 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
DNA mismatch repair (MMR)-deficient cancers accumulate high numbers of coding microsatellite mutations, which lead to the generation of highly immunogenic frameshift peptide (FSP) neoantigens. MMR-deficient cells can grow out to clinically manifest cancers either if they evade immune cell attack or if local T-cells get exhausted. Therefore, a subset of MSI cancer patients responds particularly well to treatment with immune checkpoint inhibitors. We analyzed whether immune evasion in MMR-deficient cancer mediated by loss of HLA class I or II antigens is related to local immune cell activation status. Microsatellites located in Beta2-microglobulin (B2M) and the HLA class II-regulatory genes RFX5 and CIITA were analyzed for mutations in MMR-deficient colorectal cancers (n = 53). The results were related to CD3-positive and PDCD1 (PD-1)-positive T-cell infiltration. PDCD1 (PD-1)-positive T-cell counts were significantly higher in B2M-mutant compared to B2M-wild type tumors (median: 22.2 cells per 0.25 mm2 vs. 2.0 cells per 0.25 mm2, Wilcoxon test p = 0.002). Increasing PDCD1 (PD-1)-positive T-cell infiltration was significantly related to an increased likelihood of B2M mutations (OR = 1.81). HLA class II antigen expression status was significantly associated with enhanced overall T-cell infiltration, but not related to PDCD1 (PD-1)-positive T-cells. These results suggest that immune evasion mediated by B2M mutation-induced loss of HLA class I antigen expression predominantly occurs in an environment of activated PDCD1 (PD-1)-positive T cell infiltration. If B2M mutations interfere with anti-PDCD1 (PD-1)/CD274 (PD-L1) therapy success, we predict that resistance towards anti-PDCD1 (PD-1) therapy may – counterintuitively – be particularly common in patients with MMR-deficient cancers that show high PDCD1 (PD-1)-positive T cell infiltration.
Introduction
Cancers of the microsatellite instability (MSI) phenotype are caused by deficiency of the DNA mismatch repair (MMR) system. MSI cancers are distinguished among other features by their high immunogenicity. This likely results from an exceptionally high mutational load: MMR deficiency leads to the generation of multiple insertion/etion mutations in coding microsatellites and consequently to mutation-induced frameshift peptide (FSP) neoantigens.Citation1 MSI is observed in 15% of all colorectal cancers and in a variety of extracolonic malignancies such as endometrial cancer, of which up to 30% are MSI.Citation2–4 MSI cancers can develop sporadically, most frequently through somatic epigenetic inactivation of the MMR gene MLH1.5 Alternatively, they occur in the context of the hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome that is caused by germline mutations of MMR genes, most frequently MLH1 or MSH26
MSI colorectal cancers commonly present with a dense lymphocyte infiltration, typically of an activated and cytotoxic phenotype.Citation7–10 FSP-specific tumor-infiltrating lymphocytes from MSI cancers in fact retain the potential of lysing MSI tumor cells.Citation11 Despite the evidence of local anti-tumoral immune responses, MSI tumors often grow out to large and clinically relevant size, which suggests the existence of immuno-suppressive and/or immuno-evasive mechanisms. In fact up to 30% of all MSI colorectal cancers display mutations in the B2M gene with a consecutive loss of HLA class I antigens that prevents recognition as well as killing of B2M-mutant cells by cytotoxic T cells.Citation12,13 B2M mutations, as a likely mechanism of immune evasion, are thought to provide affected MSI tumor cells with a major selection advantage as it has been reported for other tumor types such as malignant melanoma.Citation14,15 More recently, an additional mechanism has been proposed to contribute to the immune evasion of MSI tumor cells: loss of functional HLA class II antigen presentation machinery occurs in approximately one third of all MSI CRCs as a consequence of mutations inactivating the HLA class II-regulatory genes RFX5 and CIITA.Citation16 an alteration that is also associated with increased local T cell infiltration of the tumor.Citation17
Immune checkpoint molecules such as PDCD1 (PD-1) have gained attention as targets for novel immune therapy approaches.Citation18–22 The physiological role of PDCD1 (PD-1) is to preserve the equilibrium between co-stimulatory and co-inhibitory signals that regulate T cell activity and maintain self-tolerance. In tumors, PDCD1 (PD-1) can limit the potency of anti-tumoral immune responses (T cell exhaustion) upon activation by one of its ligands (CD274 [PD-L1], PDCD1LG2 [PD-L2]).Citation22,23 This may facilitate the outgrowth of even highly antigenic tumors in the presence of tumor-antigen specific immune cells. Antibody-mediated blockade of PDCD1 (PD-1) or CD274 (PD-L1) has recently emerged as a promising novel method of cancer immunotherapy, particularly in MSI cancer patients.Citation24–28 Although a substantial proportion of approximately half of advanced MSI cancer patients showed objective responses to anti-PDCD1 (PD-1) antibody therapy, even including radiographic complete responses in almost 20%, about one fourth of patients had progressive disease under therapy.Citation27 So far, markers predicting the success of anti-PDCD1 (PD-1)/CD274 (PD-L1) therapy among MSI cancer patients are not yet established. Similarly, studies are lacking that investigate the relationship of PDCD1 (PD-1)-positive T cell infiltration in MSI cancers with immune evasion phenomena such as alterations of HLA class I or II-mediated antigen presentationCitation26 Such more comprehensive information about the tumor immune status could potentially be highly relevant in the identification of patients expected to benefit from immune therapy by PDCD1 (PD-1)/CD274 (PD-L1) pathway blockade.Citation29,30 We therefore analyzed the local infiltration of MSI colorectal cancers by PDCD1 (PD-1) positive T cells and investigated the effect of local PDCD1 (PD-1) positive T cell infiltration on B2M mutation status and HLA class II antigen expression pattern of the respective tumors.
Results
B2M mutation status and HLA class II expression status of MSI colorectal cancers
In order to examine a potential influence of the infiltration of MSI colorectal cancer lesions with immune cells on B2M mutation status and/or HLA class II antigen expression status of the tumors, immunohistochemical staining was performed. Representative staining results are shown in . In total, we analyzed a series of 56 MSI colorectal cancers (sporadic MSI cancer, n = 38, Lynch syndrome-associated cancer, n = 18). Patients' characteristics are summarized in . Of the analyzed tumors, 19 (33.9%) displayed a mutation of the B2M gene. B2M mutations tended to be more frequent in Lynch syndrome-associated cancers compared to sporadic MSI cancers (9/18 vs. 10/38), but statistical significance was not achieved (p = 0.13, Fisher's exact test).
Figure 1. Representative immunohistochemical stainings with the B2M-specific mAb L368 (panel A+D), the CD3-specific mAb PS1 (panel B+E) and the PDCD1 (PD-1)-specific mAb NAT105 (panel C+F). A Homogenous B2M expression in a B2M-wild type tumor (HD04). B Representative CD3-positive T cell expression in a B2M-wild type tumor. C Low amount of PDCD1 (PD-1)-positive T cells in a B2M-wild type tumor. D Absent B2M expression on the tumor cell surface in a B2M-mutant tumor (HD43). E Representative image of CD3-positive T cell infiltration in a B2M-mutant tumor. F B2M-mutant tumor, highly infiltrated with PDCD1 (PD-1)-positive T cells. Scale bars, 100 μm.
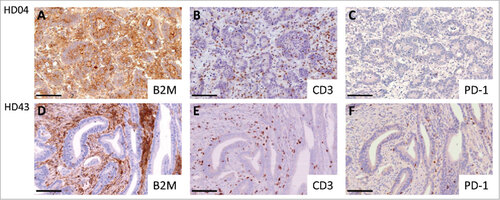
Table 1. Characteristics of MSI colorectal cancer patients.
All tumors classified as B2M-mutant by Sanger sequencing showed negative staining, and all tumors classified as B2M wild type showed homogenous positive staining. HLA class II antigen expression was ‘0’ in 19 (33.9%), ‘1’ in 10 (17.9% and ‘2’ in 27 (48.2%). 4 of the 19 (21.1%) tumors classified as lack of or barely detectable expression of HLA class II antigen displayed a mutation in the RFX5 gene.
Tumor infiltration with CD3-positive T cells and PDCD1 (PD-1)-positive T cells
Microsatellite-unstable colorectal cancer lesions were first analyzed for general lymphocyte infiltration by staining with the pan T cell marker CD3. Overall, the tumors showed CD3-positive T cell infiltration at a median number of 118.9 cells per 0.25 mm2. A significantly higher density of CD3-positive T cells was observed in hereditary compared to sporadic MSI colorectal cancers (median: 143.1 cells per 0.25 mm2 vs. 92.5 cells per 0.25 mm2, p = 0.009). Analyzing the total infiltration of PDCD1 (PD-1)-positive T cells revealed a median number of 5.2 cells per 0.25 mm2. Comparison of PDCD1 (PD-1)-positive T cell infiltration between hereditary and sporadic MSI CRCs also showed a significantly elevated number of PDCD1 (PD-1)-positive cells in hereditary tumors (median: 31.0 cells per 0.25 mm2, her., vs. 2.7 cells per 0.25 mm2, spor., p = 0.006).
Relation between immune cell infiltration and B2M mutation status
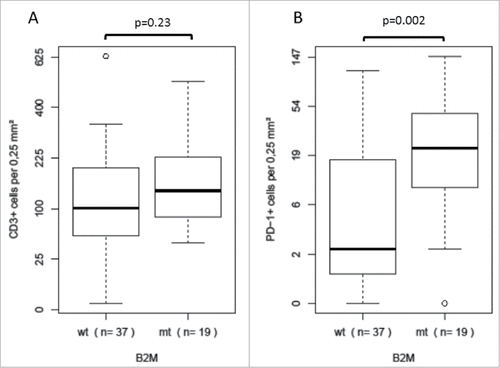
We investigated the association of general tumor lymphocyte infiltration with tumor B2M mutation status and did not observe a statistically significant change in distribution of the intratumoral CD3-positive T cell infiltration with respect to B2M mutation status (median: 101.2 B2M-wild type vs. 139.0 B2M-mutant, p = 0.23,). As a next step, we examined a potential relation of the infiltration of the tumors with PDCD1 (PD-1)-positive T cells and B2M mutation status of the tumor cells. A significantly higher number of PDCD1 (PD-1)-positive T cells was observed in B2M-mutant compared to B2M-wild type MSI colorectal cancers (median: 22.2 cells per 0.25 mm2 B2M-wild type vs. 2.0 cells per 0.25 mm2 B2M-mutant, p = 0.002, Wilcoxon test, ).
Figure 2. Distribution of specific T cell infiltration data in B2M-mutant (mt) and B2M-wild type (wt) MSI colorectal cancers A There was no statistically significant difference in intratumoral CD3-positive T cell infiltration between B2M-mutant (mt) and B2M-wild type (wt) MSI colorectal cancers B A significantly higher number of PDCD1 (PD-1)-positive T cells was observed in B2M-mutant compared to B2M-wild type MSI colorectal cancers.
In a multivariate model, PDCD1 (PD-1)-positive T cell infiltration was identified as the only statistically significant predictor being positively associated with the presence of B2M mutation (odds ratio for doubling of PDCD1 [PD-1]-positive T cell counts, OR = 1.69, ). Also after variable selection, PD-1-positive T cell infiltration remained a significant predictor in the model and showed a similar effect (OR = 1.48).
Table 2. Multivariate logistic regression model for B2M mutation status.
Relation between immune cell infiltration and HLA class II antigen expression
We observed a significantly higher overall T cell infiltration in tumors with higher HLA class II antigen expression (median 150.0 cells per 0.25 mm2 HLA class II antigen 2 vs. 133.9 cells per 0.25 mm2 HLA class II antigen 1 vs. 50.8 cells per 0.25 mm2 HLA class II antigen 0, p < 0.001, ). PDCD1 (PD-1)-positive T cell infiltration also tended to be higher in tumors with higher HLA class II antigen expression; however, the proportion of PDCD1 (PD-1)-positive T cells among all T cells was not significantly related to HLA class II antigen expression (, p = 0.0802, Jonckheere-Terpstra test).
Figure 3. Distribution of specific T cell infiltration data in tumors with HLA class II antigen expression status high, intermediate or lack of or barely detectable A We observed a significant increase in overall T cell infiltration in tumors with higher HLA class II antigen expression B PDCD1 (PD-1)-positive T cell infiltration tended to increase with higher HLA class II antigen expression, but the difference did not reach statistical significance.
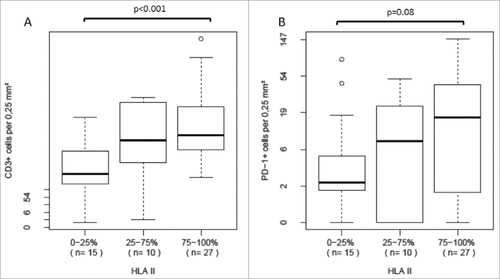
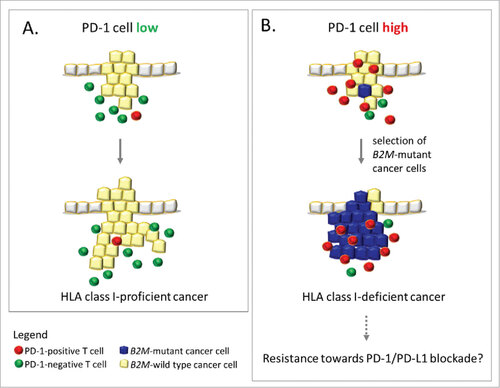
Figure 4. Influence of PDCD1 (PD-1)-positive T cell infiltration on immune evasion in MSI colorectal cancer. A (left panel): Low levels of PDCD1 (PD-1)-positive T cells within the tumor microenvironment likely reflects a condition of less stringent immune selection, which allows the outgrowth of HLA class I antigen-positive, B2M-wild type cancer cells. B (right panel): Outgrowth of B2M-mutant cancer cells (blue), which lack HLA class I antigen expression, predominantly occurs in an activated local immune environment characterized by PDCD1 (PD-1)-positive T cell (red) infiltration. This observation supports the immunoediting concept in MSI colorectal cancers. If B2M mutations interfere with anti-PDCD1 (PD-1)/CD274 (PD-L1) therapy success, MSI cancers with high PDCD1 (PD-1)-positive T cell infiltration (right panel) may be resistant towards anti-PDCD1 (PD-1) antibody therapy due to lack of HLA class I antigen expression.
In a multivariate model, CD3-positive T cell infiltration was identified as the only statistically significant predictor being positively associated with higher HLA class II antigen expressions (odds ratio for doubling of CD3-positive T cell counts, OR = 2.48, for ‘lack of or barely detectable’ vs. ‘intermediate’ as well as for ‘intermediate’ vs. ‘high’, ). Also after variable selection, CD3-positive T cell infiltration remained a significant predictor in the model, but with a somewhat smaller effect on the HLA class II antigen expression (OR = 1.88).
Table 3. Multivariate ordered logistic regression model for HLA class II antigen expression.
Discussion
A central observation of our study is that alterations to tumor antigen presentation molecules are related to the local lymphocyte infiltration patterns. Our results suggest that mutations in the B2M gene, which are the most frequent cause of complete breakdown of HLA class I-mediated antigen presentation in MSI colorectal cancer, are specifically related to a high number of tumor-infiltrating PDCD1 (PD-1)-positive T cells.
In contrast, we did not observe any significant difference of overall T cell counts between B2M-wild type and B2M-mutant tumors. This is in line with several previous studies that did not observe any correlation between immune cell infiltration and B2M mutation status.Citation17,31 except the study by de Miranda et al. who found evidence of higher counts of certain T cell subsets infiltrating B2M-mutant compared to wild type tumors.Citation32 However, increasing PDCD1 (PD-1)-positive T cell infiltration was significantly related to an increased likelihood of B2M mutation.
Our results for the first time establish B2M mutation as a consequence of high PDCD1 (PD-1)-positive T cell counts in MSI cancers. Potentially underlying this is the influence of the local immune cell milieu on the phenotype of the emerging tumors, as postulated by the immunoediting model.Citation33–36 In an environment of dense infiltration with activated PDCD1 (PD-1)-positive T cells, reflective of a more active local immune milieu and thus a stronger immunoselective pressure, emerging HLA class I-positive tumor cell clones may be eliminated more effectively. This probably favors the outgrowth of poorly immunogenic, B2M-mutant MSI colorectal cancer cells (). These no longer present FSP-derived neoantigens to the immune system via HLA class I antigens. This model is in line with the previous observation by Giannakis et al.Citation37 who reported an increased likelihood of mutations affecting B2M or other genes involved in HLA class I-mediated antigen presentation in colorectal cancers densely infiltrated by lymphocytes.
In fact, PDCD1 (PD-1)-positive T cell infiltration remained the only significant predictor of B2M mutation status in a multivariate model. No significant relationship between the proportion of PDCD1 (PD-1)-positive T cells and HLA class II antigen expression on tumor cells was observed. However, in concordance with the previously reported association of HLA class II antigen expression with CD4-positive T cell infiltrationCitation17 overall T cell density was significantly increased in tumors with higher HLA class II antigen expression.
Lynch syndrome-associated cancers showed significantly elevated immune cell infiltration compared to sporadic MSI cancers, which is in line with previous findings.Citation38,39 In addition, we here for the first time report a significantly higher absolute and relative amount of PDCD1 (PD-1)-positive T cells in Lynch syndrome vs. sporadic MSI cancer specimens. This suggests that the recurrent exposure of immune cells towards MSI-related antigens in Lynch syndrome, e.g. through mismatch repair-deficient cryptsCitation1,40,41 may lead to T cell activation and consequently an enhanced number of activated T cells in clinically manifest tumors.
The recurrent stimulation of the immune system with MSI-induced FSP neoantigens in Lynch syndrome may thus be the reason for the higher frequency of B2M mutations in Lynch syndrome-associated compared to sporadic MSI colorectal cancersCitation42 This supports the concept that stimulating the immune system against MSI-related FSP antigens, e.g. by a vaccineCitation1 may not only delay tumor formation in Lynch syndrome, but also lead to a better outcome, because B2M-mutant cancers have an excellent prognosis after surgical removal.Citation43,44
The presence of functional HLA class I antigens and therefore the presence of functional B2M is a prerequisite for recognition and killing of tumor cells by cytotoxic CD8-positive T cellsCitation45 Therefore, B2M mutations are expected to interfere with the success of immune checkpoint modulator therapy, e.g. using pembrolizumabCitation14,46–48 as has recently been observed as a mechanism of secondary resistance in a melanoma patient and in MSI cancers.Citation27,49 Accordingly, our results predict that upfront resistance towards immune checkpoint blockade may be particularly common among MSI cancers with high PDCD1 (PD-1)-positive T cell infiltration, because they commonly show B2M mutation-induced loss of HLA class I antigen presentation. This, somewhat counterintuitively, suggests that MSI cancers with low PDCD1 (PD-1)-positive T cell counts will likely respond better to anti-PDCD1 (PD-1) therapy than tumors with high PDCD1 (PD-1)-positive T cell counts.
B2M mutations have only rarely been observed in MSI colorectal cancers presenting with hematogenous metastasis formation, for example to the liver.Citation13,43 Possible mechanisms underlying this observation are enhanced NK cell killing of B2M-mutant, HLA class I antigen-negative MSI cancer cellsCitation50 or, alternatively, impaired tumor cell/platelet interaction after loss of HLA class I antigen expression.Citation51 However, B2M mutations still allow metastasis formation through local or lymphatic tumor cell spread. In fact, peritoneal metastasis through non-hematogenous tumor cell spread is a common manifestation in MSI colorectal cancer of stage M1.Citation52,53 B2M mutation status and infiltration with activated PDCD1 (PD-1)-positive T cells may be of particular predictive significance for checkpoint blockade in this clinical constellation.
In summary, our results suggest that high amounts of PDCD1 (PD-1)-positive tumor-infiltrating T cells, representing a highly active local immune milieu, may favor the outgrowth of emerging B2M-mutant, HLA class I-negative tumor cell clones. This is in line with the hypothesis that B2M mutations in MSI cancer occur as a direct result of DNA mismatch repair deficiency-induced mutations and subsequent immunoselective pressure. It is strongly suggested that all future clinical trials using PDCD1 (PD-1)/CD274 (PD-L1) checkpoint modulators should account for B2M mutation status and immune cell infiltration of the primary tumor and all available metastatic lesions as potential predictors of therapy efficacy.
Patients and methods
Patients and tumor specimens
Tumor samples represent a consecutive series of MSI colorectal cancers recorded in the Department of Applied Tumor Biology, Institute of Pathology, University Hospital Heidelberg as part of the German HNPCC Consortium. Informed consent was obtained from all patients included in this study. The Ethics Committee of the University of Heidelberg approved the study. None of these patients underwent neoadjuvant radio- and/or chemotherapy prior to surgery.
Microsatellite instability status was determined using the Bethesda standard marker panel (BAT25, BAT26, D2S123, D5S346 and D17S250/Mfd15) and CAT25 as described previously.Citation54,55 Tumors from patients with a germline mutation of the DNA mismatch repair genes were classified as ‘hereditary’. Tumors exhibiting MLH1 promoter methylation and/or the BRAF V600E mutation were classified as ‘sporadic’.
Microdissection and DNA isolation
Three to six tissue sections (2 μm, formalin fixed and paraffin-embedded) were deparaffinized and stained with hematoxylin and eosin according to standard protocols. Tumor areas were manually microdissected from HE-stained sections, and genomic tumor DNA was isolated using the Qiagen DNeasy Tissue Kit (Qiagen, Cat. No.: 69506) according to the manufacturer's instructions.
Immunohistochemical staining
Immunohistochemical staining was performed on 2 μm paraffin sections. After deparaffinization in xylol and rehydration in a decreasing series of ethanol baths, the slides were boiled 3 × 5 minutes in 10 mM citrate buffer (pH 6.0) for antigen retrieval. After boiling, the slides cooled down for 20 minutes, followed by blocking the endogenous peroxidase activity with 0.06% H2O2 (v/v in methanol) for 20 minutes. Subsequently the slides were incubated with 10% horse serum (Vector Laboratories, Cat. No.: S-2000) v/v in phosphate-buffered saline for 30 min at room temperature to prevent nonspecific antibody binding.
Mouse monoclonal antibodies specific for CD3 (1:60 dilution, Acris, Cat. No.: DM112, clone PS1), PDCD1 (PD-1) (1:50 dilution, Abcam, Cat. No.: ab52587, clone NAT105), HLA-DR-, HLA-DQ-, HLA-DP (1:200 dilution, clone LGII-612.14) and Beta-2-microglobulin (B2M) (1:50 dilution, clone L368) were used as primary antibodies at 4 °C overnight. Monoclonal antibodies LGII-612.14 and L368 were kind gifts from Prof. Soldano Ferrone and developed and characterized as described previouslyCitation56,57 After washing with PBS and incubation with biotinylated anti-mouse IgG antibodies for 30 min at room temperature, AB reagent was, following another washing step, applied for 30 minutes at room temperature (Vectastain Elite ABC HRP kit; Vector Laboratories, Cat. No.: PK-6102). Finally, antigen detection was performed by a color reaction with 3,3-di-amino-benzidine (DAB+ chromogen; Dako North America, Cat. No.: K3468) and counterstaining with Mayer's hematoxylin (AppliChem, Cat. No.: A4840).
Determination of B2M and RFX5/CIITA mutation status
Mutation analysis of the B2M gene was performed for all tumor specimens by using Sanger sequencing as described previously.Citation13 For all samples categorized as lack of or barely detectable HLA class II antigen expression, Sanger sequencing of the RFX5 and CIITA genes was performed as previously describedCitation17 PCR products were purified with the QIAquick PCR purification kit (Qiagen, Cat. No.: 28106). For the sequencing reaction the Big Dye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Cat. No.: 4337450) was used. The products for sequencing were precipitated, dissolved in 12 μl Hi-Di Formamide (Applied Biosystems, Cat. No.: 4311320) and analyzed on an ABI3130xl genetic analyzer (Applied Biosystems, Darmstadt, Germany).
Microscopic evaluation and quantification of immune cell infiltration
HLA class II antigen expression was categorized in three levels. 0 – ‘lack of or barely detectable’, less than 25% of the tumor tissue stained positive, 1 – ‘intermediate’, 25–75% positive tumor cells, 2 – ‘high’ more than 75% positive tumor cells. Immune cell infiltration of CD3-positive T cells and PDCD1 (PD-1)-positive T cells was evaluated (by J.J.) in three to five representative tumor regions (t = number of evaluated tumor regions), utilizing a 10 × 10 ocular grid (0.25 mm2) at a magnification of × 200 and a Leica DMRBE microscope (Leica Camera AG, Wetzlar, Germany). Numbers of positive cells per 0.25 mm2 (n) were calculated as the mean value of the positive cells in the individual regions (ni) using the following formula:
Pictures were scanned using a Hamamatsu NanoZoomer Digital Pathology system (Hamamatsu,Hamamatsu City, Japan). A subset of immunohistochemically stained tissue sections (20 tumor regions) was examined by a second observer (M.K.) with respect to CD3-positive T cells. With a Spearman's rank correlation coefficient of 0.96 (one-sided 95%-CI: 0.87-1), the results of the manual counting can be considered reliable.
Statistical analysis
Wilcoxon's rank sum test was applied to compare the distribution of immune cell infiltration depending on B2M mutation status and type of MSI (hereditary or sporadic). With respect to HLA class II antigen status the Jonckheere-Terpstra test was used. Fisher's exact test was applied to compare distributions of categorical data.
For the multivariate analysis with respect to the B2M mutation status, a logistic regression model was set up including age (continuous), gender, MSI type (sporadic/hereditary), CD3-positive T cells per 0.25 mm2, PDCD1 (PD-1)-positive T cells per 0.25 mm2, HLA class II antigen expression and BRAF mutation status as covariates. For the HLA class II antigen expression, an ordered logistic model was applied to the categorized version of the HLA class II antigen expression. Other than replacing the HLA class II antigen expression by the B2M mutation status, the same covariates were considered. Due to a low number of cases (53 cases for B2M mutation status; 37 wild type vs. 15 mutant; 49 cases for HLA class II antigen; 14 level 0 vs. 10 level 1 vs. 25 level 2), a backward selection was added to the multivariate analysis. The threshold for the selection was chosen at p = 0.5 according to Steyerberg et al.Citation58 as a tradeoff between selection bias and overestimation bias.
All analyses were performed on the basis of the mean of the immune cell counts over all tumor regions where measurements have been taken. P values smaller than 0.05 were considered statistically significant. The statistical analysis was performed within the statistical software environment R.Citation59
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The expert technical assistance of Nina Nelius, Petra Hoefler, and Beate Kuchenbuch is gratefully acknowledged.
Funding
The study was funded in part by grants of the Deutsche Forschungsgemeinschaft (DFG, KFO 227).
References
- Kloor M, von Knebel Doeberitz M. The immune biology of microsatellite-unstable cancer. Trends Cancer. 2016;2:121–33. doi:10.1016/j.trecan.2016.02.004. PMID:28741532
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6. doi:10.1038/nbt.1754. PMID:21221095
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi:10.1093/nar/gkq603. PMID:20601685
- Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sanchez-Heras AB, Castillejo MI, Rojas E, Barberá VM, Cigüenza S, et al. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLoS One. 2013;8:e79737. doi:10.1371/journal.pone.0079737. PMID:24244552
- Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49:151–7. doi:10.1136/jmedgenet-2011-100714. PMID:22368298
- Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–58. doi:10.1053/j.gastro.2010.01.054. PMID:20420945
- Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–13. doi:10.1016/S0002-9440(10)65436-3. PMID:10362805
- Buckowitz A, Knaebel HP, Benner A, Blaker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–53. doi:10.1038/sj.bjc.6602534. PMID:15856045
- Banerjea A, Ahmed S, Hands RE, Huang F, Han X, Shaw PM, Feakins R, Bustin SA, Dorudi S. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer. 2004;3:21. doi:10.1186/1476-4598-3-21. PMID:15298707
- Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469–75. doi:10.1002/bjs.4472. PMID:15048750
- Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–97. doi:10.1053/j.gastro.2008.01.015. PMID:18395080
- Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–7. doi:10.1016/S0960-9822(02)70795-1. PMID:8994836
- Kloor M, Michel S, Buckowitz B, Ruschoff J, Buttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121:454–8. doi:10.1002/ijc.22691. PMID:17373663
- Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007; 56:227–36. doi:10.1007/s00262-006-0183-1. PMID:16783578
- Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the beta2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61:1359–71. doi:10.1007/s00262-012-1321-6. PMID:22833104
- Michel S, Linnebacher M, Alcaniz J, Voss M, Wagner R, Dippold W, Becker C, von Knebel Doeberitz M, Ferrone S, Kloor M. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer. 2010;127:889–98. PMID:20013806
- Surmann EM, Voigt AY, Michel S, Bauer K, Reuschenbach M, Ferrone S, von Knebel Doeberitz M, Kloor M. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol Immunother. 2015;64:357–66. doi:10.1007/s00262-014-1638-4. PMID:25445815
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi:10.1038/nrc3239. PMID:22437870
- Yao S, Chen L. PD-1 as an immune modulatory receptor. Cancer J. 2014;20:262–4. doi:10.1097/PPO.0000000000000060. PMID:25098286
- Pardoll D. Cancer and the Immune system: basic concepts and targets for intervention. Semin Oncol. 2015;42:523–38. doi:10.1053/j.seminoncol.2015.05.003. PMID:26320058
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi:10.1038/nri2326. PMID:18500231
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi:10.1038/nm0902-1039c. PMID:12091876
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi:10.1084/jem.192.7.1027. PMID:11015443
- Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462–73. doi:10.1007/s10147-016-0959-z.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi:10.1056/NEJMoa1200694. PMID:22658128
- Toh JW, de Souza P, Lim SH, Singh P, Chua W, Ng W, Spring KJ. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin Colorectal Cancer. 2016;15:285–91. doi:10.1016/j.clcc.2016.07.007. PMID:27553906
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi:10.1126/science.aan6733.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi:10.1056/NEJMoa1500596. PMID:26028255
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi:10.1038/nature21349. PMID:28102259
- Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The “cancer immunogram”. Science. 2016;352:658–60. doi:10.1126/science.aaf2834. PMID:27151852
- Bernal M, Concha A, Saenz-Lopez P, Rodriguez AI, Cabrera T, Garrido F, Ruiz-Cabello F. Leukocyte infiltrate in gastrointestinal adenocarcinomas is strongly associated with tumor microsatellite instability but not with tumor immunogenicity. Cancer Immunol Immunother 2011;60:869–82. doi:10.1007/s00262-011-0999-1. PMID:21400022
- De Miranda NF, Goudkade D, Jordanova ES, Tops CM, Hes FJ, Vasen HF, van Wezel T, Morreau H. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin Cancer Res. 2012;18:1237–45. doi:10.1158/1078-0432.CCR-11-1997. PMID:22261803
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi:10.1126/science.1203486. PMID:21436444
- Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi:10.1111/nyas.12105. PMID:23651186
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi:10.1016/j.coi.2014.01.004. PMID:24531241
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi:10.1016/j.immuni.2004.07.017. PMID:15308095
- Giannakis M, Mu Xinmeng J, Shukla Sachet A, Qian Zhi R, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;17:1206. doi:10.1016/j.celrep.2016.10.009. PMID:27760322
- Shia J, Ellis NA, Paty PB, Nash GM, Qin J, Offit K, Zhang XM, Markowitz AJ, Nafa K, Guillem JG, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407–17. doi:10.1097/00000478-200311000-00002. PMID:14576473
- Shia J, Holck S, Depetris G, Greenson JK, Klimstra DS. Lynch syndrome-associated neoplasms: a discussion on histopathology and immunohistochemistry. Fam Cancer. 2013;12:241–60. doi:10.1007/s10689-013-9612-4. PMID:23435936
- Kloor M, Huth C, Voigt AY, Benner A, Schirmacher P, von Knebel Doeberitz M, Bläker H. Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: a pathological study. Lancet Oncol. 2012;13:598–606. doi:10.1016/S1470-2045(12)70109-2. PMID:22552011
- Staffa L, Echterdiek F, Nelius N, Benner A, Werft W, Lahrmann B, Grabe N, Schneider M, Tariverdian M, von Knebel Doeberitz M, et al. Mismatch repair-deficient crypt foci in Lynch syndrome–molecular alterations and association with clinical parameters. PLoS One. 2015;10:e0121980. doi:10.1371/journal.pone.0121980. PMID:25816162
- Dierssen JW, de Miranda NF, Ferrone S, van Puijenbroek M, Cornelisse CJ, Fleuren GJ, van Wezel T, Morreau H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi:10.1186/1471-2407-7-33. PMID:17316446
- Koelzer VH, Baker K, Kassahn D, Baumhoer D, Zlobec I. Prognostic impact of beta-2-microglobulin expression in colorectal cancers stratified by mismatch repair status. J Clin Pathol. 2012;65:996–1002. doi:10.1136/jclinpath-2012-200742. PMID:22859396
- Tikidzhieva A, Benner A, Michel S, Formentini A, Link KH, Dippold W, von Knebel Doeberitz M, Kornmann M, Kloor M. Microsatellite instability and Beta2-Microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer. 2012;106:1239–45. doi:10.1038/bjc.2012.53. PMID:22353804
- Bubenik J. MHC class I down-regulation: tumour escape from immune surveillance? (review). Int J Oncol. 2004;25:487–91. PMID:15254748
- Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174:1462–71. doi:10.4049/jimmunol.174.3.1462. PMID:15661905
- Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi:10.1016/j.cell.2014.12.033. PMID:25594174
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi:10.1038/ncomms10501. PMID:26883990
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375:819–29. doi:10.1056/NEJMoa1604958. PMID:27433843
- Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127:1001–10. doi:10.1002/ijc.25283. PMID:20198617
- Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, Lemmens VE, van Herk-Sukel MP, van Wezel T, Fodde R, Kuppen PJ, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174:732–9. doi:10.1001/jamainternmed.2014.511. PMID:24687028
- Fujiyoshi K, Yamamoto G, Takenoya T, Takahashi A, Arai Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y, et al. Metastatic Pattern of Stage IV Colorectal Cancer with High-Frequency Microsatellite Instability as a Prognostic Factor. Anticancer Res. 2017;37:239–47. doi:10.21873/anticanres.11313. PMID:28011498
- Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651–7. doi:10.1093/annonc/mdt591. PMID:24504447
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. PMID:9823339
- Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N, Benner A, Dondog B, Pawlita M, Dippold W, et al. T25 repeat in the 3 ′ untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005;65:8072–8. doi:10.1158/0008-5472.CAN-04-4146. PMID:16166278
- Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S. Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods. 1993;161:239–56. doi:10.1016/0022-1759(93)90300-V. PMID:8505553
- Lampson LA, Fisher CA, Whelan JP. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983;130:2471–8. PMID:6187860
- Steyerberg EW, Eijkemans MJC, Harrell FE, Habbema JDF. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Statistics in Medicine. 2000;19:1059–79. doi:10.1002/(SICI)1097-0258(20000430)19:8<1059::AID-SIM412>3.0.CO;2-0. PMID:10790680
- R Core Team. R Foundation for Statistical Computing. 2015. [accessed 2017 June 21]. https://www.r-project.org/
