ABSTRACT
Enhancement of antibody-dependent cellular cytotoxicity (ADCC) may potentiate the antitumor efficacy of tumor-targeted monoclonal antibodies. Increasing the numbers and antitumor activity of NK cells is a promising strategy to maximize the ADCC of standard-of-care tumor-targeted antibodies. For this purpose, we have preclinically tested a recombinant chimeric protein encompassing the sushi domain of the IL15Rα, IL-15, and apolipoprotein A-I (Sushi-IL15-Apo) as produced in CHO cells. The size-exclusion purified monomeric fraction of this chimeric protein was stable and retained the IL-15 and the sushi domain bioactivity as measured by CTLL-2 and Mo-7e cell proliferation and STAT5 phosphorylation in freshly isolated human NK and CD8+ T cells. On cell cultures, Sushi-IL15-Apo increases NK cell proliferation and survival as well as spontaneous and antibody-mediated cytotoxicity. Scavenger receptor class B type I (SR-B1) is the receptor for ApoA-I and is expressed on the surface of tumor cells. SR-B1 can adsorb the chimeric protein on tumor cells and can transpresent IL-15 to NK and CD8+ T cells. A transient NK-humanized murine model was developed to test the increase of ADCC attained by the chimeric protein in vivo. The EGFR+ human colon cancer cell line HT-29 was intraperitoneally inoculated in immune-deficient Rag2−/−γc−/− mice that were reconstituted with freshly isolated PBMCs and treated with the anti-EGFR mAb cetuximab. The combination of the Sushi-IL15-Apo protein and cetuximab reduced the number of remaining tumor cells in the peritoneal cavity and delayed tumor engraftment in the peritoneum. Furthermore, Sushi-IL15-Apo increased the anti-tumor effect of a murine anti-EGFR mAb in Rag1−/− mice bearing subcutaneous MC38 colon cancer transfected to express EGFR. Thus, Sushi-IL15-Apo is a potent tool to increase the number and the activation of NK cells to promote the ADCC activity of antibodies targeting tumor antigens.
Introduction
Tumor-targeting monoclonal antibodies recognize cell surface tumor antigens and elicit tumor cell death by direct or indirect mechanisms. Indirect mechanisms involve the recruitment of two types of effector immune functions. First, the complement cascade can be activated by recruitment of C1q to the Fc region of appropriate IgG subclasses.Citation1 Secondly, NK and macrophage cytotoxic activity can be triggered against tumor cells by activating Fc-receptor CD16A (FcγRIIIA). This mechanism is termed antibody-dependent cellular-mediated cytotoxicity (ADCC).Citation2
ADCC has been exploited therapeutically since the discovery of anti-tumor associated antigen directed monoclonal antibodies. One of these approved monoclonal antibodies is cetuximab,Citation3 which targets the epidermal growth factor receptor (EGFR) and is used for treatment of head and neck and colon cancer. In addition to a direct antitumor effect impairing intrinsic EGFR tyrosine kinase signaling,Citation4 cetuximab is able to mediate ADCC.Citation5
The Fc region of recently approved monoclonal antibodies has been engineered to maximize ADCC activity.Citation6,7 However, strategies to enhance immune effector mechanisms may potentiate the efficacy of monoclonal antibodies that mediate ADCC. Interleukin 15 (IL-15) is a cytokine that has been shown to enhance ADCC.Citation8 IL-15 belongs to the interleukin 2 (IL-2) family and is critical for the development of NK and NKT cells.Citation9,10 IL-15 bioactivity is optimal when complexed to IL-15Rα, which trans-presents this cytokine to IL-2Rβ/γ to surrounding lymphocytes.Citation11 Several chimeric IL-15 proteins are being developed to maximize the antitumor activity of this cytokine enhancing its transpresentation and/or increasing its half-life in circulation. Transpresented forms of IL-15 have been shown to increase ADCC by enhancing both NK cell numbers and their activity. In line with this, an IL-15 and IL-15 receptor alpha fusion protein increased the ADCC of an anti-GD2 mAb in a model of neuroblastoma.Citation1 Moreover, another fusion protein encompassing a mutated IL-15, the sushi domain of the IL-15 receptor alpha and an IgG1 Fc domain achieves strong ADCC activity in murine models and it is currently being tested in clinical trials.Citation13
We have previously described the potent antitumor activity of a triple murine fusion protein encompassing the sushi domain, IL-15, and apolipoprotein A-I (Apo A-I) upon gene transfer to the liver in mice by hydrodynamic injection. The Apo A-I moiety redirected the chimeric protein to high-density lipoproteins, increasing its half-life in circulation while the IL-15Rα sushi domain mediates cytokine transpresentation.Citation14,15 Here, we evaluate if the antitumor ADCC activity of this chimeric molecule as a purified recombinant protein can be translated to the clinical setting.
Results
Bioproduction and purification of a human Sushi-IL-15-Apo chimeric protein
We established a production procedure in eukaryotic cells to provide the natural glycosylation for the IL-15 moietyCitation16 and to minimize the endotoxin contamination of the protein. Thus, the recombinant protein was collected from the enriched medium of a stable transfectant in CHO cells.
Protein was purified by Ni-affinity chromatography followed by gel filtration to isolate monomeric protein in PBS pH 8.0. The purified protein was analyzed by Coomassie-stained SDS-PAGE under non-reducing conditions (Supp. Figure 1A). A single band of the expected molecular weight was observed. The aggregation of the protein was analyzed by analytical size-exclusion chromatography (Supp. Figure 1B). The purified protein maintained the monomeric state upon long-term storage at -80ºC. The endotoxin level, measured by the LAL test, was under 0.5EU/mg.
Recombinant Sushi-IL15-Apo has IL-15 bioactivity
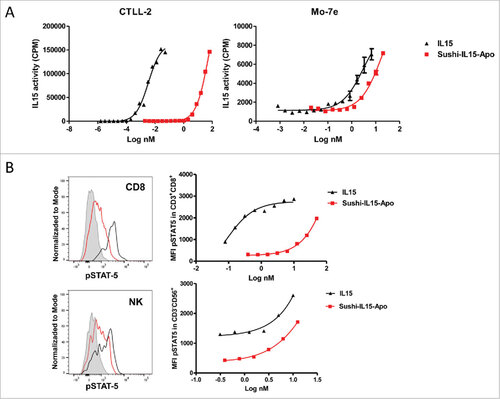
Sushi-IL15-Apo bioactivity was tested analyzing the capacity to induce proliferation in the IL-15-dependent cell lines CTLL-2 and Mo-7e. CTLL-2 express IL-15Rα while Mo-7e does not (Supp. Figure 2).Citation17,18 As shown in , Sushi-IL15-Apo was bioactive in both assays. In these experiments, Sushi-IL15-Apo activity was studied using the WHO international standard of IL15 (NIBSC code 95/554) as the positive control. To test the bioactivity of Sushi-IL15-Apo on human CD8+ T and NK cells, STAT5 phosphorylation was determined in the cell lines following 30 min in cultures in presence of the cytokines (). Sushi-IL15-Apo was also bioactive on primary CD8+ T and NK cells in a dose-dependent manner.
Figure 1. Sushi-IL15-Apo bioactivity (A) IL-15 activity upon stimulation of IL-15Rα-expressing and non-expressing responding cells, measured as cell proliferation. CTLL2 and Moe7 cells were labeled with tritiated thymidine and treated with different concentrations of IL-15 or Apo-IL15-Sushi. Nuclear incorporation of tritiated thymidine was measured at 48 h. (B) STAT-5 phosphorylation in CD8+ and NK cells as induced by IL-15 and Apo-IL15-Sushi. pSTAT-5 was stained and its expression studied by flow cytometry. PBMCs from volunteers were incubated with different concentrations of IL-15 or Sushi-IL15-Apo at 37ºC during 30 min. Histograms show pSTAT-5 expression in cells treated with 10 nM of Sushi-IL15-Apo (black) or 10 nM of IL-15 (red). The grey shadow histograms correspond to the non- stimulated cells.
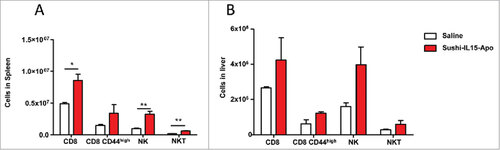
Sushi-IL15-Apo i.v. administration increases numbers of NK and CD8+ T cells in mice
Sushi-IL15-Apo is an amphipathic molecule that was designed to become part of high-density-lipoproteins in order to optimize the pharmacokinetic profile of IL-15.14 As human IL-15 is cross-reactive on mouse lymphocytes, 20 µg of Sushi-IL15-Apo were i.v. administered to mice and after 5 days, spleen and liver lymphocyte populations were studied ( and ). The absolute number of splenic and liver CD8+ T, NK and NKT cells was higher in mice that received Sushi-IL15-Apo than in mice receiving control. This effect was comparable to observations in mice that received by hydrodynamic injection 10 µg of a plasmid coding for Sushi-IL15-Apo (Supp. Figure 3). In this case, lung lymphocytes were also studied, and a marked increase in lung NK cell population was observed in comparison with mice receiving a plasmid coding for apolipoprotein A-I. Importantly, at day 5, mice treated with Sushi-IL15-Apo (either plasmid or protein) showed no noticeable signs of toxicity (Supp. Figure 3).
Sushi-IL15-Apo increases absolute numbers, survival and proliferation of human NK cells in culture
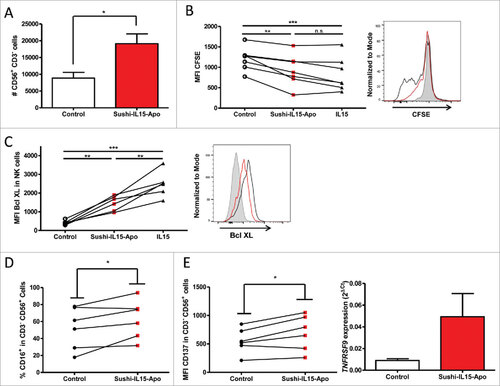
Since the main objective of this study was to determine the capacity of Sushi-IL15-Apo to increase ADCC in NK cells, we studied the effect of the fusion protein on cultured NK cells. Firstly, we observed an increment in the absolute number of NK cells in cultures of peripheral mononuclear cells (PBMCs) from healthy volunteers cultured with 10 nM of Sushi-IL15-Apo for 72h (). To explore the mechanisms behind this effect, NK cells purified from PBMCs by immunomagnetic negative selection were used. CFSE stained NK cells treated with Sushi-IL15-Apo proliferated more than NK cells cultured without cytokines. The level of proliferation induced by Sushi-IL15-Apo was similar to the proliferation observed in NK cells cultured with IL15 (). To determine if pro-survival effects were better in the cultures treated with the fusion protein, expression of the anti-apoptotic protein Bcl-XL was studied (). Sushi-IL15-Apo enhanced Bcl-XL expression in NK cells, although the expression was higher in IL15-cultured NK cells. It is of note that NK cells treated with Sushi-IL15-Apo presented a slightly higher expression of CD16A, an important molecule for ADCC, on their membrane (). Moreover, Sushi-IL15-Apo enhanced the presence of the co-stimulatory molecule CD137 on NK cells, as shown in .
Figure 3. Sushi-IL15-Apo increases absolute numbers of human NK cells in culture and increases their survival and proliferation. (A) PBMCs from volunteers were cultured without cytokines or with 10 nM of Sushi-IL15-Apo. At 72h cells were counted and CD3−CD56+ percentages determined by flow cytometry. (B) Purified NK cells were pre-stained with CFSE and cultured without cytokines or with 10 nM of Sushi-IL15-Apo or IL15 during 72 h and proliferation was monitored by flow cytometry. (C and D) NK cells from volunteers were isolated using a negative selection immunomagnetic kit and cells were cultured without cytokines or with 10 nM of Sushi-IL15-Apo for 6 days. The indicated markers were studied by flow cytometry. Each dot represents the result in individual subjects. (E) Surface CD137 expression was studied by flow cytometry on purified NK cultured as in B and C. Furthermore, RNA was isolated and CD137 expression analyzed by real time RT-PCR. # = p < 0.05, ## = p < 0.01 and ### = p < 0.001 in a paired student t test.
Sushi-IL15-Apo increases NK-cell-mediated ADCC and natural cytotoxicity
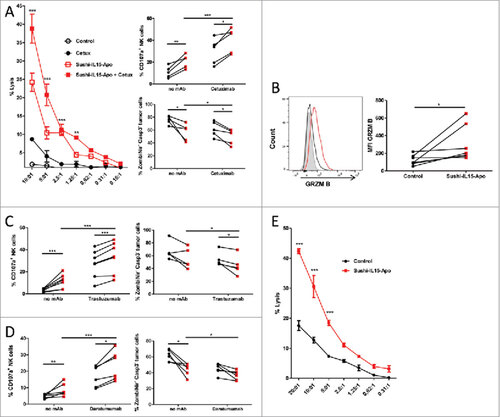
To explore the ADCC performance of NK cells treated with Sushi-IL15-Apo, chromiun51 release assays were performed with purified NK cells cultured during 48h with Sushi-IL15-Apo. In a first stage, the target was the EGFR+ colon carcinoma-derived cell line HT-29 and the tumor-cell coating antibody eliciting ADCC was cetuximab. As shown in , Sushi-IL15-Apo increased natural cytotoxicity against HT-29 cells, but also the measurable ADCC effect. This activity was also observed using the EGFR+ cell line HCT116 as a target (Supp. Figure 4). Enhancement of ADCC was also addressed analyzing surface expression of CD107a on NK cell membrane during the cytotoxicity assay as an estimate of degranulation. More intense expression was seen in NK-HT-29 co-cultures treated with Sushi-IL15-Apo with or without cetuximab, than in their respective controls. This effect was confirmed using two additional experimental settings co-culturing NK cells with antibody-coated tumor cells: the Her2+ breast cancer derived cell line BT474 treated with trastuzumab (Herceptin, Roche) () and the CD38+ multiple myeloma-derived cell line MM1S coated with the anti-CD38 mAb daratumumab (Darzalex, Janssen) (). In both cases, Sushi-IL15-Apo increased NK cell degranulation and decreased viability in tumor cells, indicating that the Sushi-IL15-Apo enhancement of ADCC is independent of the target or the tumor-cell coating antibody. Next, an enhancement in natural cytotoxicity of NK cells treated with Sushi-IL15-Apo was observed in a 4 hour chromiun51 release assay using the erythroleukemia derived cell line K562 as the canonical NK target (). The increase in cytotoxicity of NK cells cultured with Sushi-IL15-Apo is likely associated with the enhancement of granzyme B expression ().
Figure 4. Sushi-IL15-Apo increases NK-cell mediated ADCC and natural cytotoxicity in vitro (A) Purified NK cells that had been cultured for 48h with Sushi-IL15-Apo or without cytokines were subsequently cocultured with cells from the colon carcinoma derived cell line HT-29 previously labeled with chromiun51 in the presence or absence of cetuximab. % specific cytotoxicity was measured in 4 h chromium release assays. Right top panel: CD107a expression on NK cell membranes after isolation, activation as in A and coculture with HT-29 cells in the presence or absence of cetuximab. Right bottom panel: Percentage of dead or apoptotic tumor cells. (B) Intra-cellular expression of granzyme B in NK cells cultured for 48 h with 10 nM of Sushi-IL15-Apo. (C and D) Percentage of CD107a+ NK cells and dead and apoptotic tumor cells were determined in a flow cytometry assay as in A, but using the HER2+ cell line BT474 with trastuzumab (C) or the CD38+ multiple myeloma-derived cell line MM1S with daratumumab (D) to stimulate the cultures. (E) NK cells treated as in A were cocultured for 4 h with chromiun51 labeled K562 cells to study % of cytotoxicity in chromium release assays. ###< p0.001, ## = p < 0.01 and # = p < 0.05 in a two-way ANOVA test comparing the Sushi-IL15-Apo + cetuximab group vs. cetuximab group in A or Sushi-IL15-Apo vs. control in C.
Sushi-IL15-Apo binds to SR-B1 in tumor cells and is trans-presented to CD8 T and NK cells
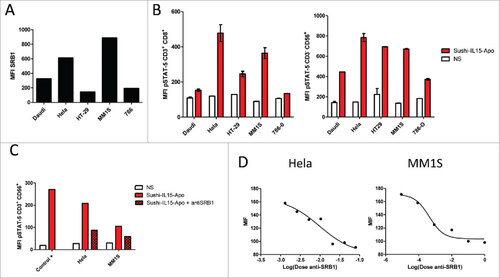
First-in-human clinical trials with IL-15 have shown unfavorable safety causing fever and hypotension at low doses, probably related to bolus infusion and systemic exposure.Citation19 One potential solution could be IL-15-constructs targeting the tumor but retaining the ability to activate CD8+ T and NK cells. SR-B1 is the main receptor of apolipoprotein A-I and it is highly overexpressed on tumor cells.Citation20 We tested if Sushi-IL15-Apo becomes targeted to tumor cells because of binding to SR-B1. Firstly, tumor cell lines derived from different types of cancer were selected and the expression of SR-B1 was examined by flow cytometry. As shown in , all the cell lines studied expressed SR-B1 on the plasma membrane at different densities. To show the trans-presentation functionality of SR-B1, we allowed Sushi-IL15-Apo to become bound to tumor cells which following extensive washing were co-cultured with human PBMCs. After 30 min co-culture, cells were fixed to observe the STAT5 phosphorylation in gated CD8+ T and NK cells by flow cytometry. As a control, PBMCs co-cultured with tumor cells that had not been pretreated with Sushi-IL15-Apo were used. All the tumor cells tested were able to induce STAT5 phosphorylation, and therefore, to trans-present IL-15 to CD8+ T and NK cells (). However, given the amphipathic nature of Sushi-IL15-Apo, unspecific adsorption on tumor cell membrane had to be ruled out. Thus, experiments of trans-presentation were performed pre-treating tumor cells with an antibody that blocks SR-B1 binding to apolipoprotein A-I. As shown in and , the presence of the blocking antibody curtailed STAT5 phosphorylation in CD8+ T and NK cells in a dose-dependent manner, suggesting that the transpresentation of IL-15 by tumor cells is specific to SR-B1-apolipoprotein A-I binding
Figure 5. Sushi-IL15-Apo binds to SR-B1 on tumor cells and IL-15 is trans-presented to CD8T and NK cells (A) SRB1 membrane expression on different tumor cell lines as measured as the mean of fluorescence intensity by flow cytometry. (B) STAT5 phosphorylation in NK and CD8+ T cells cocultured with tumor cells that had been previously incubated with 50 nM of Sushi-IL15-Apo protein and washed three times. (C) STAT5 phosphorylation in NK cells treated as in B, but with a group in which SR-B1 was blocked by a specific antibody. The positive control was STAT5 phosphorylation in NK cells directly treated with 50 nM of Sushi-IL15-Apo. (D) STAT5 phosphorylation in NK cells incubated with Hela and MM1S washed three times after 30 min incubation with 100 nM of Sushi-IL15-Apo testing different dilutions of an anti-SR-B1 blocking antibody.
Sushi-IL15-Apo increases ADCC in a transiently humanized murine model
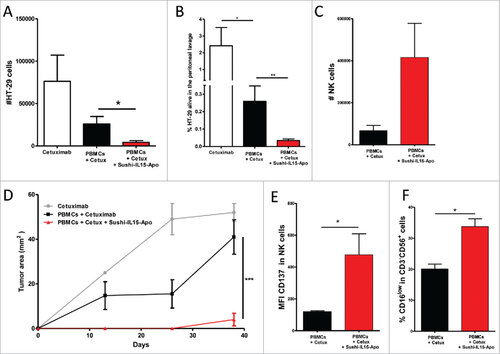
Sushi-IL15-Apo capacity to increase ADCC in vivo was tested in a transiently humanized murine model in which cells from the EGFR+ colon carcinoma-derived cell line HT-29 were stained with CFSE and injected i.p. into immunodeficient mice. At the same time, PBMCs and Sushi-IL15-Apo were administered intraperitoneally. Cetuximab was given to the mice the next day and after 36h peritoneal lavage was performed to assess the number of HT-29 cells remaining in the peritoneum. and show the number and percentage of HT-29 recovered from the peritoneal lavage of mice that received Sushi-IL15-Apo. Both parameters were significantly reduced in comparison with mice that received only PBMCs and cetuximab, whereas the absolute number of NK cells in the peritoneum was enhanced in mice that received the Sushi-IL15-Apo protein (). It is of note that there was a slight increase in natural cytotoxicity when Sushi-IL15-Apo was administered without cetuximab (Supp. Figure 5A). However, the largest effect against HT-29 was obtained when the two compounds were co-administered. Following lavage, mice were kept alive, and subcutaneous tumors grew at the peritoneal lavage puncture site. These tumors were absent or very small in mice which received Sushi-IL15-Apo (figure 76D). These experiments allowed us to study the NK cells recovered from the peritoneal cavity. The NK cells were not only more abundant in mice treated with Sushi-IL15-Apo () but also expressed CD137 more intensely on the membrane while less CD16A was detectable ( and ). These results suggest that NK cells had engaged in ADCC on cetuximab coated carcinoma cells. However, the increased CD137 expression on the membrane was independent of cetuximab (sup. Fig. 5B).
Figure 6. Sushi-IL15-Apo increases ADCC in a transient humanized murine model. (A) Absolute numbers of living HT-29 cells recovered from the peritoneal lavage of mice intraperitoneally given 106 HT-29 cells and 106 human PBMCs, 20 µg of Sushi-IL15-Apo protein and 50 µg of cetuximab. Pooled data from 4 independent experiments with 2–5 mice per group are shown in the graph. (B) Percentage of living HT-29 cells recovered in experiment A. (C) Absolute numbers of NK cells recovered in the peritoneal lavages. (D) Subcutaneous tumor growth observed in mice from the experiment A at the tumor lavage puncture site. (E and F) CD137 expression and percentage of CD16low cells in NK cells recovered in the peritoneal lavages. ## = p < 0.01 and # = p < 0.05 in U-Mann Whitney or Kruskal-Wallis test.
Sushi-IL15-Apo exerts anti-tumor effects in combination with an anti-EGFR mAb in a syngeneic model
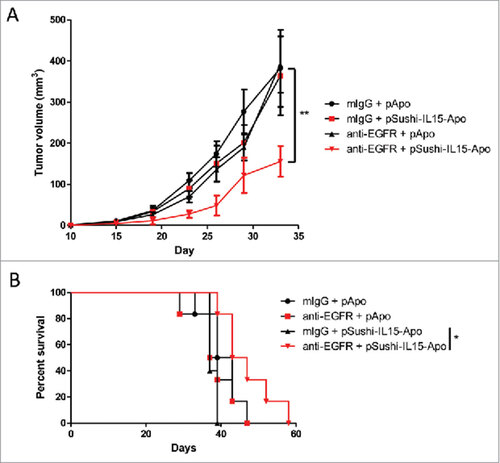
The long-lasting anti-tumor effect of Sushi-IL15-Apo inducing ADCC was tested in subcutaneous tumors derived from the colon cancer cell line MC38 transfected to express EGFR (Supp. Figure 6). Tumor cells were injected into Rag1−/− mice and 10 days after tumor inoculation, mice were treated with an anti-EGFR murine antibody and 1 µg of plasmid coding for Sushi-IL15-Apo hydrodynamically transfected. A delay in tumor growth was observed in mice treated with anti-EGFR and the Sushi-IL15-Apo plasmid (), that led to a delayed animal death ().
Figure 7. Sushi-IL15-Apo increases the anti-tumor effect of an anti-EGFR mAb in a subcutaneous syngeneic model. (A) Volume follow-up of subcutaneous tumors derived from MC38 colon cancer expressing EGFR treated with an anti-EGFR murine antibody or control and 1 µg of expression plasmid coding for Sushi-IL15-Apo that was hydrodynamically injected. ## indicates p<0.01 in a repeated measured ANOVA test comparing the tumor sizes between the group receiving anti-EGFR + pApo and the group treated with anti-EGFR and pSushi-IL15-Apo. (B) Kaplan-Meier survival curves. #indicates p<0.01 in a Gehan-Breslow-Wilcoxon test comparing the tumor sizes between the group receiving anti-EGFR + pApo and the group treated with anti-EGFR and pSushi-IL15-Apo.
Discussion
The therapeutic efficacy of IL2 is believed to be due to stimulation on T and NK lymphocytes and led to the approval of this cytokine for the treatment of advanced renal cell carcinoma and metastatic melanoma.Citation21,22 However, the serious adverse events associated with the high dose of this cytokine complicate wide clinical use.Citation23 Nevertheless, it is an invaluable tool for in vitro culture and expansion of T and NK lymphocytes and for prolonging the engraftment of adoptively transferred lymphocytes.Citation24
Among the different alternatives to IL2 treatment, IL15 is particularly appealing due to its reduced effects on T regulatory cellsCitation25 and the transpresentation mechanism.Citation11 As mentioned above, IL15 is trans-presented to target lymphocytes by the interleukin 15 receptor alpha (IL15Rα) subunit expressed on the IL15 producing cell. The IL15- IL15Rα complex is then optimally able to trigger the intracellular signaling cascade in the target cell that expresses the common beta and gamma receptor of the IL2 receptor.Citation11 For these reasons, IL15 may be more suitable for cancer immunotherapy.
IL15 is hindered by a short plasma half-life due to renal filtration and elimination. Thus, optimal therapeutic agents based on IL15 must consist of chimeric molecules that overcome the transpresentation mechanism due to the provision of the sushi domain of the IL15Rα in cis and provide an increased half-life in circulation. Sushi-IL15-Apo fulfills these two requirements.Citation14,15 Here, we developed a eukaryotic production system to obtain a glycosylated-IL15-based chimeric protein. This protein was able to sustain the proliferative capacity of CTLL-2 although the specific activity was reduced when compared with an international standard of IL15. Of note, this difference was minimized in the Mo-7e cell line, a cell line deficient in IL15Rα that allowed us to measure the contribution of the IL15Rα sushi domain.
An increase in ADCC mediated by tumor-targeted monoclonal antibodies upon combination with different IL15 variants has been recently reported.Citation12,13 Our results with the chimeric Sushi-IL15-Apo fully support these previous findings. The novel chimeric protein enhanced the number of NK cells due to an increase in survival and proliferation. In addition, the chimeric protein increased the cytotoxic potential on a per cell basis. Thus, the combination of increased numbers of effector cells and their cytotoxic performance resulted in enhanced ADCC as mediated by cetuximab. This effect was not only demonstrated in vitro, but also observed in immunodeficient mice xenografted with EGFR+ HT-29 tumor cells in which adoptive transfer of PBMCs in combination with cetuximab and the Sushi-IL15-Apo attained maximal tumor cell elimination, and in a syngeneic model of a subcutaneous MC38 colon cancer expressing EGFR.
One advantage of the use of the apolipoprotein A-I moiety is its tumor-targeting property via SR-B1. In line with this, reconstituted high-density lipoproteins and apolipoprotein A-I-decorated nanoparticles have been extensively used to target lipophilic compounds to tumors.Citation26 Tumor cells overexpress the main apolipoprotein A-I receptor, the scavenger receptor class B type I to sustain their high requirements for cholesterol and phospholipids.Citation20 The targeting effect was mimicked in culture and was abrogated by a blocking anti-SR-B1 antibody. Therefore, apolipoprotein A-I converts tumor cells in IL15 presenting cells and this is an advantage of this chimeric molecule over the IL15 fusion protein with the Fc domain of immunoglobulins.
In conclusion, Sushi-IL15-Apo is an IL15 derivative with unique characteristics. The combination of reduced specific activity, sustained half-life in circulation and tumor targeting may increase the therapeutic window. This is an important issue when developing IL15 chimeric proteins since overdosing may produce a lethal NK-mediated pneumonitis in mice.Citation14 Thus, Sushi-IL15-Apo constitutes a new agent with translational potential particularly as a partner in combined strategies with tumor-targeting Ab such as cetuximab.
Material and methods
Recombinant Sushi-IL15-Apo protein production and purification
Codon-optimized cDNA synthesis of Sushi-IL15-Apo with a His-tag in the N-terminus was ordered from Genewiz (Suzhou, China). cDNA was cloned into a pQMCF expression vector.
Protein production and purification were performed at Icosagen (Tartu, Estonia). CHOEBNALT85 cells were transfected with 1μg of the expression plasmid. 48 h after the transfection, 700 μg/ml of G418 was added to select the plasmid containing cell population.
For production, temperature was shifted to 30°C. The production phase lasted 10 days. At the end of the production, the culture supernatant was clarified by centrifugation (1000 g, 30 min, 15°C), PMSF was added and the supernatant was frozen and kept at -20°C prior to purification.
The supernatant was filtered through a glass fiber prefilter and Durapore 0.22 μm GV (PVDF) filter. The chimeric protein was purified by Ni-affinity chromatography. HisTrap FF (5ml, GE Healthcare, Chalfont St Giles, UK) was used for purification. The column was equilibrated with PBS pH 7.4. 0.5 M imidazole in PBS was used for elution. Äkta Prime was used for loading; FPLC was used for washing and elution.
Three fractions were pooled and desalted into PBS 7.4 using a HiPrep 26/10 Desalting column (GE Healthcare). Desalted fractions were further purified by gel-filtration (Superdex 200, GE Healthcare). Fractions from the gel filtration runs were pooled, sterile filtered, and the final concentration was measured on a NanoDrop 2000C device.
The Endpoint Chromogenic LAL test (Lonza, Walkersville, MD) was used for endotoxin level measurement
Mice, cell lines
C57BL/6 mice (6–10 weeks old), Rag1−/− and Rag2−/− IL-2Rγ−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facility of CIMA of the University of Navarra under the guidelines of Ethics Committee of the center.
Cell lines were maintained at 37°C in 5% CO2 and were grown in RPMI medium (RPMI 1640) with Glutamax (Gibco, Invitrogen, Carlsbad, CA) containing 10% heat-inactivated FBS (Gibco, Invitrogen), 100 IU/mL penicillin and 100 g/mL streptomycin (Biowhittaker, Walkersville, MD). MC38-EGFR cell line was kindly provided by Dr. Umaña (Roche Pharma). CTLL-2 is a stable subclone of cytotoxic T-lymphocytes originally isolated from a C57BL/6 mouse and 200 U/mL of IL2 (Preprotech, Hamburg, Germany) were added to the culture medium. Mo-7e is derived from a human acute myeloid leukemia and was cultured with 10ng/mL of GM-CSF.
PBMCs and NK purification and culture
PBMCs were obtained from healthy volunteers' blood after gradient centrifugation with Ficoll-Paque (GE Healthcare). NK cells were purified by negative selection using magnetic beads and LS selection columns following the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany).
Sushi-Apo-IL15 bioactivity assay
CTLL-2 and Mo-7e cells were washed three times with PBS and 50 μl of cells RPMI medium/well were plated on a 96-well plate (104cells/well). Recombinant IL15 (NIBSC, Hertfordshire, UK) or Sushi-IL15-Apo proteins were added at the described concentrations and the cultures were maintained for 48h. The cells were then pulsed with 0.5 μCi of tritiated thymidine (3H-TdR) 8h before being harvested with a Micro Beta Filter Mate-96 harvester (Perkin Elmer, Waltham, MA). 3H-TdR incorporation into the nuclei adsorbed onto the filters was measured using an automated Topcount liquid scintillation counter (Packard, Meriden, CT).
pSTAT-5 detection after culture with Sushi-IL15-Apo
PBMCs were incubated 20 min at 37ºC in RPMI medium and IL15 or Sushi-IL15-Apo protein at the indicated concentrations. Then the cells were fixed with an equal volume of 37ºC pre-warmed BD Cytofix Buffer (BD Biosciences, San Diego, CA) for 10 min. Cells were then permeabilized with BD Phosflow perm Buffer III (BD Biosciences) for 30 min in ice-cold solution and were stained with the different antibodies and analyzed by flow cytometry.
Isolation of mononuclear cells from spleen, lung, and liver in mice treated with Sushi-IL15-Apo
Mice were injected i.v. with 20 µg of recombinant Sushi-IL15-Apo or with 10 µg of Sushi-IL15-Apo plasmid by hydrodynamic injection. At day 5, spleens, lungs, and livers were surgically harvested. The organs were mechanically disrupted and passed through a 70 μm nylon mesh filter (BD Falcon, BD Biosciences). Dissociated cells from livers and lungs were centrifuged with Percoll© (GE Healthcare) at 35% (500 g, 10 min, 20ºC) establishing a gradient in order to eliminate parenchymal cells. In all cases, erythrocytes were lysed with ACK buffer and all the cells were washed before further use. Total cell number was counted with a Neubauer chamber and marker expression studied by flow cytometry.
Chromium51 release Cytotoxicity assays
Target cells (K562, HT-29 and HCT116) were incubated with 50 µCi of Chromium51 in RPMI during 1 h before being washed twice and seeded on a 96 well plate (5000 cells per well). Freshly purified NK cells cultured for 48h with or without 10 nM of Sushi-IL15-Apo were added together with 10 µg/mL of cetuximab (Erbitux©, Merk) for ADCC assay. After 4h of cell culture, supernatants were recovered and the chromium51 was measured using an automated Topcount liquid scintillation counter (Packard). Maximum lysis was calculated adding 1% of Triton X-100 (Sigma-Aldrich, Poole, Dorset, UK) to the labeled cells, and spontaneous lysis was calculated measuring a supernatant of labeled cells without NK cells or antibody. The percentage of lysis of each sample was calculated with the following formula: % lysis = (sample counts-spontaneous lysis counts) x 100/(maximum lysis counts – spontaneous lysis counts).
Antibodies and flow cytometry
Murine cells were pretreated with Fc-Block (anti-CD16/32, eBioscience, San Diego, CA) and human cells were pretreated with 10 µg/mL of beriglobin (CSL Behring, Marburg, Germany) to reduce nonspecific staining. Monoclonal antibodies to the mouse or human antigens were conjugated to fluorescein isothiocyanate, phycoerythrin (PE), PerCP-Cy5.5, allophycocyanin (APC), AlexaFluor 488, Alexa Fluor 647, Brillant Violet 510 or Brillant Violet 421. Antibodies anti- mCD3 (145-2C11), NK1.1 (PK-136), mCD8 (53-6.7), mCD44 (IM7), hCD3 (UCHT1), hEGFR (AY13) and hGrzmB (6B11) were obtained from Biolegend (San Diego, CA). Anti-hCD137 (4B4-1) and CD107a (H4A3) were purchased from Pharmigen. Anti-hCD56 (NCAM16.2), hCD16 (3G8), Active Caspase 3 (C92-605), and pSTAT-5 (pY694) were obtained from BD. The antibody anti-BclXL (7B2.5) was purchased from Southern Biotech (Birmingham, AL) and the anti-IL15Rα is from R&D. SR-B1 staining was done with the polyclonal anti-SR-B1 antibody NB400-113 from Novus Biologicals (Littleton, CO) and an anti-Rabbit secondary antibody from Biolegend. Intracellular staining was performed with the BD Cytofix/Cytoperm™ solution or BD Cytofix and BD Perm Buffer III respectively, following the manufacturer's protocol. A FACSCanto II cytometer was used for cell acquisition, and data analysis was performed using FACS DiVa (BD Biosciences) and FlowJo 7.2.1 (Tree Star Inc., San Carlos, CA).
In vivo ADCC experiments
Rag2-/-γc-/- mice were grafted with freshly human PBMCs short term in the peritoneal cavity with or without 20 µg of Sushi-IL15-Apo. At the same time, HT-29 cells prestained with CFSE were also injected into the peritoneal cavity in a ratio 1:10 (tumor: PBMC). After 40h, 50 µg of cetuximab was administered in the indicated groups, and 24 h later peritoneal lavages were performed. 5mL of saline solution was injected i.p and the lavage was recovered by gravity. The percentage of live HT-29 and NK cells was studied by flow cytometry, gating out murine cells. Absolute numbers were calculated with Perfect-Count Microspheres (Cytognos, Santander, Spain).
To set up a syngeneic tumor model, 2 × 106 hEGFR-transfected MC38 cells were subcutaneously administered to Rag1−/− mice. 10 days later, mice received 20 µg of a murine anti-hEGFR mAb (528, Bioxcell) or an irrelevant mouse IgG. At the same timepoint, mice received 1 µg of a plasmid coding for Sushi-IL15-Apo or for apolipoprotein A-I administered by hydrodynamic injection to achieve expression in hepatocytes.
Conflict of interest
I.M. has participated in advisory boards serving Roche-Genentech, Bristol-Myers Squibb, Incyte, Medimmune, Novartis, Alligator Biosciences, Bioncotech, Lilly, MSD, and LeadArtis. J.P.M and P.W. are former U3 Pharma employees. The rest of the authors have no conflict of interest to declare.
2017ONCOIMM0473R-s01.pptx
Download MS Power Point (225 KB)Acknowledgments
This work was supported by Worldwide Cancer Research Grant under Grant 15–1146, Asociación Española Contra el Cancer (AECC) Foundation under Grant GCB15152947MELE, Red Temática de Investigacion Cooperativa en Cancer under Grants RD12/0036/0040 and RD12/0036/0062, Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional (FEDER) under Grants PI14/01686, PI13/00207, PI16/00668, and H2020 PROCROP project under Grant 635122. P.B. is supported by Miguel Servet II (CPII15/00004) contract from Instituto de Salud Carlos III. EP-R is supported by the Carmen Lavigne training program of Asociación Española contra el Cancer and by Consejeria de Salud de la Junta de Andalucía. We thank Dr. Pablo Umaña for providing EGFR+-MC38 cells.
References
- Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–84. doi:10.4049/jimmunol.164.8.4178.
- Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inoges S, Melero I, Berraondo P. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95:347–55. doi:10.1038/icb.2017.6. PMID:28138156.
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi:10.1056/NEJMoa071834. PMID:18003960.
- Chen G, Kronenberger P, Teugels E, Umelo IA, De Greve J. Targeting the epidermal growth factor receptor in non-small cell lung cancer cells: the effect of combining RNA interference with tyrosine kinase inhibitors or cetuximab. BMC medicine. 2012;10:28. doi:10.1186/1741-7015-10-28. PMID:22436374.
- Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:3712–8. doi:10.1200/JCO.2006.08.8021. PMID:17704420.
- Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng. 2001;74:288–94. doi:10.1002/bit.1119. PMID:11410853.
- Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Tsumoto K, Kumagai I, Shitara K. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336:1239–49. doi:10.1016/j.jmb.2004.01.007. PMID:15037082.
- Moga E, Alvarez E, Canto E, Vidal S, Rodriguez-Sanchez JL, Sierra J, Briones J. NK cells stimulated with IL-15or CpG ODN enhance rituximab-dependent cellular cytotoxicity against B-cell lymphoma. Exp Hematol. 2008;36:69–77. doi:10.1016/j.exphem.2007.08.012. PMID:17959301.
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi:10.1084/jem.180.4.1395. PMID:7523571.
- Ochoa MC, Mazzolini G, Hervas-Stubbs S, de Sanmamed MF, Berraondo P, Melero I. Interleukin-15 in gene therapy of cancer. Curr Gene Ther. 2013;13:15–30. doi:10.2174/156652313804806561. PMID:23157547.
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–17. doi:10.1016/j.immuni.2007.03.006. PMID:17398124.
- Vincent M, Bessard A, Cochonneau D, Teppaz G, Sole V, Maillasson M, Birklé S, Garrigue-Antar L, Quéméner A, Jacques Y. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int J Cancer. 2013;133:757–65. doi:10.1002/ijc.28059. PMID:23354868.
- Rosario M, Liu B, Kong L, Collins LI, Schneider SE, Chen X, Han K, Jeng EK, Rhode PR, Leong JW, et al. The IL-15-Based ALT-803 Complex Enhances FcgammaRIIIa-Triggered NK Cell Responses and In Vivo Clearance of B Cell Lymphomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:596–608. doi:10.1158/1078-0432.CCR-15-1419. PMID:26423796.
- Ochoa MC, Fioravanti J, Rodriguez I, Hervas-Stubbs S, Azpilikueta A, Mazzolini G, Gúrpide A, Prieto J, Pardo J, Berraondo P, et al. Antitumor immunotherapeutic and toxic properties of an HDL-conjugated chimeric IL-15 fusion protein. Cancer research. 2013;73:139–49. doi:10.1158/0008-5472.CAN-12-2660. PMID:23149919.
- Ochoa MC, Fioravanti J, Duitman EH, Medina-Echeverz J, Palazon A, Arina A, Dubrot J, Alfaro C, Morales-Kastresana A, Murillo O, et al. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PloS One. 2012;7:e52370. doi:10.1371/journal.pone.0052370. PMID:23285013.
- Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem. 2000;275:30653–9. doi:10.1074/jbc.M002373200. PMID:10869346.
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi:10.1016/S1074-7613(02)00429-6. PMID:12433361.
- Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. The Journal of biological chemistry. 2006;281:1612–9.
- Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:74–82. doi:10.1200/JCO.2014.57.3329. PMID:25403209.
- Vasquez M, Simoes I, Consuegra-Fernandez M, Aranda F, Lozano F, Berraondo P. Exploiting scavenger receptors in cancer immunotherapy: Lessons from CD5 and SR-B1. European journal of immunology. 2017;41:1108–1118. doi:10.1002/eji.201646903. PMID:28504304
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17:2105–16. doi:10.1200/JCO.1999.17.7.2105. PMID:10561265.
- Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Annals of surgery. 1989;210:474–84; discussion 84–5. doi:10.1097/00000658-198910000-00008. PMID:2679456.
- Siegel JP, Puri RK. Interleukin-2 toxicity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1991;9:694–704. doi:10.1200/JCO.1991.9.4.694. PMID:2066765.
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi:10.1126/science.aaa4967. PMID:25838374.
- Marshall D, Sinclair C, Tung S, Seddon B. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol. 2014;193:5525–33. doi:10.4049/jimmunol.1402144.
- Fioravanti J, Medina-Echeverz J, Berraondo P. Scavenger receptor class B, type I: a promising immunotherapy target. Immunotherapy. 2011;3:395–406. doi:10.2217/imt.10.104. PMID:21395381.