ABSTRACT
Haploidentical Natural Killer (NK) cells have been shown as an effective and safe alternative for the treatment of haematological malignancies with poor prognosis for which traditional therapies are ineffective. In contrast to haematological cancer cells, that mainly grow as single suspension cells, solid carcinomas are characterised by a tridimensional (3D) architecture that provide specific surviving advantages and resistance against chemo- and radiotherapy. However, little is known about the impact of 3D growth on solid cancer immunotherapy especially adoptive NK cell transfer. We have recently developed a protocol to activate ex vivo human primary NK cells using B lymphoblastic cell lines, which generates NK cells able to overcome chemoresistance in haematological cancer cells. Here we have analysed the activity of these allogeneic NK cells against colorectal (CRC) human cell lines growing in 3D spheroid culture and correlated with the expression of some of the main ligands regulating NK cell activity. Our results indicate that activated NK cells efficiently kill colorectal tumour cell spheroids in both 2D and 3D cultures. Notably, although 3D CRC cell cultures favoured the expression of the inhibitory immune checkpoint PD-L1, it did not correlate with increased resistance to NK cells. Finally, we have analysed in detail the infiltration of NK cells in 3D spheroids by microscopy and found that at low NK cell density, cell death is not observed although NK cells are able to infiltrate into the spheroid. In contrast, higher densities promote tumoural cell death before infiltration can be detected. These findings show that highly dense activated human primary NK cells efficiently kill colorectal carcinoma cells growing in 3D cultures independently of PD-L1 expression and suggest that the use of allogeneic activated NK cells could be beneficial for the treatment of colorectal carcinoma.
Introduction
Natural Killer cells (NK), originally identified as immune cells with natural citotoxicity against transformed cells,Citation1–5 are granular lymphocytes belonging to the innate immune system that recognise and eliminate viral-infected and tumour cells.Citation1–5 NK cell development, activation and effector function is regulated by a complex balance between activating and inhibitory signals without prior sensitization and MHC restriction.Citation6,7 Accordingly, manipulation of NK cells has been the focus of several immunotherapy protocols to eliminate cancer cells that are resistant to chemotherapy or to T cell-based immunotherapy.Citation8
The main NK cell inhibitory signal is the level of expression of MHC-I in target cells, meanwhile several activating ligands, also known as stress ligands, have been described.Citation6,7 During tumour progression, some cells escape from NK cell-mediated immunosurveillance contributing to cancer development and disease. One of the main immune-evasion mechanism is a high level of MHC-I expression. Indeed, although adoptive cell therapy (ACT) employing autologous NK cells has shown poor anti-tumour cytotoxicity and low clinical effectiveness, the use of haploidentical hematopoietic transplantation from KIR ligand–mismatched donors has provided good results, especially for the treatment of blood-borne cancers.Citation8–11
Further attempts to increase the number and cytotoxic activity of transferred NK cells lead to the development of several protocols for ex vivo expansion and activation of human NK cells.Citation12 Recent works have shown that the anti-tumour activity of human NK cells greatly depends on the activating stimuli,Citation13 which is of special relevance during the elimination of chemo- and radio-resistant cancer cells of haematological origin.Citation14,15 Thus, the selection of a proper protocol to efficiently activate allogeneic NK cells is critical for cancer immunotherapy success.
An efficient activation of NK cells may be particularly important when considering solid tumours, which are considered to be more resistant to NK cells than haematological cancer cells.Citation16 Indeed, there is little evidence of clinical benefit of NK ACT in solid carcinomas.Citation8,17 There exist several potential explanations for this low efficacy, all of them related to the intrinsic characteristics of solid tumours:Citation16,18 i) tumour microenvironment generates immunosuppressant conditions impairing the anti-tumoral activity of immune cells and favouring immunoediting, ii) elimination of tumour cells requires NK cell extravasation and infiltration into the solid mass to engage target cells and release cytolytic granules and iii) microregions generated inside the tumour due to hypoxic conditions and nutrient restriction influence tumour heterogeneity, differentiation and growth and might affect its sensitivity to NK cells.Citation19
All these limitations are in part due to the intrinsic properties of cells growing in three dimensions (3D) as it happens during development of solid carcinomas in vivo. However, little is known about how 3D architecture affects the anti-tumoral cytotoxic potential of NK cells since most studies using human solid carcinoma cells have employed traditional 2D cultures based on monolayers that do not reproduce the physiological behaviour.
Here we have analysed the ability of human allogeneic NK cells activated with B lymphoblastic cell lines (LCLs) to kill and infiltrate human colorectal carcinoma (CRC) cells growing in 3D spheroid cell cultures in comparison to tradition monolayer cell cultures.
Results
Analyses of the effect of 3D colorectal cancer cell culture on MHC-I, ICAM- 1 and PD-L1 expression
We have selected three human CRC cells lines expressing different t mutational status concerning TP53, Ras/Raf and PI3K pathways (). On this way, we could analyse if the most common mutations found in CRC and known to modulate resistance to chemotherapy and mAb therapy (EGFR1) affect the tumour sensitivity to NK cells in both 2D and 3D cell cultures. First of all, we analysed cell viability in 3D spheroid cell cultures and confirmed that 97% of cells were viable by annexin-V/7-AAD staining in all cell lines analysed (). No necrotic core was observed in any of the spheroids analysed even after 96h of incubation ().
Table 1. Classification of human colorectal cancer cell lines according to oncogenic mutational status and microsatellite stability.
Figure 1. Spheroid characterization. (A) Dot-Plot of a representative spheroid control showing the percentage of viable cells after 4 days (Ann-V−/AAD−) for the three cell lines. PS exposure on plasma membrane (annexin-V) and membrane permeabilization (7AAD) were analysed by flow cytometry as described in Material and Methods. (B) CRC spheroid area evolution in control group shows tumour progression in absence of treatment along the study time course. (C) Monocellular suspensions from 2D or 3D cultures were analysed by flow cytometry for the expression of HLA-ABC, ICAM-1 and PD-L1. Bar charts represent the Mean Fluorescence Intensity in 2D (white) and 3D (black) conditions. Histograms show a representative determination for HCT116 cell line. The dotted line corresponds to isotype control, the black line to 2D conditions and the grey line to 3D conditions. Data in the graphics are represented as the mean±SEM of at least 5 independent experiments where duplicate measures were determined as described in Materials and Methods. n.s. no statistically significance or #p<0.05, ##p<0.01, ###p<0.001 statistically significance was analysed by t-test.
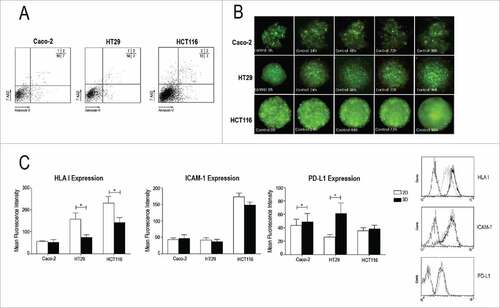
Recognition of target cells by NK cells involves several activating and inhibitory interactions. In this complex network three molecules present a notable role in regulating NK-cell mediated cytotoxicity: MHC-I, ICAM-1 and the inhibitory immune checkpoint PD-L1.Citation21,22 Thus, we have analysed the effect of spheroid 3D culture on the expression level of MHC-I, ICAM-1 and PD-L1 in Caco-2, HT29 and HCT116 cells in comparison with cells growing in 2D monolayers.
As shown in ICAM-1 expression was lower, although not significantly different, on 3D cell cultures than on 2D cell cultures. In contrast, HLA-I expression was significantly downregulated (40%) in CRC spheroids in comparison to 2D cell cultures in HCT116 and HT29 cells. This difference was not significative in Caco-2 cells, although it should be noted that they express very low levels of HLA-I. This reduction was not due to the protocol employed to dissociate 3D spheroids since it was the same as the one used for 2D cell cultures. Notably, when we analysed the expression of PD-L1 we found that Caco-2 and HT29 cell lines, but not HCT116 cells, significantly increased the expression of this inhibitory ligand on the cell membrane.
Activated NK cells are able to kill colorectal tumour cells in 3D and 2D cultures
We have previously shown that specific activation of NK cells using accessory EBV+ R69-LCLs is required to effectively destroy haematological tumour cell lines, including apoptosis-resistant mutants. Now we have analysed whether activated NK cells were able to kill 2D and 3D CRC cell cultures employing Caco-2, HT-29 and HCT116 CRC cell lines. To this aim we compared NK cell-mediated cytotoxicity (analysed by AnnexinV/7AAD staining) at low (3:1) and high (9:1) effector to target (e:t) ratio. As shown in , cell death induced by freshly isolated nNK cells in 2D cell cultures was low at both low and high e:t ratios in all cell lines. In contrast, nNK cells from some donors were able to kill CRC cells growing as 3D spheroids after 48h at both low and high e:t ratio. This effect was restricted to HT29 and Caco-2 cells meanwhile HCT116 spheroids were resistant to nNK cells.
Figure 2. Apoptosis induced by R69-LCL activated NK cells on 2D and 3D HCT116 cell cultures. NK cells were enriched by MACS from fresh PBMC or after activation for 5 days with R69-LCLs (aNK). Subsequently they were incubated with Caco-2, HT29 and HCT116 cells seeded in monolayers for 4 hours (A) or spheroids for 48.hours (B) at low (up to 3:1) or high (between 6:1 and 9:1) e:t ratios. PS exposure on plasma membrane (annexin-V) and membrane permeabilization (7AAD) were analysed by flow cytometry as described in Materials and Methods. Results are presented as mean +/− SEM SEM of at least 3 independent donors
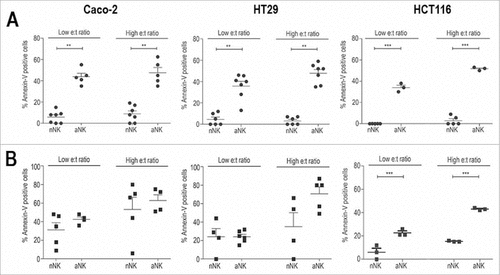
Next we analysed cell death induced by activated (a)NK cells obtained from the same donors as nNK cells. In 2D model, cell death induced by aNK cells was significantly higher than that induced by nNK cells (). In contrast to nNK cells, aNK cells were able to kill CRC 3D cell cultures from all cell lines irrespectively of the NK cell donor. In this case cell death induced by aNK cells was in general higher than that induced by nNK cells, although only in HCT116 cells was statistically significant. The differences between the cytotoxic potential of nNK and aNK cells were more pronounced at 24h (data not shown).
These results indicate that aNK cells are able to kill Caco-2, HT29 and HCT116 cells growing in 3D cultures. In addition, they suggest that expression of PD-L1 is not a critical factor modulating the cytotoxic potential of NK cells in this model.
Activated NK cells kill HCT116 cell spheroids depending on the NK cell density
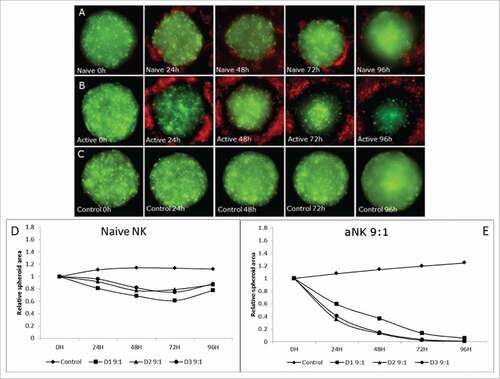
Once we showed and quantified by flow cytometry that aNK cells kill different CRC cells lines growing in both 2D and 3D, we decided to analyse in detail the elimination of 3D spheroids by conventional and confocal fluorescence microscopy. By this means spheroid cell death together with NK cell infiltration can be analysed and compared. To this aim we selected HCT116 cells expressing the Green Fluorescent Protein (GFP). All spheroids showed similar levels of viability, being always over 90%. In addition, all spheroids, formed by 1000 cells, showed a similar size after two days and formation of a necrotic was not detected. The area obtained for the spheroids was of 333.323,15 +/− 23.209,66 µm2.
As shown in at high e:t ratio, nNK cells from 3 different donors demonstrated partial cytotoxicity (15 to 20% spheroid reduction after 24h) during the first 3 days. However, this effect was not observed at longer incubation time (4 days), but an increase in the spheroid size was observed. In contrast, aNK cells from the same donors were able to induce a significantly higher level of cell death, achieving 40 to 60% spheroid reduction after 24h and almost a complete elimination after 4 days in culture (more details in supplementary ). These data confirm the results obtained by flow cytometry.
Figure 3. Cytotoxic effect of NK R69-LCL activated cells on HCT116 spheroid compared with naïve NK cells at high e:t ratio. (A, B and C) Fluorescent images of HCT116 cells (green) and NK cells (red) in a high e:t ratio during 96 hours treatment. Area evolution of CRC spheroids after treatment with nNK (A), activated NK cells (B), and control group with no NK presence (C). (D,E) A representative experiment from at least three experiments performed with 3 different donors is shown. The relative spheroid area was measured (D) Naïve nNK cells were able to reduce partially the tumour size but after 72h, spheroids star to grow and no clear effect is observed after 96h. (E) Activated NK cells at high e:t ratio are able to diminish drastically CRC spheroid size until almost complete elimination of GFP signal expressed by CRC cells.
When a lower e:t ratio was used different results were obtained ( and supplementary ). Tumor spheroids cultured in presence of aNK cells showed a small reduction in their area after 24–48 hours. However, after 4 days those spheroids reached a size similar to the one of non-treated controls. Similar results were achieved by increasing the e:t ratio to 6:1, a reduction in spheroid area after 48 hours and an ulterior increase in size. In this case the initial reduction was more intense than that observed using 3:1 ratio and spheroid area did not reach the original size (). As expected nNK cell-mediated cytotoxicity at low e:t ratio was not higher than that found at high e:t ratio (data not shown).
Figure 4. The e:t Ratio determines the cytotoxic effect of activated NK cells. A representative experiment from at least three experiments performed with 3 different donors is shown (A, B, C and D) Fluorescent images of HCT116 cells (green) and NK cells (red) in different e:t ratio after 96 hours of co-culture. (E, F and G) Relative area progression of the spheroids after treatment with different e:t ratio of activated NK cells from 3 different donors. (B and E) correspond to a 3:1 ratio of activated NK cells, (C and F) shows results obtained from the treatment of NK cells at a 6:1 ratio. (D and G) describe the 9:1 ratio activated aNK cell cytotoxic response.
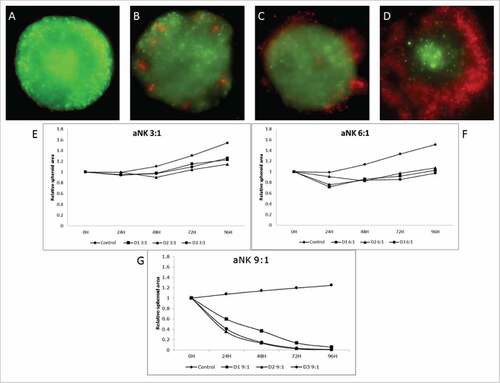
Human colon carcinoma spheroids are infiltrated by aNK at low density
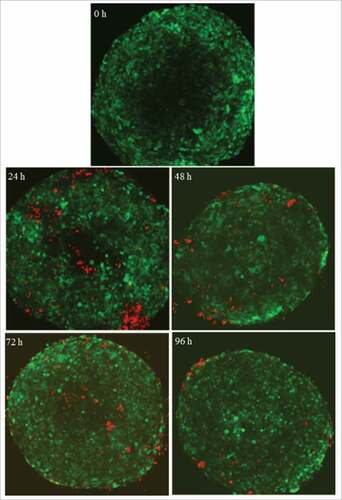
Analysing in more detail the previous data () we noticed that there was an inverse correlation between NK cell infiltration and e:t ratio. When high e:t ratio was used, aNK cells were able to kill tumor spheroids with apparently no infiltration (). In contrast, at lower e:t ratios aNK cells did not reduce significantly the tumour area (), but they were able to infiltrate more deeply into the spheroid. Indeed, after 24 hours at low e:t ratio it is possible to find aNK cells inside of spheroids (). Infiltrated cells are still present after 48 and 72 hours, although a reduced number is observed in comparison with 24 hours with no dark areas. After 96 hours, most of the aNK cells are in the periphery of the spheroid. A potential explanation for these findings is that at high e:t ratio there are enough NK cells to kill all spheroid tumour cells before infiltration can be detected. In contrast, smaller NK cell number is not enough to kill the tumour cells and in those conditions infiltration inside the living spheroid is observed.
Figure 5. activated NK cells infiltrate the tumor spheroid at low e:t ratios. Time lapse of the central layers of CRC spheroids (green) obtained by confocal microscopy during the aNK (red) in vitro co-culture at 3:1 e:t ratio. After 24h in co-culture, aNK cells are able to infiltrate the tumor spheroid. This infiltration is observed till the 4th day in which NK cells tend to migrate towards the periphery.
Discussion
NK cells are critical regulators of tumour immunosurveillance and lysis of malignant cells. Thus, NK cell-based immunotherapy has arisen as a promising alternative for patients with refractory or recurrent tumours. Allogenic NK cells have been shown to be very effective for treating haematological malignancies with bad prognosis, Citation9,10,23although clinical trials performed in solid tumours have produced modest results. Tumour architecture itself and the surrounding microenvironment contribute to generate immune-evasion conditions affecting clinical outcomes.Citation8,18 Here we have shown that human allogenic NK cells activated in the presence of mitomycin C- inactivated R69 lympoblastoid B cell line efficiently kill colorectal cancer (CRC) cells not only in 2D cultures, but in addition in a more physiological 3D cell spheroid culture condition. Of note we report that PDL1, a critical molecule that inhibits the anti-tumoural activity of immune effector cells like CD8+T cells, is upregulated in 3D cell cultures of CRC cells expressing MSI and BRAF mutations. However, NK cell-mediated elimination of CRC cells in 3D cultures is not affected by neither the presence of cancer critical gene mutations or microsatellite instability (MSI) nor by the level of expression of the inhibitory immune checkpoint PD-L1.
Previous works have analysed the susceptibility of CRC to NK cells.Citation24,25 These studies showed that differentiated CRC cells are resistant to NK cells in contrast to cancer initiating cells that were efficiently eliminated by NK cells.Citation25 However, it is important to note here that in most of the studies that have analysed the sensitivity of solid tumours to NK cells, freshly isolated NK cells or NK cells activated with cytokines like IL2 and/or IL15 were employed. Citation24–27We have confirmed here that in most cases freshly isolated allogeneic NK cells are not able to kill CRC cells in 2D or ·3D cultures. However, it should be noted that naïve NK cells from some donors were able to kill Caco-2 and HT29 cells in 3D cultures after 48 hours. A potential explanation for the sensitivity of these cells to freshly isolated NK cells could be a lower level of expression of HLA-I in 3D cell cultures in comparison to HCT116 cells. Moreover, this effect could be restricted to donor-CRC cell combinations that present KIR/HLA-I mismatch.
In contrast to freshly isolated NK cells, activated NK cells from all donors were able to kill all CRC cell lines in both 2D and 3D cultures indicating that 3D spheroid cell culture do not alter the ability of NK cells to kill CRC cells. This finding is in apparent contradiction with the abovementioned studies suggesting that only cancer initiating cells are sensitive to NK cells. Here it should be pointed out that activation and activity of NK cells depends on the stimulus employed and that different activation protocols renders NK cells with different cytotoxic potential as previously shown by our recent studies.Citation13–15 These works showed that NK cells activated in the presence of stimulating lymphoblastoid cell lines presented higher cytotoxicity against hematological cancer cells than those activated in the presence of IL2 and/or IL15 alone or together with K562 cells. This finding may help to explain why we have found that mature differentiated colorectal carcinoma cells show a good sensitivity to activated NK cells.
Not only the type of NK cells employed but the conditions of cancer cell cultures in vitro should be carefully analysed when testing the susceptibility of solid carcinomas to NK cells. NK cell-mediated cytotoxicity has been analysed against several cancer cells including renal, melanoma, oral and colon carcinoma mostly cultured under traditional monolayer cell cultures.Citation24,28,29 Tumour microenvironment has been proposed to play a role in generating the most favourable condition for altered cells to grow and disseminate. Hence, although colon adenocarcinomas show reduced levels of classical MHC-I and upregulate stress ligands such us NKG2D ligands, low infiltration rates of NK cells have been detected in patients.Citation30,31 Thus, although T cells are present inside tumours, the NK cell population would remain in the outer stroma. Accordingly, and because of discrepancies in phenotype markers, the correlation between higher infiltration rates of NK cells and better clinical outcomes is not as clear as in the case of T cells.Citation30,31
This context leads to consider some factors may be influencing NK cell recruitment and activity acting as mechanisms of resistance (i.e. the chemokine profile of the tumour microenvironment, hypoxic conditions or receptor blocking ligands shed by tumour cells). For this reason, we have generated multicellular tumour spheroids in a hydrogel matrix to recreate tumour architecture and microenvironment and mimic the conditions for NK cell migration, interaction with and penetration into solid tumours. Besides, previous works have already shown that this technique provides a proper scenario for studying immunosurveillance and NK cell effector functions in other types of solid carcinomas.Citation26,27,32,33 In this study three CRC cell lines (Caco-2, HT29 and HCT116) were defined as representative models of colorectal cancer, since they present different mutational status for critical genes involved in CRC progression and resistance to treatment (see ). Phenotypical characterisation of three representative NK cell ligands in 2D and 3D conditions by flow cytometry revealed a different regulation of their expression. The level of expression of the intercellular adhesion molecule ICAM-1did not change, but a downregulation of the classical MHC-I in spheroid cultures was observed. Notably, the expression of the inhibitory immune checkpoint PDL1 was increased in HT29 and HCT116 3D cell cultures. The interaction of LFA-1 with ICAM-1 and of NK cell inhibitory receptors with MHC-I have been shown to be the most critical processes that regulates NK-cell immunological synapse formation and killing of target cells.Citation21,22,34 The high levels of ICAM-1 observed here are in agreement with other observations in patient samples where malignant but not normal colon tissue upregulates this molecule.Citation31 MHC-I serves as the main inhibitory signal for NK cells. In fact, a recent study in Ewing's sarcoma spheroids shows that the sensibility of target cells is dependent on a threshold regulated by this ligand.Citation27 Here, MHC-I downregulation observed in the more physiological 3D conditions could be related with the loss of MHC-I in the colon carcinoma lesions compared to normal epithelia, as it has been described.Citation31,35,36 In a similar way, the upregulation of PD-L1 observed in 3D cell cultures of Caco-2 and HT29 cells resembles a recent observations in CRC patient biopsies indicating that expression of PD-L1 in CRC cells is related with the presence of MSI and BRAF mutation. Indeed, HT29 cells express a BRAF mutation and a higher PD-L1 level.Citation37
As indicated it has been shown a low level of NK cell infiltration in solid carcinomas including CRC.Citation31,38 In addition, maintaining of high levels of MHC-I is the main immune evasion mechanism employed by solid cancer to overcome NK cell attack. Thus, autologous NK cells could be impaired in migration, recognition and elimination of the tumor. Here our results indicate that employing allogenic NK cells could overcome resistance of CRC cells expressing high levels of MHC-I and PDL1. Moreover, our previous results indicate that NK cells activated by B-LCLs increased the expression chemokine receptors suggesting that this activation may enhance the ability of NK cells to migrate and infiltrate solid carcinomas.Citation13 In this line, we show here that allogenic activated NK cells efficiently infiltrate CRC spheroids, a process that was more evident at low e:t ratios. Moreover, we have recently shown that allogenic NK cells activated with R69 LCLs are able to migrate and penetrate CRC cell spheroids employing an in vitro biomimetic microfluidic chip.Citation20 Recent studies also evaluated the infiltration of NK cells in spheroids of human cervical carcinoma cell lines at 24h. In contrast to our data a low percentage of infiltrating cells, which would be the ones responsible for the partial destruction of the spheroid was found.Citation26 However our results show a dynamic process regulating NK cell location in cell spheroid at different times, which might explain these apparent discrepancies. In addition the use of NK cells activated with other stimuli like IL2 might also explain the different results.Citation26,27
In summary, this study shows that in vitro activated allogeneic NK cells can kill CRC cells in spheroid cultures irrespectively of the presence of mutations conferring bad prognosis and drug resistance as well as independently of the level of expression of PDL1. During adoptive cell transfer immunotherapy, the anti-tumour effect of transferred NK cells will not only be dependent on the level of exogenous NK cell activation, but in addition on the in vivo microenvironmental conditions that NK cells will find a in cancer patients. Our model set the basis for further studies considering other microenvironment components (extracellular matrix, stromal and immune cells or soluble factors) and physico-chemical conditions (fluid flow, oxygen, pH or glucose) that will be useful to predict the efficacy of immunotherapy protocols to treat colorectal cancer and other solid carcinomas.
Material and methods
Cell culture
The next human colorectal carcinoma cell lines were used: HCT116, HT29 and Caco2. HCT116 and HT29 were routinely cultured in high glucose Dubelcco´s modified Eagles´s medium (DMEM) (Lonza) supplemented with 10% foetal bovine serum (HyCloneTM), penicillin/streptomycin (Sigma-Aldrich) and 2mM ultraglutamine (Lonza). Caco-2 cells were maintained in Dubelcco´s modified Eagles´s medium (DMEM) (Lonza) supplemented with MEM Non-Essential Amino Acids Solution (Gibco), 10% foetal bovine serum (HyCloneTM), penicillin/streptomycin (Sigma-Aldrich) and 2mM ultraglutamine (Lonza). Cells were incubated at 37°C and 5% CO2.
For fluorescent cell labelling, HCT116 cell line was stably transfected with EGFP using lentiviral vectors provided by Dr. Prats (University Paul Sabatier, Toulouse, France) as described.Citation20 In case of HT29 and Caco2 cell lines, Dio Vybrant® lipophilic cell membrane dye (Life Technologies) was used to label cells following the manufacturer´s instructions. Cells were stained prior to spheroid formation.
Spheroid generation and analysis of cell viability
Spheroids were generated by the hanging drop method using methylcellulose. Cell suspension was mixed with methylcellulose stock solution at a 4:1 ratio. 25 µl droplets containing 1000 cells per droplet were placed on the lid of a Petri dish and incubated for 48 hours. Following this methodology, a single well-defined spheroid was generated per droplet.
To assess cell viability, spheroids were disaggregated with Trypsin-EDTA (Sigma-Aldrich) and phosphatidylserine translocation and membrane permeabilization were analysed by Annexin-V and 7-Amino-Actinomycin (7-AAD) staining and flow cytometry (FACScalibur). Identification of both markers are widely used for the detection of dead cells, thereby viable cells remain unstained.
Immune characterization of spheroid cells
NK cell-ligand expression on cell membrane of colorectal cancer cells growing in spheroids or their monolayer cell counterparts was analysed by flow cytometry. After cell dissociation with Trypsin-EDTA, cellular suspensions were labeled with the following antibodies: HLA-ABC-FITC (clone W6/32; eBioscience), ICAM-1-APC (clone HA58; BD Bioscience) and PD-L1-APD (clone 29E.2A3; Biolegend). All antibodies were diluted in PBS with 5% FCS and 0.1% Sodium Azide for cell staining.
Isolation and activation of human NK cells
Human primary NK cells were enriched by using anti-CD56 antibodies attached to magnetic beads (MACS, Miltenyi) from either freshly isolated PBMCs (freshly isolated NK cells –nNK-) or from PBMCs cultures activated in vitro (activated NK cells –aNK-) as previously described.Citation14 PBMCs were obtained by Ficoll gradient centrifugation of blood from healthy donors provided by the Blood and Tissue Bank of Aragon (with the approval of the Ethical Committee of Clinical Research of Aragon, number: C.I.PI11/006).
Activation of human NK cells was pursued by culturing PBMCs in RPMI-1640 medium (Lonza) supplemented with 10% FCS (HyCloneTM), 100 U/ml penicillin, 0.1 mg/ml streptomycin (Sigma-Aldrich), 2 mM ultraglutamine (Lonza) and mitomycin C-inactivated R69 cells at a 10:1 ratio (PBMC: stimulator cells) for 5 days as described.Citation14
Cytotoxicity assays
NK cells were fluorescently labelled with eFluor670 (eBioscience) following the manufacturer's instructions, and incubated with target cells at different effector:target (e:t) ratios and times. Cytotoxicity in 2D cell cultures was measured by flow cytometry. Target cells were seeded in flat bottom 96-multiwell plates for 24 hours and subsequently they were incubated with labelled NK cells for 4 hours-. Cell death was analysed by monitoring phosphatidylserine translocation and membrane permeabilization by Annexin-V and 7-Amino-Actinomycin (7-AAD) staining and flow cytometry in the eFluor670 negative target cell population.
In case of 3D cell cultures, cytotoxicity was measured by flow cytometry and fluorescence microscopy. For cytometry analysis, labelled NK cells were co-cultured with two days old spheroids in round bottom 96 well plates and cell death was determined at 24 and 48 hours. Spheroids were disaggregated with Trypsin-EDTA and cell death was analyzed as described above. For fluorescence microscopy assays cell death was analyzed for 96 hours every 24 hours, as described below. Six spheroids for each NK cell donor and condition (activated and freshly isolated) were seeded plus six spheroids without NK cells as control. Three different healthy donors were used per cytotoxicity assay.
Image analysis
Laser confocal and fluorescence images were acquired using a Nikon Eclipse Ti-E C1 confocal microscope. Cell analysis was performed using ImageJ software. Images were taken at different focal planes (200 µm in the “z” direction every 5 µm from the center of the spheroid). Z stack photo shoots were taken in two different wave lengths (GFP and Cy5) as well as bright field. Maximum intensity reconstruction of the Z stack in both wave length were merged to measure the diameter of the spheroid using the Fiji software.
Statistical analysis
Data were analysed using GraphPad Prism 5.0. The normal distribution was tested by the Kolmogorov-Smirnov test, and the t-Student´s t-test or the Wilconxon test were used for the data analysis depending on the properties of the experimental groups. Statistical significance was set at p<0.05.
Abbreviations
| aNK | = | Activated Natural Killer cells |
| HCC | = | Human Colon carcinoma |
| HCT 116 | = | Human Colon tumor cells |
| MHC I | = | Mayor Histocompatibility complex one |
| NK | = | Natural Killers Cells |
| nNK | = | Freshly isolated Natural Killers Cells |
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
supp_data.zip
Download Zip (106.8 MB)Acknowledgments
We would like to acknowledge the use of Servicios Cientifico-Tecnicos (SCT) del CIBA (Instituto Aragones de Ciencias de la Salud, Universidad de Zaragoza, Fundacion IIS Aragón).
Additional information
Funding
References
- Haller O, Kiessling R, Orn A, Karre K, Nilsson K, Wigzell H. Natural cytotoxicity to human leukemia mediated by mouse non-T cells. Int J Cancer. 1977;20:93–103.
- Herberman RB, Holden HT. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–77.
- Kiessling R, Bataillon G, Lamon EW, Klein E. The lymphocyte response to primary Moloney sarcoma virus tumors: definition of a non-specific component of the in vitro cellular hyporeactivity of tumor-bearing hosts. Int J Cancer. 1974;14:642–8.
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7.
- Karre K. Natural killer cell recognition of missing self. Nat Immunol. 2008;9:477–80.
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502.
- Bottino C, Moretta L, Moretta A. NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol. 2006;298:175–82.
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–52.
- Velardi A. Natural killer cell alloreactivity 10 years later. Curr Opin Hematol. 2012;19:421–6.
- Locatelli F, Pende D, Mingari MC, Bertaina A, Falco M, Moretta A, et al. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: role of alloreactive NK cells. Front Immunol. 2013;4:15.
- Murphy WJ, Parham P, Miller JS. NK cells-from bench to clinic. Biol Blood Marrow Transplant. 2012;18:S2–7.
- Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med. 2009;29:89–96.
- Sanchez-Martinez D, Krzywinska E, Rathore MG, Saumet A, Cornillon A, Lopez-Royuela N, et al. All-trans retinoic acid (ATRA) induces miR-23a expression, decreases CTSC expression and granzyme B activity leading to impaired NK cell cytotoxicity. Int J Biochem Cell Biol. 2014;49:42–52.
- Sanchez-Martinez D, Azaceta G, Muntasell A, Aguilo N, Nunez D, Galvez EM, et al. Human NK cells activated by EBV lymphoblastoid cells overcome anti-apoptotic mechanisms of drug resistance in haematological cancer cells. Oncoimmunology. 2015;4:e991613.
- Sanchez-Martinez D, Lanuza PM, Gomez N, Muntasell A, Cisneros E, Moraru M, et al. Activated Allogeneic NK Cells Preferentially Kill Poor Prognosis B-Cell Chronic Lymphocytic Leukemia Cells. Front Immunol. 2016;7:454.
- Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun. 2011;3:355–64.
- Chan CJ, Andrews DM, Smyth MJ. Can NK cells be a therapeutic target in human cancer? Eur J Immunol. 2008;38:2964–8.
- Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202.
- Loeffler DA, Juneau PL, Heppner GH. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int J Cancer. 1991;48:895–9.
- Ayuso JM, Virumbrales-Munoz M, Lacueva A, Lanuza PM, Checa-Chavarria E, Botella P, et al. Development and characterization of a microfluidic model of the tumour microenvironment. Sci Rep. 2016;6:36086.
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–12.
- Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–66.
- Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–61.
- Bhat R, Rommelaere J. NK-cell-dependent killing of colon carcinoma cells is mediated by natural cytotoxicity receptors (NCRs) and stimulated by parvovirus infection of target cells. BMC Cancer. 2013;13:367.
- Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–90.
- Giannattasio A, Weil S, Kloess S, Ansari N, Stelzer EH, Cerwenka A, et al. Cytotoxicity and infiltration of human NK cells in in vivo-like tumor spheroids. BMC Cancer. 2015;15:351.
- Holmes TD, El-Sherbiny YM, Davison A, Clough SL, Blair GE, Cook GP. A human NK cell activation/inhibition threshold allows small changes in the target cell surface phenotype to dramatically alter susceptibility to NK cells. J Immunol. 2015;186:1538–45.
- Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP, Jr., Takahashi Y, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170–7.
- Jewett A, Tseng HC, Arasteh A, Saadat S, Christensen RE, Cacalano NA. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr Drug Deliv. 2012;9:5–16.
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
- Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–89.
- Christakou AE, Ohlin M, Onfelt B, Wiklund M. Ultrasonic three-dimensional on-chip cell culture for dynamic studies of tumor immune surveillance by natural killer cells. Lab Chip. 2015;15:3222–31.
- Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal. 2011;5:239–48.
- Núñez D, Domingo MP, Sánchez-Martínez D, Cebolla V, Chiou A, Velázquez-Campoy A, et al. Recombinant production of human ICAM-1 chimeras by single step on column refolding and purification. Process Biochemistry. 2013;48:708–15.
- Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–85.
- Haworth KB, Leddon JL, Chen CY, Horwitz EM, Mackall CL, Cripe TP. Going back to class I: MHC and immunotherapies for childhood cancer. Pediatr Blood Cancer. 2015;62:571–6.
- Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104–12.
- Larsen SK, Gao Y, Basse PH. NK cells in the tumor microenvironment. Crit Rev Oncog. 2014;19:91–105.
