BD) was added at a dilution of 1:200 after the first hour of incubation. Cells were wash, stained with surface marker antibodies, then fixed and permeabilized with fixation/permeabilization and permeabilization buffer (eBioscience) and intracellularly stained for IFN-γ, IL-2. Cell were analyzed on the BD FACSVerse flow cytometer, and data analyzed using Flowjo software.
Graph and statistical analysis
The survival curves were estimated using the Kaplan-Meier method. The log-rank test was performed for the significance determined. FACS results were analyzed with Flowjo software. Differences in variables between any 2 groups were analyzed using the Mann-Whitney test or two-tailed t-test. All graphs and statistical analysis were undertaken with Graph Pad Prism version 5.0. All data are presented as mean±SEM. P values less than 0.05 were considered to be statistically significant. #p < 0.05, ##p < 0.01, ###p < 0.001.
Grants
This project was supported by the National Natural Science Foundations of China (No. 81472524, 81630025, 81602383), Research Grant Council, Hong Kong (#17114814, #17121616, General Research Fund), (Theme-based Research Scheme: T12-401/13-R), and the Start-up Fund from the School of Biomedical Sciences, Faculty of Medicine, the Chinese University of Hong Kong.
Competing interests
VWYL serves as a scientific consultant for Novartis, Hong Kong. All other authors declare that no competing interests.
2017ONCOIMM0595R-f07-z-4c.tif
Download TIFF Image (5.2 MB)References
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489. doi:10.1038/nature10673. PMID:22193102
- Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017;66(5):551-564. doi:10.1007/s00262-017-1954-6. PMID:28213726
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. doi:10.1038/nature04444. PMID:16382236
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127r-137r. doi:10.1126/scitranslmed.3003689.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. doi:10.1056/NEJMoa1200694. PMID:22658128
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. doi:10.1056/NEJMoa1305133. PMID:23724846
- Bagley SJ, Bauml JM, Langer CJ. PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. Clin Adv Hematol Oncol. 2015;13:676-683 PMID:27058572
- Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Martignoni G, Brunelli M, Piva F, Berardi R, Montironi R, et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015;41:114-121. doi:10.1016/j.ctrv.2014.12.013. PMID:25586601
- Fuereder T. Immunotherapy for head and neck squamous cell carcinoma. Memo. 2016;9:66-69. doi:10.1007/s12254-016-0270-8. PMID:27429658
- Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655-667. doi:10.1016/j.oraloncology.2005.10.013. PMID:16448841
- Hawkins BL, Heniford BW, Ackermann DM, Leonberger M, Martinez SA, Hendler FJ. 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck. 1994;16:424-432. doi:10.1002/hed.2880160506. PMID:7960739
- Ide F, Oda H, Nakatsuru Y, Kusama K, Sakashita H, Tanaka K, Ishikawa T. Xeroderma pigmentosum group A gene action as a protection factor against 4-nitroquinoline 1-oxide-induced tongue carcinogenesis. Carcinogenesis. 2001;22:567-572. doi:10.1093/carcin/22.4.567. PMID:11285190
- Ma G, Ikeda H, Inokuchi T, Sano K. Effect of photodynamic therapy using 5-aminolevulinic acid on 4-nitroquinoline-1-oxide-induced premalignant and malignant lesions of mouse tongue. Oral Oncol. 1999;35:120-124. doi:10.1016/S1368-8375(98)00066-9. PMID:10211320
- Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301-313. doi:10.1158/1078-0432.CCR-0999-3. PMID:14734483
- Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856-861. doi:10.1016/j.intimp.2011.01.030. PMID:21315783
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-2454. doi:10.1056/NEJMoa1200690. PMID:22658127
- Supsavhad W, Dirksen WP, Martin CK, Rosol TJ. Animal models of head and neck squamous cell carcinoma. Vet J. 2016;210:7-16. doi:10.1016/j.tvjl.2015.11.006. PMID:26965084
- Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila). 2009;2:27-36. doi:10.1158/1940-6207.CAPR-08-0147. PMID:19139015
- Li J, Liang F, Yu D, Qing H, Yang Y. Development of a 4-nitroquinoline-1-oxide model of lymph node metastasis in oral squamous cell carcinoma. Oral Oncol. 2013;49:299-305. doi:10.1016/j.oraloncology.2012.10.013. PMID:23187306
- Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389-395. doi:10.1038/cmi.2010.28. PMID:20514052
- Malaspina TS, Gasparoto TH, Costa MR, de Melo EF, Jr, Ikoma MR, Damante JH, Cavassani KA, Garlet GP, da Silva JS, Campanelli AP. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:965-974. doi:10.1007/s00262-011-1007-5. PMID:21442435
- Waki K, Yamada T, Yoshiyama K, Terazaki Y, Sakamoto S, Matsueda S, Komatsu N, Sugawara S, Takamori S, Itoh K, et al.. PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci. 2014;105:1229-1235. doi:10.1111/cas.12502. PMID:25117757
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350-354. doi:10.1038/nature05115. PMID:16921384
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al.. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198-1202. doi:10.1038/nm1482. PMID:16917489
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733-1741. doi:10.1158/0008-5472.CAN-12-2384. PMID:23288508
- Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501-6505 PMID:14559843
- Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627-632. doi:10.1016/j.oraloncology.2014.04.003. PMID:24819861
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. doi:10.1056/NEJMoa1504627. PMID:26028407
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-314. doi:10.1007/s00262-004-0593-x. PMID:15599732
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467-477. doi:10.1038/nri2326. PMID:18500231
- Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887-896. doi:10.1002/ijc.25397. PMID:20473887
- Quan L, Chen X, Liu A, Zhang Y, Guo X, Yan S, Liu Y. PD-1 Blockade can restore functions of T-Cells in Epstein-Barr Virus-Positive Diffuse large B-cell lymphoma in vitro. PLoS One. 2015;10:e136476. doi:10.1371/journal.pone.0136476.
- Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209-5218. doi:10.1158/0008-5472.CAN-12-1187. PMID:22915761
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636-5646. doi:10.1158/1078-0432.CCR-13-0458. PMID:23873688
- Mkrtichyan M, Chong N, Abu ER, Wallecha A, Singh R, Rothman J, Khleif SN. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15. doi:10.1186/2051-1426-1-15. PMID:24829751
- Belai EB, de Oliveira CE, Gasparoto TH, Ramos RN, Torres SA, Garlet GP, Cavassani KA, Silva JS, Campanelli AP. PD-1 blockage delays murine squamous cell carcinoma development. Carcinogenesis. 2014;35:424-431. doi:10.1093/carcin/bgt305. PMID:24031027
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-571. doi:10.1038/nature13954. PMID:25428505
- Yu GT, Bu LL, Huang CF, Zhang WF, Chen WJ, Gutkind JS, Kulkarni AB, Sun ZJ. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPalpha axis in HPV negative head and neck squamous cell carcinoma. Oncotarget. 2015;6:42067-42080. doi:10.18632/oncotarget.5955. PMID:26573233
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537-1544. doi:10.1182/blood-2008-12-195792. PMID:19423728
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175-2186. doi:10.1084/jem.20100637. PMID:20819923
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691-10696. doi:10.1073/pnas.0307252101. PMID:15249675
- Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223-1234. doi:10.1093/intimm/dxm091. PMID:17898045
- Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, Ostrand-Rosenberg S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol. 2014;96:1109-1118. doi:10.1189/jlb.3A0414-210R. PMID:25170116
- Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5:e1247135. doi:10.1080/2162402X.2016.1247135. PMID:28123883
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781-790. doi:10.1084/jem.20131916. PMID:24778419
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439-2453. doi:10.1084/jem.20100587. PMID:20876310
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693-5701. doi:10.4049/jimmunol.0900092. PMID:19380816
- Mougiakakos D, Johansson CC, Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113:3542-3545. doi:10.1182/blood-2008-09-181040. PMID:19050306
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828-835. doi:10.1038/nm1609. PMID:17603493
