ABSTRACT
Gliomas are lethal brain tumors that resist standard therapeutic approaches. Immunotherapy is a promising alternative strategy mostly developed in the context of glioblastoma. However, there is a need for implementing immunotherapy for grade II/III gliomas, as these are the most common CNS tumors in young adults with a high propensity for recurrence, making them lethal despite current treatments. We recently identified HLA-A2-restricted tumor-associated antigens by peptide elution from glioblastoma and formulated a multipeptide vaccine (IMA950) evaluated in phase I/II clinical trials with promising results. Here, we investigated expression of the IMA950 antigens in patients with grade II/III astrocytoma, oligodendroglioma or ependymoma, at the mRNA, protein and peptide levels. We report that the BCAN, CSPG4, IGF2BP3, PTPRZ1 and TNC proteins are significantly over-expressed at the mRNA (n = 159) and protein (n = 36) levels in grade II/III glioma patients as compared to non-tumor samples (IGF2BP3 being absent from oligodendroglioma). Most importantly, we detected spontaneous antigen-specific T cell responses to one or more of the IMA950 antigens in 100% and 71% of grade II and grade III patients, respectively (27 patients tested). These patients displayed T cell responses of better quality (higher frequency, broader epitope targeting) than patients with glioblastoma. Detection of spontaneous T cell responses to the IMA950 antigens shows that these antigens are relevant for tumor targeting, which will be best achieved by combination with CD4 epitopes such as the IDH1R132H peptide. Altogether, we provide the rationale for using a selective set of IMA950 peptides for vaccination of patients with grade II/III glioma.
Introduction
WHO grade II and III glioma include astrocytoma, oligodendroglioma and ependymoma and are the most common central nervous system (CNS) tumors in young adults. Grade II and III astrocytoma almost inevitably evolve to secondary glioblastoma (grade IV), a disease associated with very poor prognosis.Citation1,2 Tumors deriving from ependymal cells (i.e. ependymoma) are rarer but some of them display a similar poor prognosis,Citation3,4 advocating for alternative therapeutic strategies. One of the pillars of cancer therapy is immunotherapy, with the recent development of immune checkpoint inhibitors that have the potential to synergize with cell therapy and cancer vaccines.Citation5,6 Among vaccines, most of them have targeted glioblastoma (GBM) so far, most likely due to the limited knowledge about antigen expression by grade II/III tumors. However, recent studies of peptide vaccines in grade II glioma patients showed promising resultsCitation7,8 and additional trials are ongoing, including one with a peptide spanning the IDH1R132H mutation found in the majority of low-grade glioma and secondary GBM (sGBM).Citation9
Implementing vaccines as complementary treatments for patients with grade II/III glioma is a very attractive strategy considering the characteristics of these incurable tumors. Indeed, even when gross total resection is achieved, they ultimately relapse due to their impressive invasive properties, and/or transform into high-grade tumors.Citation10,11 In addition quality of life is often worsened by the tumor itself, its progression, or the side effects related to the different treatments.Citation12,13 Finally, grade II/III glioma patients have a longer life expectancy than grade IV patients, may receive less concomitant treatments (at least for grade II) and seem to face less systemic immunosuppression, offering more time to elicit strong and long-lasting anti-tumor immune responses. Therefore, vaccines have the theoretical potential to achieve valuable clinical benefit, such as delaying tumor progression, transformation, or improving quality of life, autonomy and maintaining social inclusion.
We recently identified nine glioma-associated antigens by peptide elution from the surface of HLA-A#0201+ GBM samples which were overexpressed on tumor versus normal tissues, detected in a majority of patients and which were immunogenic.Citation14 These antigens, together with two HLA class II-restricted peptides, have been formulated in a multipeptide vaccine (called IMA950), which was shown to be well tolerated and immunogenic in combination with GM-CSFCitation15 and is currently being tested in combination with poly-ICLC (NCT01920191).Citation16 Here, in order to broaden the use of vaccines to grade II and III glioma patients, we investigated expression of the IMA950 antigens (which encompasses peptides derived from the BCAN, CSPG4, FABP7, IGF2BP3, NLGN4X, NRCAM, PTPRZ1 and TNC proteins), as well as additional glioma- or melanoma-associated antigens (gp100, Her2, IL13Rα2, SOX2 and TRP2) in a series of patients presenting with grade II or III astrocytoma, oligodendroglioma and ependymoma, at the mRNA (n = 159), protein (n = 36) and peptide (n = 6) levels. In addition, we explored the presence of spontaneous T cell responses to these antigens in patients with grade II and III glioma.
Results
Most of glioma-associated antigens identified in grade IV tumors are expressed in grade II and III glioma
We analysed mRNA expression levels of the antigens targeted by the IMA950 vaccine (Supplementary Table S1) in grade II (n = 49) and grade III (n = 41) astrocytoma, grade II (n = 30) and grade III (n = 27) oligodendroglioma and ependymoma (n = 12) using the Nanostring nCounter technology (probesets are listed in Supplementary Table S2). The IMA950 vaccine incorporates 9 HLA-A2-restricted tumor-eluted peptides derived from BCAN, CSPG4, FABP7, IGF2BP3, NLGN4X, NRCAM, PTPRZ1 (2 peptides) and TNC (Supplementary Table S1). Expression of gp100, Her2, IL13Rα, SOX2 and TRP2 was also analyzed as these antigens were shown to be overexpressed in GBM and/or melanoma and HLA-A2-restricted peptides recognized by T cells have been described.Citation17–20 We also included 29 de novo and 16 s GBM samples as well as 12 non-tumor brain samples (8 epilepsy samples, 2 post-mortem brain samples and 2 commercial brain RNA) as controls.
Expression of BCAN at the mRNA level among all samples tested is shown as a representative example in . Box plot analysis shows that BCAN mRNA is significantly upregulated as compared to non-tumor samples in grade II and III astrocytoma, grade II and III oligodendroglioma, ependymoma, sGBM and de novo GBMCitation14 (, p < 0.01 for all comparisons except ependymoma: p < 0.05). Analysis of all target antigens revealed that five of them (i.e., BCAN, CSPG4, PTPRZ1, TNC and SOX2) were significantly overexpressed in all tumor groups as compared to the non-tumoral control samples (). IGF2BP3 was overexpressed in grade II and III astrocytoma and grade IV samples (). FABP7 was upregulated in grade IV tumors only. Furthermore, gp100, Her2, IL13Rα and TPR2 were mainly upregulated in ependymoma whilst only TRP2 was significantly upregulated in grade II/III astrocytoma samples (). The NLGN4X and NRCAM genes were detected at similar levels in control and tumor samples (except for ependymoma, ). Finally, we did not observe significant differential expression of any gene between grade II and grade III astrocytoma or oligodendroglioma (-); BCAN was significantly decreased in primary GBM as compared to all other tumors (P < 0.01 for all comparisons).
Figure 1. BCAN RNA expression on grade II, III astrocytoma, oligodendroglioma and ependymoma samples. (A) RNA counts using the Nanostring technology for the BCAN gene. Tumor samples are grouped by type and non-tumor control samples (8 epilepsy samples, 2 normal brains, 2 commercial RNAs) are shown on the right (ctrl). sGBM: secondary GBM. (B) Box plots analysis of expression of the BCAN gene on the different tumor groups showing the median, lower quartile (25th percentile) and upper quartile (75th percentile). The bars indicate the lower adjacent value and the upper adjacent value. Points indicate outliers. AII: grade II astrocytoma, AIII: grade III astrocytoma, ODII: grade II oligodendroglioma, ODIII: grade III oligodendroglioma, EP: ependymoma, sGBM: secondary GBM, ctrl: non-tumor samples. ##: p < 0.01, #: p < 0.05.
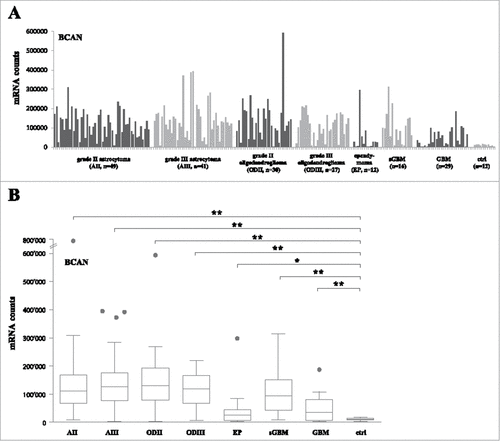
Figure 2. Antigen expression on grade II, III astrocytoma, oligodendroglioma and ependymoma samples and recurrent sample pairs. Box plots analysis of expression of the BCAN, CSPG4, PTPRZ1, TNC and SOX2 (A), FABP7 and IGF2BP3 (B), NLGN4X and NRCAM (C), IL13Rα2, gp100, Her2 and TRP2 (D) genes on the different tumor groups showing the median, lower quartile (25th percentile) and upper quartile (75th percentile). The bars indicate the lower adjacent value and the upper adjacent value. #: p < 0.05 versus non-tumor samples, ##: p < 0.01 versus non-tumor samples.. AII: grade II astrocytoma, AIII: grade III astrocytoma, ODII: grade II oligodendroglioma, ODIII: grade III oligodendroglioma, EP: ependymoma, sGBM: secondary GBM, ctrl: non-tumor samples. (E) mRNA counts for the IGF2BP3 gene in paired (pairs of 2, 3 or 4, each separated by a space) recurrent samples. AII➔AII: grade II astrocytoma recurring as grade II astrocytoma, AII➔AIII: grade II astrocytoma recurring as grade III astrocytoma, AII➔sGBM: grade II astrocytoma recurring as secondary GBM, AIII➔sGBM: grade III astrocytoma recurring as secondary GBM.
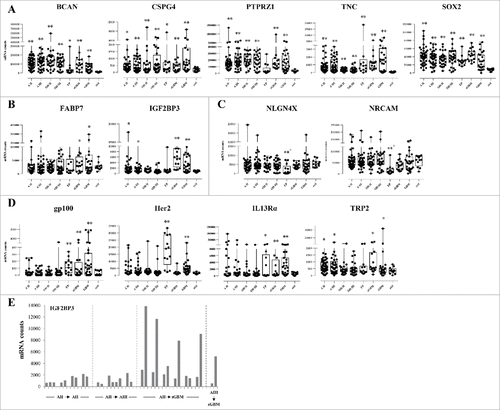
Interestingly, we were able to analyze paired samples obtained from the same patient at primary surgery and progression, with or without change to higher grade. We observed that progression from grade II or III glioma to sGBM was associated with a significant increase in expression of the IGF2BP3 gene (p < 0.05, ), whereas no significant difference was observed for the other genes (data not shown). This was consistent with overexpression of IGF2BP3 in GBM when all (including non-paired) samples were considered ().
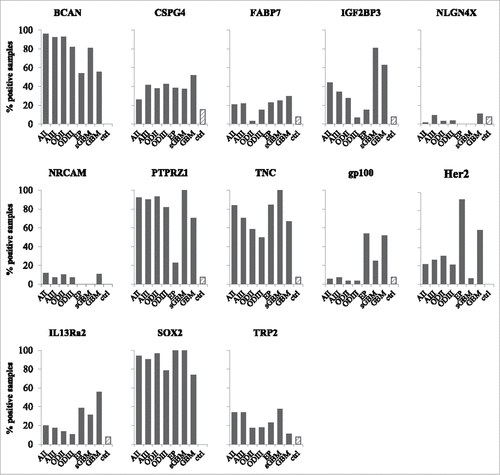
We next then determined the percentage of positive samples within each tumor type, taking the percentage of samples with mRNA counts higher than the mean + 2 standard deviations of mRNA counts in non-tumor samples as the cutoff. As shown in , the BCAN, PTPRZ1, TNCCitation21 and SOX2 gene mRNA were over-expressed in the majority of astrocytoma, oligodendroglioma and ependymoma samples tested (with the exception of PTPRZ1, which was expressed in 20% of ependymoma only). Expression of the CSPG4, FABP7, IGF2BP3, gp100, Her2, IL13Rα2 and TRP2 genes was more heterogeneous. The percentage of NLGN4X- or NRCAM-positive tumors was low. In order to get appraisal of gene expression within individual tumor types, the same data were plotted per tumor type (Supplementary Figure S1) and data obtained for all genes and tumor groups summarized in Supplementary Table S3.
Figure 3. Proportion of positive samples for the tested antigens. Percentage of samples positive for BCAN, CSPG4, FABP7, IGF2BP3, NLGN4X, NRCAM, PTPRZ1, TNC, gp100, Her2, IL13Rα2, SOX2 and TRP2, determined as mRNA counts > mean mRNA counts for non-tumor samples + 2 standard deviations. AII: grade II astrocytoma, AIII: grade III astrocytoma, ODII: grade II oligodendroglioma, ODIII: grade III oligodendroglioma, EP: ependymoma, sGBM: secondary GBM, ctrl: non-tumor samples (hatched bars).
IMA950 antigens are detected at the protein and peptide levels in grade II/III glioma
Confirmation of antigen expression at the protein level was performed for the IMA950 antigens using a tissue microarray incorporating 24 grade II and 12 grade III astrocytoma samplesCitation22 (see representative examples in ). The BCAN, CSPG4, FABP7, IGF2BP3 and NLGN4X, NRCAM and PTPRZ1 proteins were detected in grade II and II astrocytoma samples as well as in sGBM. Expression of TNC and SOX2 was previously reported.Citation21,23 Importantly, these proteins were expressed in the majority (>80%) of grade II and III astrocytoma samples tested (), suggesting that the majority of patients may benefit from vaccination with the IMA950-derived antigens.
Figure 4. Expression of IMA950 antigens at the protein and peptide levels. (A) Representative IHC staining for the BCAN, CSPG4, FABP7 and IGF2BP3 proteins on grade II and III astrocytoma and secondary GBM (sGBM). Inserts show higher magnification. Bar scale: 100 μm (B) Percentage of positive tumors. (C) Presentation of the PTPRZ1195-203 peptide at the surface of normal samples including normal brain samples, grade II/III samples and GBM. Upper panel: Shown is the MS signal intensity, reflecting peptide presentation at the cell surface, including box plots analysis showing the median, lower quartile (25th percentile) and upper quartile (75th percentile). The box plot bars indicate the lower adjacent value and the upper adjacent value. Lower panel: peptide detection frequency on the tested tissues.
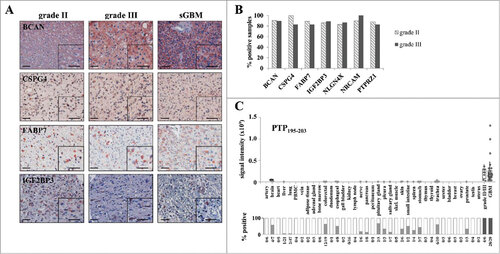
In order to further ensure cell surface presentation of the IMA950 antigenic peptides, we submitted grade II (n = 2) and III (n = 4) astrocytoma and grade II (n = 2) and III (n = 1) oligodendroglioma samples from HLA-A2+ patients to peptide elution and identification. Normal tissues as well as de novo GBM samples were used for comparison of peptide presentation levels. Three samples (2 grade II astrocytoma and 1 grade II oligodendroglioma) yielded very few (<400) peptides and were not further analyzed. Shown as an example (), the PTPRZ1195-203 peptide was detected in the one remaining grade II samples and in the 5 analyzable grade III samples, whereas it was detected at very low levels in a small subset of normal tissue samples only. The mass spectrometry signal intensity (reflecting amount of peptide presentation at the cell surface) of the PTPRZ1195-203 peptide was similar in grade II/III and GBM samples (p = 0.42). This was the case also for the CSPG421-29, PTPRZ11347-1355 and TNC3-11 peptides (supplementary Figure S2). The FABP7118-126 peptide was detected in one sample only whereas the BCAN478-486, IGF2BP3552-560, NLGN4X131-139 and NRCAM692-700 peptides were not detected in these samples.
The majority of patients with grade II/III glioma display spontaneous immune responses to the IMA950 antigens
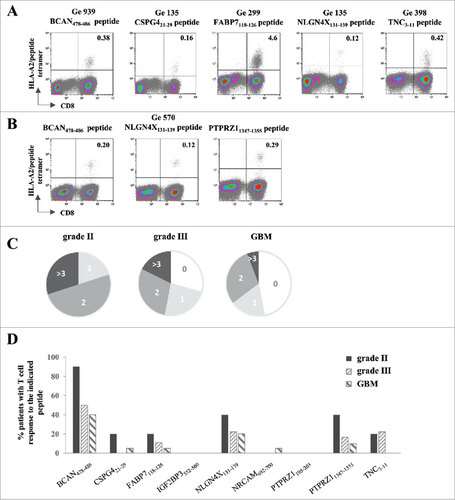
In order to determine if such pre-existing T cell responses also exist in patients with grade II/III tumors, we incubated PBMC with a cocktail of the IMA950 HLA-A2-binding peptides and assessed the presence of specific T cells by MHC/peptide multimer staining after one week of culture. HLA-A2+ patients with grade II astrocytoma (n = 8), grade II oligodendroglioma (n = 2), grade III astrocytoma (n = 14), grade III oligodendroglioma (n = 3) and primary GBM (n = 17) were tested. The majority of patients with grade II or grade III (100% and 71%, respectively) glioma displayed specific immune responses to one or more of the IMA950 antigens (representative examples in ), with occasionally high magnitude of CD8+ T cells detected. In addition, as shown in for a grade II astrocytoma patient, CD8 T cell responses to more than one peptide (up to 5) were detected in 60% and 47% of patients with grade II or III tumors, respectively (). Importantly, detection of T cell response to multiple (>3) peptides was greater in patients with grade II and III tumors as compared with patients with GBM (). This was not due to the intrinsic inability of T cells isolated from patients with GBM to proliferate, as proliferation assays to CD3/CD28 beads showed no differences in proliferation capacity between patients with grade II, III or IV tumors (12 patients were tested per group, data not shown).
Figure 5. Spontaneous T cell responses to the IMA950 antigens are detected in patients with grade II/III tumors. (A) Representative examples of T cell responses in four different grade II/III astrocytoma (Ge 939, Ge 135, Ge 299)/oligodendroglioma (Ge 398) patients specific for 5 different antigens. The number of CD8+ HLA-A2/peptide tetramer+ T cells are shown in the upper right quadrant. (B) T cell responses towards 3 different antigens are shown for grade II astrocytoma patient Ge 570. The number of CD8+ HLA-A2/peptide tetramer+ T cells are shown in the upper right quadrant. (C) Proportion of grade II (n = 10) and III (n = 17) astrocytoma/oligodendroglioma and primary GBM (n = 17) patients with spontaneous T cell responses to 0, 1, 2, 3 or more antigens. (D) Pattern of spontaneous T cell responses in grade II, III astrocytoma and oligodendroglioma and primary GBM patients to the tested antigens.
Discussion
Glioma vaccines are being investigated for many years and the recent successful development of CTLA-4 and PD-1 antibodies is likely to give them a new impetus.Citation24 To date, peptide vaccines have been developed mostly for grade IV tumors but are now slowly being implemented for grade II/III glioma. One study showed induction of robust and poly-epitopic T cell responses in grade II astrocytoma/oligoastrocytoma patients after vaccination with a cocktail of four glioma-associated antigens (EphA2, WT1, IL13Rα2 and survivin in Montanide) adjuvanted with poly-ICLC.Citation7 The same peptide cocktail (excluding WT1) was tested in paediatric patients with recurrent low-grade gliomaCitation25 and a phase II study in the same patient population is ongoing (NCT02358187). Further studies using a peptide spanning the IDH1R132H mutation in IDH1 mutated grade II/III/IV astrocytoma patients (NCT02193347 and NCT02454634) are ongoing and will provide useful information on the potential of peptide vaccines in grade II/III glioma.
The present results contribute to this field as they provide a detailed analysis of the expression profile of nine antigens composing the IMA950 vaccine in grade II/III astrocytoma and oligodendroglioma patients. We show that the BCAN, CSPG4, IGF2BP3, PTPRZ1 and TNC proteins are significantly over-expressed at the mRNA and protein levels in grade II/III glioma patients as compared to non-tumor samples (IGF2BP3 being absent from oligodendroglioma), making peptides derived from these proteins candidates for therapeutic vaccination for these patients. Importantly, the majority (>50%) of grade II/III patients had tumors positive for BCAN, PTPRZ1 and TNC and 20–50% of them displayed expression of CSPG4 and IGF2BP3. Adding the latter antigens to the multipeptide vaccine is of importance to target all tumor cells and avoid tumor escape by antigen downregulation.Citation26
Regarding NLGN4X and NRCAM, data are less definitive. Despite a high mRNA level in grade II/III glioma and GBM, NLGN4X and NRCAM were not overexpressed as compared to non-tumor samples. These results were confirmed in qPCR analysis (Supplementary Figure S3), suggesting that these two gene mRNAs are expressed at significant levels in normal brain tissues. However, we previously showed that the peptides derived from the NLGN4X and NRCAM proteins are detected at the surface of GBM cells but not non-tumor brain cells, possibly due to different protein processing and presentation by tumor cells.Citation14 This discrepancy between mRNA expression levels and peptide presentation at the cell surface is not unexpected and has been specifically investigated.Citation27 It was shown that a significant number of peptides presented predominantly showed no or only minor changes in mRNA expression level. Therefore, absence of increased mRNA levels does not imply that these peptides are absent from the surface of grade II/III glioma samples. To address this important issue, available grade II and III glioma samples were analyzed by liquid chromatography and mass spectrometry. We could detect five of the IMA950 peptides (CSPG421-29, IGF2BP3552-560, PTPRZ1195-203, PTPRZ11347-1355 and TNC3-11). These peptides were presented at a level comparable to that observed for GBM and at higher levels as compared to normal brain samples. Their detection ensures that these peptides can function as antigens recognized by T cells, a level of analysis usually not attained for other glioma-associated antigens, for which immune responses induced upon vaccination might prove inefficient due to lack of the cognate target. The other peptides, including those derived from the NLGN4X and NRCAM proteins, were not detected. The low number of samples available for such an analysis could explain these results. Indeed, elution of a sufficient number of peptides for sensitive analysis requires large samples with high tumor content, two parameters that are not always obtained concomitantly despite extended surgery in grade II/III glioma.
On the other hand, the detection of NLGN4X-specific T cell responses in patients with grade II/III tumors suggests that the tumor elicited a response to this peptide. The presence of small amounts of peptide, below the detection limit of liquid chromatography-mass spectrometry technologies used for peptide elution, might be enough to trigger a T cell response in vivo.Citation28 Additionally, we previously showed in vitro that NLGN4X was one of the most immunogenic peptides composing the IMA950 vaccine,Citation14 suggesting that potent NLGN4X-specific T cell responses elicited upon vaccination will be able to detect small amounts of peptide in vivo. Nevertheless, although the NLGN4X and NRCAM peptides are interesting targets for vaccine implementation in grade II/III glioma, we recommend waiting for peptide presentation data to be available before inclusion in vaccination protocols. Regarding the other peptides (BCAN478-486, CSPG421-29, IGF2BP3552-560, PTPRZ1195-203, PTPRZ11347-1355 and TNC3-11), mRNA expression and peptide presentation data provide evidence for their use in a therapeutic peptide vaccine, taking as well into account the absence of peptide-specific autoimmune adverse events observed in clinical trials investigating the use of the IMA950 antigens in GBM.Citation15,16
Expression of gp100, Her2, IL13Rα,Citation7,29,30 SOX2 and TRP231 was analyzed as these antigens were shown to be overexpressed in GBM and/or melanoma and HLA-A2-restricted peptides recognized by T cells are available for potential vaccination.Citation17–20 Only SOX2 displayed strong over-expression in grade II/III astrocytoma/oligodendroglioma and ependymoma, with > 80% positive samples. An HLA-A2-resticted SOX2-derived peptide (SOX2118-127) eliciting glioma-specific T cells has been describedCitation20 and future knowledge of presentation of SOX-2-derived peptides at the surface of grade II/III glioma could promote its use together with the selected IMA950 peptides in future vaccination trials.
In this study, we had the opportunity to study a few cases of ependymoma, a rare and heterogeneous tumor with variable responses to treatment. Immunotherapeutic strategies are starting to being developed for this disease,Citation32,33 based on the observations that ependymoma is infiltrated by immune cellsCitation34 and that this correlates with good prognosis in pediatric patients.Citation35 Our results offer new targets for this tumor entity. IMA950 antigen expression in ependymoma was somewhat different as compared to astrocytoma and oligodendroglioma, with TNC being expressed in almost 90% of samples, an observation which is not surprising given the cell of origin of this malignancy.Citation36 We observed a constant downregulation of NLGN4X and NRCAM in ependymoma samples tested, whereas other studies associated NRCAM with highly-proliferative ependymomaCitation37 We also detected high levels of Her2 expression at the mRNA level in this group of patients, but analysis of protein expression did not display increased expression as compared to normal brain tissues (not shown). Although the number of samples studied here is limited, our results offer new antigens for the study of ependymoma. Only a few HLA-A2+ patients (n = 4) with ependymoma were available for investigating the presence of spontaneous T cell responses to the IMA950 antigens. Among these, two patients displayed low but detectable T cell responses to the BCAN478-486 and PTPRZ11347-1355 antigens (not shown), suggesting that, although further analysis is needed, patients with ependymoma might as well be able to mount spontaneous antitumor immune responses.Citation14
The detection of spontaneous (i.e. not induced by vaccination) CD8 T cell responses to several IMA950 antigens in grade II and III glioma patients further supports the use of these antigens in a therapeutic vaccine. Spontaneous T cell responses to glioma-associated antigens have previously been reported in one grade III astrocytoma patientCitation38; our observations now further indicate that these responses are frequent and directed towards multiple glioma-associated antigens in patients with grade II/III astrocytoma and oligodendroglioma. Interestingly, patients with grade II/III tumors displayed T cell responses of better quality than patients with GBM, as determined by a higher frequency and a broader epitope targeting. This might reflect the fact that patients with grade II/III tumors are less immunosuppressed as compared to patients with grade IV tumors, suggesting that vaccination in this patient population could be more efficient.
Based on the results obtained in this study, we propose to use selected peptides from the IMA950 vaccine depending on the tumor type. Patients suffering from grade II/III astrocytoma or from ependymoma would receive a vaccine composed of the BCAN478-486, CSPG421-29, IGF2BP3552-560, PTPRZ1195-203, PTPRZ11347-1355 and TNC3-11 peptides, whereas patients suffering from oligodendroglioma would be vaccinated with BCAN478-486, CSPG421-29, PTPRZ1195-203, PTPRZ11347-1355 and TNC3-11 (supplementary Figure S4). The majority of samples used in this study having been collected over the last 20 years, the IDH1/2 mutation as well as the 1p/19q LOH status are not available for most of the samples. Although worth investigating, expression of the antigens examined in this study is unlikely to correlate with the molecular profile of the tumor. Importantly, these vaccines would benefit from combination with HLA-DR-restricted epitopes, such as those currently included in the IMA950 vaccine (Supplementary Table S1) but, more interestingly, with the glioma-specific IDH1R132H mutant peptide mentioned above.Citation9 There is indeed considerable evidence for the crucial need to integrate both CD4 and CD8 T cell responses in designing immunotherapies.Citation39–42 Due to the high frequency of the IDH1 mutation in patients with grade II/III glioma,Citation1 the IDH1R132H-derived CD4 peptide is the epitope of choice to integrate with the herein-described CD8 T cell antigens. Furthermore, the fact that spontaneous T cell responses to both the IDH1R132H CD4 epitopeCitation9 and to the IMA950 CD8 antigens are detected suggests that tolerance to these antigens has not been induced and opens the possibility of further augmenting the magnitude of the response to see whether antitumor effector function can be achieved through vaccination in combination with immune checkpoint inhibitors or molecules targeting the tumor microenvironment. Finally, in the light of the results of the recent trials of adoptive transfer of GBM-specific chimeric antigen receptor T cells,Citation43–46 our results indicate that adoptive transfer of TCR-transgenic T cells specific for the epitopes we describe in this study would be an additional attractive approach to explore. These rationally designed trials will hopefully improve progression-free survival, life expectancy and quality of life of patients suffering from grade II/III glioma.
Methods
Patients and samples
Two-hundred and fourteen patients were included in this study, which conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of all centers involved. All patients gave written informed consent. Patients with grade II (n = 49), III (n = 41) astrocytoma, grade II (n = 30) and III (n = 27) oligodendroglioma, primary (n = 29) and secondary GBM (n = 16), ependymoma (n = 12), and patients with epilepsy (n = 8) were seen at the Geneva University Hospital, University of Heidelberg and the Tumor Research Centre in San Francisco. Tumor or epilepsy samples were collected at time of initial or recurrent surgery. Initial surgery was performed prior to any radiotherapeutic or chemotherapeutic treatment. Ependymoma were obtained from all grades (grade I, II and III: 4, 6 and 2 patients, respectively). Tumor and epilepsy samples were processed immediately and frozen in liquid nitrogen for further analysis. Normal brain samples (n = 2) were obtained from cadaveric brain donation from GBM patients in the Geneva University Hospital after informed consent and processed similarly. They were taken at distance from the tumor site and confirmed by the pathologist to be tumor-free. sGBM samples were all progression from astrocytoma except for two samples that were progression from oligodendroglioma. Two commercial brain RNA samples (Amsbio 1 whole brain ref R1234035-P, 1 cerebellum, ref R1234039-50) were purchased.
RNA isolation
Tumor tissues were homogenized and RNA extraction was performed using an RNEasy Mini Kit (Qiagen, ref 74104). RNA quality was tested using a BioAnalyzer (Agilent Technologies) and quantified using a spectrophotometer. When necessary, DNase (Sigma, ref AMPD1-1KT) treatment was performed to remove contaminating DNA.
qPCR analysis
Expression of the BCAN, NLGN4X and NRCAM genes was verified by qPCR. Two pairs of primers per gene (Supplementary Table S4) were designed, one covering the Nanostring probesets (primers_n) and one matching that used in Affymetrix HG-U13314 (primers_a). Housekeeping genes used were GAPDH, GusB and TBP. Eight tumor as well as 8 non-tumor (CD8 sorted T cells, 2 commercial brain RNA, 1 post-mortem brain and 4 epilepsy) samples were tested.
Tissue microarray and immunohistochemistry
The tissue microarray contained 24 astrocytoma grade II and 12 astrocytoma grade III as well as four normal brain samples as described.Citation22 Informed consent was obtained from each patient according to the research proposals approved by the Institutional Review Board at Heidelberg Medical Faculty. Antigen retrieval, incubation with primary and secondary antibodies as well as detection with VECTASTAIN Laboratories Elite ABC Kit (Vector Laboratories, ref PK-6100) was carried out as described.Citation22 Each tumor biopsy was evaluated at x20 magnification by two independent investigators.
Peptide elution from brain specimens
Nine HLA-A#0201+ tumor biopsies samples were analysed and compared to a panel of 244 non-tumoral body tissue including 7 brain tissues. Shock-frozen tumor samples were essentially processed as described previouslyCitation47 according to standard protocols.Citation48 Briefly, HLA-A#02 peptide pools from shock-frozen tissue samples were obtained by immune precipitation from solid tissue using HLA-specific antibodies, acid treatment and ultrafiltration. To obtain samples containing HLA-A#02-restricted peptides the antibody BB7.2 was used.Citation49 The HLA peptide pools as obtained were separated according to their hydrophobicity by reversed-phase chromatography (nanoAcquity UPLC system, Waters) and the eluted peptides were analysed in an LTQ Orbitrap hybrid mass spectrometer (Thermo Fisher Scientific) equipped with an electrospray ionization source. The LC–MS data were collected and automatically processed by analyzing the LC–MS survey (mass signals of unfragmented peptides) as well as the tandem-MS (MS/MS) data (fragment spectra containing peptide sequence information). Automated data analysis had been optimized and adapted for identification and quantification of HLA-restricted peptides.
Assessment of spontaneous antigen-specific immune responses
HLA-A2+ patients with grade II, III and IV astrocytoma (including sGBM) or grade II and III oligodendroglioma were selected for analysis of spontaneous T cell responses to the IMA950 antigens. The majority of patients were sampled at the time of tumor resection. Nine patients (3 grade II, 3 grade III, 3 GBM patients) had received temozolomide and/or radiotherapy more than 3 years before PBMC sampling and 10 patients (2 grade II, 3 grade III and 5 GBM patients) were under or less than 3 months away from treatment with either chemotherapy or radiotherapy. PBMC (5 × 106) were incubated with a mix of the IMA950 peptides (BCAN478-486 ALWAWPSEL; CSPG421-29 TMLARLASA; FABP7118-126 LTFGDVVAV; IGF2BP3552-560 KIQEILTQV; NLGN4X131-139 NLDTLMTYV; NRCAM692-700 GLWHHQTEV; PTPRZ1195-203 AIIDGVESV; PTPRZ11347-1355 KVFAGIPTV; TNC3-11 AMTQLLAGV, 1 μM each) for 7 days in the presence of 100 IU/ml IL-2 (Proleukine, Novartis) before staining with MHC/peptide tetramers (kindly provided by Immatics Biotechnologies GmbH, Germany) and CD8 (Beckman Coulter, ref 6607102). Dead cells were excluded using a Live/Dead dye (Invitrogen, ref L34959). In order to assess tetramer background and set a threshold for positive tetramer staining, we analyzed nine HLA-A2 negative patients suffering from grade II (n = 2), III (n = 4) or IV (n = 3) tumors with the same protocol. A tetramer staining was considered positive if (i) the number of tetramer+CD8+ T cells observed in flow cytometry was >20, (ii) the percentage of tetramer+ CD8+ T cells was superior to the mean percentage of tetramer+CD8+ T cells in HLA-A2 negative individuals + 2 SD, (iii) the median of tetramer+CD8+ T cells was superior to the median of tetramer+CD8+ T cells in HLA-A2 negative individuals + 2 SD.
Statistical analysis
Differentially expressed genes (tumors vs. ctrl, recurrent vs. primary and astrocytoma vs. secondary GBM) were identified by using the marker selection feature of GENE-E (http://www.broadinstitute.org/cancer/software/GENE-E/index.html). Difference in expression between the classes was calculated using a t-test that estimates the significance (permutation p-values) of the test score. It was then corrected for multiple hypotheses testing (MHT) by computing the false discovery rate (FDR). FDR was estimated using the Benjamini and Hochberg procedure.Citation50 Correlation coefficients were considered significant when p-value <0.01.
Conflict of interest
T.W. with affiliation immatics biotechnologies GmbH is an employees of this company which also has a commercial interest in a glioma vaccine comprising the antigens described here (IMA950). The other authors have no conflict of interest to declare.
supp_material.zip
Download Zip (708 KB)Acknowledgments
We would like to thank Mrs. Francesca Corlazzoli for excellent technical assistance in sample preparation and Anny Shai and Joanna Philips at UCSF BTRC Tissue Core for providing tissue. We additionally thank the iGE3 Genomics Platform of the University of Geneva for technical assistance in Nanostring and real-time PCR analysis.
Funding
This work was supported by the Association Marietta, the Fond Lionel Perrier, the Association Frédéric Fellay, as well as by Fond'action and the Ligue genevoise contre le cancer (PYD). This work was as well partly supported by NIH/NINDS R21NS093654 (HO and JC), NIH/NCI P50CA097257 (HO), the Cancer Research Institute (HO), loglio funds from the Dabierre Foundation (HO and JC) and a Research Fund of the Department of Internal Medicine of the University Hospital and the Faculty of Medicine of Geneva (VD, this Fund receives an unrestricted grant from AstraZeneca Switzerland).
References
- Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. CurrNeurolNeurosciRep. 2013;13(5):345.
- Duffau H, Taillandier L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. NeuroOncol. 2015;17(3):332–342.
- Shonka NA. Targets for therapy in ependymoma. Target Oncol. 2011;6(3):163–9. doi:10.1007/s11523-011-0170-0. PMID:21445635
- Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506(7489):451–5. doi:10.1038/nature13109. PMID:24553141
- Dietrich PY, Dutoit V, Walker PR. Immunotherapy for glioma: from illusion to realistic prospects? AmSocClinOncolEducBook. 2014:51–9.
- Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. NatRevNeurol. 2015;11(9):504–14.
- Okada H, Butterfield LH, Hamilton RL, Hoji A, Sakaki M, Ahn BJ, Kohanbash G, Drappatz J, Engh J, Amankulor N, et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. ClinCancer Res. 2015;21(2):286–94.
- Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Normolle DP, Connelly AK, Dibridge S, Mason G, Whiteside TL, et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol. 2016;18(8):1157–1168.
- Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–7. doi:10.1038/nature13387. PMID:25043048
- Jaeckle KA, Decker PA, Ballman KV, Flynn PJ, Giannini C, Scheithauer BW, Jenkins RB, Buckner JC. Transformation of low grade glioma and correlation with outcome: an NCCTG database analysis. J Neurooncol. 2011;104(1):253–259. doi:10.1007/s11060-010-0476-2. PMID:21153680
- Park CK, Park I, Lee S, Sun CH, Koh Y, Park SH, Kim JE, Yun H, Lee SH. Genomic dynamics associated with malignant transformation in IDH1 mutated gliomas. Oncotarget. 2015;6(41):43653–66. doi:10.18632/oncotarget.6189. PMID:26524630
- Delgado-Lopez PD, Corrales-Garcia EM, Martino J, Lastra-Aras E, Duenas-Polo MT. Diffuse low-grade glioma: a review on the new molecular classification, natural history and current management strategies. Clin Transl Oncol. 2017;19(8):931–944. doi:10.1007/s12094-017-1631-4.
- Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn MJ, Hassel MB, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–32. doi:10.1016/S1470-2045(16)30313-8. PMID:27686946
- Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, Dorsch K, Flohr S, Fritsche J, Lewandrowski P, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135(Pt 4):1042–54. doi:10.1093/brain/aws042. PMID:22418738
- Rampling R, Peoples S, Mulholland PJ, James A, Al-Salihi O, Twelves CJ, McBain C, Jefferies S, Jackson A, Stewart W, et al. A Cancer Research UK First Time in Human Phase I Trial of IMA950 (Novel Multipeptide Therapeutic Vaccine) in Patients with Newly Diagnosed Glioblastoma. Clin Cancer Res. 2016;22(19):4776–85. doi:10.1158/1078-0432.CCR-16-0506. PMID:27225692
- Migliorini D, Dutoit V, Walker PR, Dietrich PY. IMA950 peptide-based vaccine adjuvanted with poly-iclc in combination with standard therapy in newly diagnosed HLA-A2 glioblastoma patients: preliminary results. Neuro-Oncology. 2016;18(suppl 6):vi22. doi:10.1093/neuonc/now212.086.
- Castelli C, Rivoltini L, Andreola G, Carrabba M, Renkvist N, Parmiani G. T-cell recognition of melanoma-associated antigens. JCell Physiol. 2000;182(3):323–31. doi:10.1002/(SICI)1097-4652(200003)182:3%3c323::AID-JCP2%3e3.0.CO;2-.
- Kono K, Rongcun Y, Charo J, Ichihara F, Celis E, Sette A, Appella E, Sekikawa T, Matsumoto Y, Kiessling R. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. IntJCancer. 1998;78(2):202–8.
- Okano F, Storkus WJ, Chambers WH, Pollack IF, Okada H. Identification of a novel HLA-A#0201-restricted, cytotoxic T lymphocyte epitope in a human glioma-associated antigen, interleukin 13 receptor alpha2 chain. ClinCancer Res. 2002;8(9):2851–55.
- Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M, et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. BrJCancer. 2007;96(8):1293–301.
- Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner HH. Clinical impact and functional aspects of tenascin-C expression during glioma progression. IntJCancer. 2002;98(3):362–9.
- Campos B, Bermejo JL, Han L, Felsberg J, Ahmadi R, Grabe N, Reifenberger G, Unterberg A, Herold-Mende C. Expression of nuclear receptor corepressors and class I histone deacetylases in astrocytic gliomas. Cancer Sci. 2011;102(2):387–92. doi:10.1111/j.1349-7006.2010.01792.x. PMID:21143702
- Wan F, Herold-Mende C, Campos B, Centner FS, Dictus C, Becker N, Devens F, Mogler C, Felsberg J, Grabe N, et al. Association of stem cell-related markers and survival in astrocytic gliomas. Biomarkers. 2011;16(2):136–43. doi:10.3109/1354750X.2010.536256. PMID:21323603
- Bloch O. Immunotherapy for malignant gliomas. Cancer TreatRes. 2015;163:143–58.
- Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Normolle DP, Connelly AK, Dibridge S, Mason G, Whiteside TL, et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. NeuroOncol. 2016;18(8):1157–68.
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. JClinOncol. 2010;28(31):4722–29. doi:10.1200/JCO.2010.28.6963.
- Weinzierl AO, Lemmel C, Schoor O, Muller M, Kruger T, Wernet D, Hennenlotter J, Stenzl A, Klingel K, Rammensee HG, et al. Distorted relation between mRNA copy number and corresponding major histocompatibility complex ligand density on the cell surface. MolCell Proteomics. 2007;6(1):102–13. doi:10.1074/mcp.M600310-MCP200.
- Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4(6):565–71. PMID:8673703
- Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. JClinOncol. 2011;29(3):330–6.
- Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Potter DM, Connelly AK, Dibridge SA, Whiteside TL, Okada H. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic Acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. JClinOncol. 2014;32(19):2050–58.
- Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, Ma W, Hoa N, Minev B, Delgado C, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. ClinCancer Res. 2007;13(2 Pt 1):566–75.
- Pollack IF, Jakacki RI, Butterfield LH, Okada H. Ependymomas: development of immunotherapeutic strategies. ExpertRevNeurother. 2013;13(10):1089–98.
- Ursu R, Taillibert S, Banissi C, Vicaut E, Bailon O, Le RE, Guillamo JS, Psimaras D, Tibi A, Sacko A, et al. Immunotherapy with CpG-ODN in neoplastic meningitis: A phase I trial. Cancer Sci. 2015;106(9):1212–18. PMID:26094710
- Kong LY, Wei J, Haider AS, Liebelt BD, Ling X, Conrad CA, Fuller GN, Levine NB, Priebe W, Sawaya R, et al. Therapeutic targets in subependymoma. JNeuroimmunol. 2014;277(1–2):168–75.
- Donson AM, Birks DK, Barton VN, Wei Q, Kleinschmidt-Demasters BK, Handler MH, Waziri AE, Wang M, Foreman NK. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. JImmunol. 2009;183(11):7428–40.
- Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119(1):55–73. PMID:20024659
- Zangen I, Kneitz S, Monoranu CM, Rutkowski S, Hinkes B, Vince GH, Huang B, Roggendorf W. Ependymoma gene expression profiles associated with histological subtype, proliferation, and patient survival. Acta Neuropathol. 2007;113(3):325–37. doi:10.1007/s00401-006-0190-5. PMID:17265049
- Beckhove P, Warta R, Lemke B, Stoycheva D, Momburg F, Schnolzer M, Warnken U, Schmitz-Winnenthal H, Ahmadi R, Dyckhoff G, et al. Rapid T cell-based identification of human tumor tissue antigens by automated two-dimensional protein fractionation. JClinInvest. 2010;120(6):2230–42.
- Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174(5):2591–601. doi:10.4049/jimmunol.174.5.2591. PMID:15728465
- Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164(7):3902–12. doi:10.4049/jimmunol.164.7.3902. PMID:10725753
- Hoepner S, Loh JM, Riccadonna C, Derouazi M, Maroun CY, Dietrich PY, Walker PR. Synergy between CD8 T cells and Th1 or Th2 polarised CD4 T cells for adoptive immunotherapy of brain tumours. PLoSOne. 2013;8(5):e63933. doi:10.1371/journal.pone.0063933.
- Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165(11):6047–55. doi:10.4049/jimmunol.165.11.6047. PMID:11086036
- Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017;3(8):1094–101. doi:10.1001/jamaoncol.2017.0184. PMID:28426845
- Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375(26):2561–69. doi:10.1056/NEJMoa1610497. PMID:28029927
- Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T cells in Patients with Recurrent Glioblastoma. ClinCancer Res. 2015;21(18):4062–4072.
- O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399–413). PMID:28724573
- Schirle M, Keilholz W, Weber B, Gouttefangeas C, Dumrese T, Becker HD, Stevanovic S, Rammensee HG. Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. EurJImmunol. 2000;30(8):2216–25.
- Falk K, Rotzschke O, Deres K, Metzger J, Jung G, Rammensee HG. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. JExpMed. 1991;174(2):425–34. doi:10.1084/jem.174.2.425.
- Parham P, Brodsky FM. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. HumImmunol. 1981;3(4):277–99.
- Benjamini Y, Hochberg.Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. In: Royal Statistical S, editor. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. 57: Wiley; 1995. p. 289–300.
