ABSTRACT
Small-cell lung cancer (SCLC) is often associated with paraneoplastic syndromes. To assess the role of anti-neuronal autoantibodies (NAAs) as biomarkers of treatment outcome, we assessed NAAs in serial samples from SCLC patients treated with chemoimmunotherapy compared to chemotherapy alone. We evaluated 2 cohorts: in cohort 1 (C1), 47 patients received standard platinum/etoposide, and in cohort 2 (C2), 38 patients received ipilimumab, carboplatin and etoposide. Serum samples at baseline and subsequent time points were analyzed for the presence of NAAs. NAAs were detected at baseline in 25 patients (53.2%) in C1 and in 20 patients (52.6%) in C2 (most frequently anti-Sox1). NAA at baseline was associated with limited disease (75% vs 50%; p: 0.096) and better overall survival (15.1 m vs 11.7 m; p: 0.032) in C1. Thirteen patients (28.9%) showed 2 or more reactivities before treatment; this was associated with worse PFS (5.5 m vs 7.3 m; p: 0.005) in patients treated with chemoimmunotherapy. NAA titers decreased after therapy in 68.9% patients, with no differential patterns of change between cohorts. Patients whose NAA titer decreased after treatment, showed longer OS [18.5 m (95% CI: 15.8 – 21.2)] compared with those whose NAA increased [12.3 m (95% CI: 8.1 – 16.5; p 0.049)], suggesting that antibody levels correlate to tumor load. Our findings reinforce the role of NAAs as prognostic markers and tumor activity/burden in SCLC, warrant further investigation in their predictive role for immunotherapy and raise concern over the use of immunotherapy in patients with more than one anti-NAA reactivity.
Introduction
Small-cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer.Citation1,Citation2 Although robust and often dramatic clinical responses are achieved with platinum-based first-line treatment, recurrence/progression usually occurs early and is resistant to available treatments.Citation3 Outcomes in patients with stage IV disease remain poor with a median overall survival that rarely exceeds one year.Citation1,Citation4
Immunotherapy has led to a paradigm-shift in the treatment of solid tumors.Citation5,Citation6 SCLC seems a particularly attractive target for immunotherapy. Paraneoplastic neurological syndromes (PNSs) are common in SCLC and are characterized by anti-cancer immune responses, which become clinically visible by off-tumor immune consequences against neuronal antigens, ectopically expressed by cancer cells and physiologically by the normal nervous system.Citation7 The better cancer specific survival outcomes in patients with autoimmune disease points to a protective effect, linked to these cross-reactive immune responses.Citation8,Citation9 Moreover, high mutational load has been linked to benefit from immunotherapyCitation10 and SCLC falls into this category,Citation2,Citation11,Citation12 most likely as a result of the tight association with tobacco smoking. Therefore, harnessing the immune system to fight SCLC seems a potentially promising strategy, although the best approach is currently uncertain.
Ipilimumab is a fully human immunoglobulin G1 monoclonal antibody that blocks CTLA-413, and has been tested in SCLC. A phase II trial,Citation14 showed a trend to improved overall survival with phased ipilimumab added to a platinum doublet. However, the confirmatory phase III trial Citation15 comparing ipilimumab and chemotherapy vs chemotherapy did not confirm better overall survival after chemoimmunotherapy. More recently, anti-PD-1 agents have also been tested in SCLC. Nivolumab (alone and in combination with ipilimumab) showed antitumor activity in SCLC patients with relapsed disease.Citation16 In a phase I trial, Pembrolizumab use led to objective responses greater than 30% of patients.Citation17
Nonetheless, the majority of patients with SCLC do not benefit from checkpoint inhibitors and the absence of predictive biomarkers for patient selection may have contributed to negative trial results.Citation15 Additionally, clinically relevant autoimmune toxicity has been reported, including severe neurological PNSCitation14,Citation16,Citation18 increasing the level of caution that is needed when investigating immunotherapy in SCLC. PD-L1 immunohistochemical expression, which is predictive for benefit from modulation of PD1/PDL1 in Non-Small Cell Lung cancer, does not appear to correlate with outcome in SCLC.Citation16,Citation17 A confounding effect for the evaluation of PD-L1 status in the tumor is limited access to tissue due to small biopsy material or cytology diagnostic specimens, a well-recognized problem in SCLC.Citation19 Therefore, the need to develop predictive biomarkers remains an urgent priority in SCLC.
With the aim of identifying potential biomarkers, we previously performed an exploratory analysis of the phase II ICE trial with a single cohort of patients treated with chemotherapy and ipilimumab. In this single arm study, positivity of autoantibodies at baseline was associated with improved outcomes.Citation18
In the current study we added a ‘control’ cohort treated with standard chemotherapy in order to assess the predictive vs. prognostic role of the neuronal autoantibodies. We also analyzed changes in autoantibody detection during treatment to evaluate the potential utility of serial sampling.
Results
Patients’ characteristics and outcomes
We included 85 SCLC patients: 47 treated with standard chemotherapy (cohort 1 herein) and 38 with standard chemotherapy plus ipilimumab (cohort 2 herein). Patients’ characteristics are shown in . The main differences between both cohorts were that cohort 1 (C1) included more men (74.5% vs 65.8%), 25% patients had limited stage and 15% patients presented with performance status (PS) 2 versus none in cohort 2. None of the patients had any neurological symptoms at diagnosis.
Table 1. Patients' characteristics.
Objective response rate (ORR) was 93.6% in C1 and 81.6% in C2. Median progression free survival (PFS) was 6.8 months (m) (95% CI: 6.1-7.6) in C1 and 6.9 m (95% CI: 6.3-7.6) in C2 (p = 0.847). Overall survival (OS) was 13.3 m (95% CI: 10.1-16.5) in C1 vs 17 m (95% CI: 4.5-29.5) in C2 (p = 0.987). When we selected patients with stage IV in C1, OS was 11.7 m (95% CI: 7.6 – 15.7).
We assessed the impact of clinical variables on survival. illustrates the association between clinical variables and OS combining both cohorts. PS:0 vs. PS>0 (p = 0.019), limited stage (p = 0.003) and response to first line chemotherapy (p<0.001) were associated with a significantly improved survival ().
Table 2. Univariate analysis for overall survival including both cohorts (Log-Rank test).
Table 3. Neuronal autoantibody detection at baseline.
Neuronal autoantibody detection
Neuronal autoantibodies (NAA) were detected at baseline in 25 patients (53.2%) in C1 and in 20 patients (52.6%) in C2. In both cohorts, the most prevalent autoantibody was anti-Sox1 (38.3% in C1 and 34.2% in C2), followed by anti-HuD and anti-Yo (, – left).
Figure 1. Neuronal autoantibodies prevalence in patients with SCLC. On the left, percentage and absolute numbers of neuronal autoantibody detected at baseline in each cohort. In the center, Benn's diagrams illustrating the different NAA multi reactivities in patients from both cohorts. On the right we show the combination of NAA with 2, 3 or 4 reactivities. Numbers at the intersection of Benn's diagrams are number of patients with that combination.
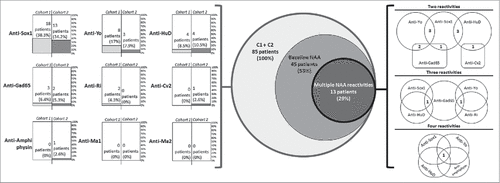
Globally, 13 patients out of the 45 with baseline NAA (28.9%) showed 2 or more reactivities at any time-point (Supplementary Table 1); of these, 11 patients (24.5%), had 2 or more types of autoantibody at baseline. Eight patients (17.8%) in C1 showed 2 or more reactivities; one of these patients was positive at baseline for anti-Sox1, but became also positive for anti-Yo after 3 cycles of chemotherapy. Five patients (11.1%) in C2 showed 2 or more reactivities; one of these patients was positive at baseline for anti-Yo, and became also positive for anti-Gad65 after 8 weeks of treatment. Anti-Yo and Anti-Sox1 detection were associated with another NAA in 7/12 patients (58.3%) and 9/32 patients (28.1%), respectively. One patient showed reactivity for 4 NAA: anti-Sox1, anti-amphiphysin, anti-Ri and anti-HuD. In (right) we illustrate the different multi-reactivities of NAA in patients of both cohorts.
Association between neuronal autoantibodies and clinical characteristics and outcomes
We next evaluated the association of the detection of NAA with clinical variables. Of 12 patients with limited stage, 9 patients (75%) had detectable NAAs at baseline versus 36/72 (50%) of patients with stage IV disease (p = 0.096). This trend was also observed for anti-Sox1 where 7/12 (58.3%) of patients with limited stage had detectable anti-Sox1 at baseline compared to 24/72 (33.3%) patients with stage IV (p = 0.092). The presence of anti-HuD and anti-Yo did not show any correlation with stage. There was no association between sex, age or PS and detection of neither any baseline NAA nor specific autoantibodies.
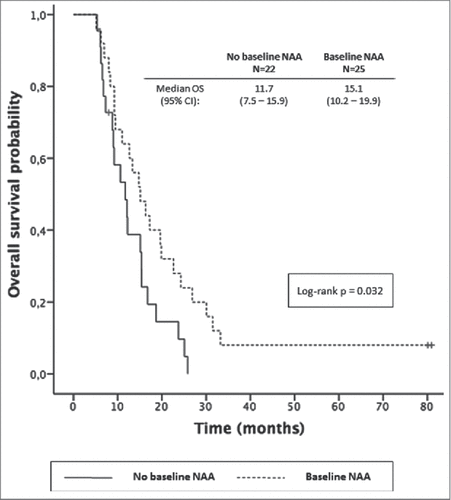
We assessed the association of NAAs with clinical outcomes. In patients with pre-treatment NAAs we observed a trend for better overall survival when both cohorts were analyzed together [14.8 m (95% CI: 10.6-19) vs 12.1 m (95% CI: 8.2-16); p: 0.089]. In patients treated with chemotherapy alone, this association was significant [15.1 m (95% CI: 10.2-20) vs 11.7 m (95%CI: 7.5-15.9); p = 0.032] (). This association was lost when the analysis was restricted to stage IV patients and was not observed in patients from C2. No effect from baseline autoantibody presence was observed on objective response rates or PFS ().
Table 4. Objective response rate, progression free survival and overall survival in patients with at least 1 baseline positive NAA vs those with no detectable NAAs.
Figure 2. Overall survival in patients treated with chemotherapy alone (n = 47) with or without any baseline NAA.
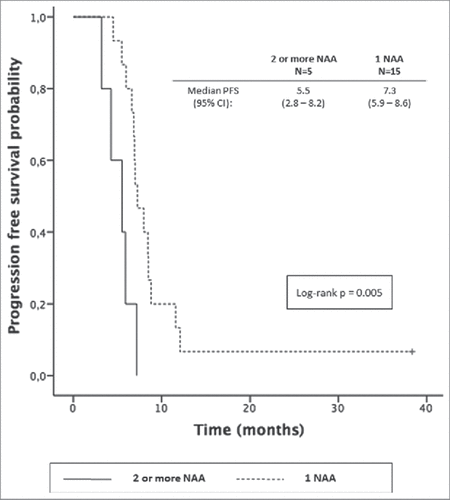
We then tried to gain insight whether the breadth rather than simply examining presence or absence of reactivity against NAAs might link to outcomes. We segregated patients with reactivity to exactly one, as opposed to >1 NAA. Unexpectedly, the presence of only 1 NAA vs >1 NAA was associated with better PFS [7.3 m (95% CI: 5.9-8.6) vs 5.5 m (95% CI: 2.8-8.2); p = 0.005] in patients treated with chemo-immunotherapy (); a trend was also visible in OS [12.3 m (95% CI: 4.7-19.9) vs 7.9 m (95% CI: 4.9-11); p = 0.180]. No such association was observed in the chemotherapy only cohort. The detection of multiple vs. single reactivities was not associated with ipilimumab-related adverse events in cohort 2 (colitis, fatigue and rash) nor neurological events.
Figure 3. Progression free survival in patients treated with ipilimumab plus chemotherapy with presence of 1 NAA vs 2 or more (n = 20).
We then evaluated the association between NAA against individual antigens and clinical outcomes. In patients treated with ipilimumab and chemotherapy, OS was 7.8 m (95% CI 2.2-13.4) when anti-Yo was positive, and 17 m (8.7-25.4) when it was negative (p = 0.015); PFS was 4.3 m (95% CI: 2.5-6) when anti-Yo was positive, and 6.9 m (95% CI: 6.4-7.5) when anti-Yo was negative (p = 0.081). No other associations were observed between other individual NAAs and clinical outcomes.
In the multivariate analysis in C2 for OS, including poor PS and lack of response to first line treatment, having 2 or more vs 1 NAA, remained independently associated with poor outcome (HR: 3.4; p-value: 0.033).
Titers of NAA change upon response to treatment
We quantified the titers of NAAs at each time point (Supplementary Fig. 1). Overall in 31 (68.9%) of the 45 patients with NAAs at baseline, titers decreased after treatment initiation (). In 22 out of these (48.9%), titers remained lower than baseline at the 3rd time point (progression) and in 9 patients (20%) they increased again. In 6 patients (13.4%), titers increased after treatment; of these, 2 patients (4.5%) maintained higher titers at progression and in 4 patients (8.9%) titers decreased. Autoantibody titers remained unchanged in 2 patients (4.5%). Variable patterns of change were observed in the 6 (13.4%) remaining patients (example shown in Supplementary Fig. 2).
Figure 4. Changes in autoantibody titers at different time-points in both cohorts. A: Patients treated with chemotherapy, whose NAA titers decreased after treatment was started. B: Patients treated with chemotherapy and ipilimumab, whose NAA titers decreased after treatment was started. C: Patients treated with chemotherapy, whose NAA titers increased after treatment was started. D: Patients treated with chemotherapy and ipilimumab, whose NAA titers increased after treatment was started.
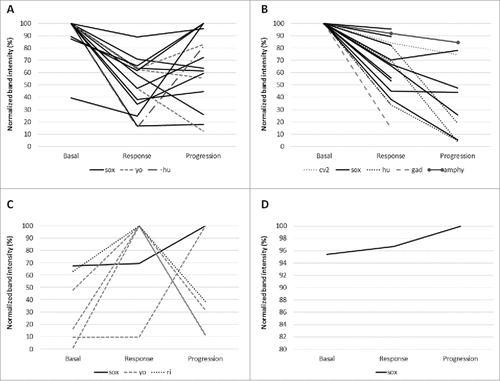
We then assessed the correlation between the pattern of change of NAAs and response to treatment. Of 45 patients with baseline NAA, 42 (93.3%) responded to treatment while 3 (6.7%) did not. At progression, 25 (59.5%) of previous treatment responders showed an ongoing decrease in NAA; all 3 primary non-responder patients showed an increase of titer of NAA (p = 0.080).
Next, we evaluated the correlation between survival and evolution of NAA's titers. In patients treated with chemotherapy alone, those whose NAAs increased from baseline to first response assessment (N: 6), showed a longer PFS [12 m (95% CI: 11.4-12.6)] compared to those showing a decrease in titers (N: 15) [6.9 m (95% CI: 6.1-7.6)] (p: 0.016). This difference was not observed in the chemoimmunotherapy cohort. Patients of both cohorts, whose NAA had decreased or remained stable at the end of the treatment (third time point) showed an OS of 18.5 m (95% CI: 15.8 – 21.2) and those whose NAA had increased showed an OS of 12.3 m (95% CI: 8.1 – 16.5; p: 0.049). When analyzed separately by treatment, this trend was present in the ipilimumab plus chemotherapy [18.5 m (95% CI: 9.1-27.8) vs 9.5 m (95% CI: 7.0-12.0); p: 0.143] and in the chemotherapy alone group [17.2 m (95% CI: 12.4-22.1) vs 12.6 m (95% CI: 8.3-17); p: 0.090].
Discussion
SCLC is an aggressive disease with poor outcome. Tumor-associated immunological events have been classically described in SCLC in the form of paraneoplastic syndromes, which clinically lead to neurological adverse events.Citation20 Autoantibodies directed to neuronal antigens abnormally expressed in the tumor are detected in patients with PNS and are used as a diagnostic tool in this setting.Citation21,Citation22 NAAs have been described to be immune effectors of neurological dysfunction in the case of membranous antigens (i.e. calcium channel antibodies).Citation23 Intracellular antigens may also be subject to a cytotoxic T-cell response, where the accompanying antibody production may not be per se pathogenic.Citation24-27 Notably, a proportion of patients have detectable NAAs in absence of neurological symptoms, although generally at lower titers. Dalmau et al. had previously reported a prevalence of around 16% of SCLC patients without neurological PNS who have detectable titers of anti-Hu.Citation28 Around 1% of positivity of anti-HuD was reported in patients diagnosed of PNS.Citation28,Citation29 In a more recent study, Gozzard et al. found 9.6% of SCLC patients without neurological PNS with positivity for anti-Hu, but when testing also for other NAAs (Sox2, HuD, Gad65, Amphyphisin, Ri, Cv2, Ma2 and Yo), 41% patients harbored at least one NAA.Citation30 Consistent with these observations and in line with previous evidence, 53% of our patients had detectable levels of at least one NAA and none had neurological symptoms.Citation22,Citation28-33 All but one patient with positive detection of anti-HuD, showed lower titers of NAAs in comparison with the patient with known anti-HuD related PNS (positive control) (data not shown).
Consistent with previously reported series,Citation22,Citation30 in the current study anti-Sox1 was the most prevalent tumor-associated NAA. Unexpectedly, anti-Yo was the second most prevalent NAA; although it is usually associated with ovarian and breast cancer, it has also been described in lung cancer.Citation34,Citation35 Moreover, anti-Gad65, usually linked to autoimmune diabetes,Citation36 was detected in 3 patients in C1 and in 2 patients in C2. None of these patients had a history of type 1 diabetes in our study. It could be argued that these detections could be due to technical issues with the immune-blot assay. However, this method has been used in previous studies and shows good performance in ‘screening’ settings such as our work.Citation37,Citation38 Furthermore, the fact of having serial samples for the same patient allowed us to use these as internal controls for the positive cases (Supplementary Fig. 1).
The presence of NAAs has been linked to early stages and in turn to better outcome.Citation28,Citation29,Citation32,Citation39 The concomitant cytotoxic T cell responseCitation27,Citation40-42 might contribute to a degree of tumor control, sparing the immune privileged nervous system.Citation43,Citation44 In the case of SCLC-associated PNS, the diagnosis of the neurological disease often antedates that of the tumor,Citation20,Citation45 suggesting that the immune system is able to detect the presence of SCLC before the cancer develops metastases.Citation39,Citation46 This concept is also supported in other studies where the presence of autoantibodies was independently associated with earlier stages.Citation29,Citation47 Accordingly, in our series we observed a trend towards an association of NAAs (specifically anti-Sox1) with earlier stage.
When we analyzed the presence of multiple against single NAA reactivities, we observed that 28% of patients had more than one reactivity. This is consistent with previous literature. In a large series of 60,000 subjects with suspicion of PNS, 553 (0.9%) were positive for any NAA Citation21; among these, 31% had more than 1 autoantibody. Intriguingly, in our series, patients with multiple reactivities had a significantly shorter PFS and OS when treated with the combination of chemotherapy and ipilimumab. The presence of coexisting autoantibodies reflects a multifaceted response to the different inmunogenic onconeural proteins expressed in tumors. The impact of multiple vs. single reactivities has not been reported previously. Although small numbers are included in this analysis, if multiple reactivities reflected heterogeneity within the tumor and consequently subclonal T cell responses, it has been described that checkpoint inhibitor treatment might be less effective in this scenario,Citation48 but this is hypothesis generating and requires validation.
We analyzed the patterns of change in NAA titers in patients treated with chemotherapy vs. those treated with chemoimmunotherapy. In most patients the NAA titers decreased after initiation of therapy. We note that the addition of ipilimumab to chemotherapy did not appear to affect this outcome; this is in contrast with boosting of humoral response to vaccination against infectious agents that has previously been reported Citation49 and also to the effect of ipilimumab on tumor-specific T cells that appear to be induced de novo.Citation50 Our data are in line with clinical observations in patients with cancer-associated PNS, where in treatment-responders antibodies can decrease or even normalize.Citation51,Citation52 In a series of 50 cancer patients with PCD and at least one NAA, Shams'ili et al. report resolution of PCD in 9/42 patients (21.4%) after successful tumor treatment.Citation53 In 5 neurological responder patients where the authors evaluated changes in NAA-titer no consistent pattern was observed. Additional immunosupressants for PNS may have also confounded the interpretation in this study. In patients with anti-Yo related PNS, antibody titers decreased after surgical removal of the tumor without resolution of the neurological symptoms.Citation54,Citation55 Overall NAA levels appear to decrease following removal of tumor antigen; the observation of worsening PNS with disease recurrenceCitation32,Citation56 and increase risk of relapse in HPV+ patients whose HPV antibodies persistCitation57 both support this conclusion. Whether levels of NAA are important clinically or simply ‘bystanders’ of cellular immune consequences of treatment is uncertain.
An intriguing question arises over the location of the antibody forming cells that drive the production of NAA. More widely in oncology, for example in patients with thymoma, improvement of paraneoplastic myasthenia is observed after, and is the clinical aim of surgical thymectomy. These data point to the production of antibody within the tumor microenvironment and there is indeed an emerging literature that identifies B-cells and tertiary lymphatic structures as common constituents in the cancer mass in a wide variety of solid tumors.Citation58-61 In contrast, persistence of the neurological paraneoplasia in some cases even after radiological complete removal of cancer by treatment both in thymomas and SCLC suggests that the antibody forming cells may not be located in the cancer tissue (only) in all cases. In line with this, one immunohistochemical study assessing the presence of T and B-cells in SCLC surgical specimens, demonstrated a small numbers of B-cells infiltrating the tissue with 40% of cases with absence of this cell subtype,Citation62 although this might change in extensive disease. Moreover, we cannot rule out here that the SCLC chemotherapy may have also removed B-cells that are producing the NAA irrespective of the effect on tumor load.
Consistent with the concept that removal of immunogen is linked with a decrease in antibody titer, we observed a trend to a longer OS in those patients whose titer of NAA decreased progressively after treatment with chemotherapy; this was irrespective of whether ipilimumab was administered.
Intriguingly however, our data also raise the possibility of a quite different immunological consequence: subgroup analysis of the small number of patients with increasing titers after initiation of chemotherapy identified a longer than median PFS; this raises the question of whether enhanced immune response due to intracellular antigen exposure after cytotoxic chemotherapy may lead to a better T-cell response and in turn to increased antibody production. A dataset in melanoma reported by the Wolchok group is consistent with this concept, as the presence or development of antibodies and T-cell responses to NyEso1 appeared to identify a group of patients, who did well after ipilimumab treatment.Citation63 We conclude that further detailed studies are needed, which link the humoral responses and cellular responses in cancer patients, both after standard treatment and immunotherapy.
In summary, we were able to confirm the prognostic role of NAA probably related with disease control in early stage derived from tumor directed immune response. To our knowledge, we demonstrate for the first time that treatment with ipilimumab combined with chemotherapy in SCLC does not induce differential changes in NAA titers and their levels appear to mainly reflect the disease burden. The predictive role of NAAs and B cell immune response warrants further investigation in SCLC.
Patients and methods
Study populations
We retrospectively analyzed the data and serum from 85 SCLC patients from two independent cohorts. The first cohort included 47 patients with SCLC treated with first-line standard chemotherapy (platinum – etoposide), recruited within research projects in Hospital del Mar, Barcelona, and previously reported.Citation64-66 We included patients with early and locally advanced disease as well as stage IV patients, in order to investigate the impact of stage in the detection of NAAs. From this series we had a control population, from age- and sex-matched healthy donors (N: 30) to the study population. The second cohort were patients included in the ICE trial,Citation18 where 38 chemo-naïve stage IV SCLC patients were treated with ipilimumab in combination with carboplatin-etoposide. Serum samples at baseline and subsequent time points (before, during and after treatment) were collected from each patient. Complete clinical data and follow up information was available for every patient.
Sample analysis was approved for the cohort 1 by the local ethics committee and for cohort 2 within the trial ethics committee.
Sampling
Serum samples were collected for C1 before treatment initiation (baseline), at first response assessment and at progression. For C2, samples were collected at baseline, at 3 and 6 months when feasible. All patients in C1 had 3 serial serum samples (baseline, first response assessment and progression timepoints). Out of the 38 patients in C2, 20 had 2, 11 had 3, and 6 had 4 serial timepoints (all included baseline). One patient in C2 had no available serum samples. Serum from 3 healthy volunteers was used as negative controls and serum from a SCLC patient with a known anti-HuD associated PNS as a positive control.
Whole blood samples were collected by standard venipuncture techniques using serum separator tubes. Samples were allowed to clot for 30 minutes at room temperature before centrifugation for 10 minutes at 1000 g at 4ºC. Following centrifugation, the supernatant (serum) was immediately removed and assayed immediately or aliquoted and stored frozen at −80ºC until further use.
Assessment of autoantibodies
A commercial immunoblotting assay (Ravo PNS-Blot, Ravo Diagnostika) consisting of a membrane strip coated with the recombinant intracellular neuronal antigens Gad65, Sox1, Ma1, Ma2, Amphiphysin, CRMP5, Ri, Yo and HuD was used. Patient and control sera were diluted 1:200 as established in the current version of the instructions for use (01/2017) and processed following manufacturer's protocol.
Incubated line blots were scanned and processed for quantification with ImageJ 1.51 h (NIH). A distinctive band corresponding to the actual position of the antigen in the line blot was considered as a positive signal. Background was subtracted from band intensity. This value was normalized with the positive control and the percentage of variation between each time-point was calculated. A percentage of variation of more than 5% was considered as change (increase/decrease) of titer of NAA. Prism 6.0 (GraphPad) was used for plot construction.
Statistical analysis
Statistical analysis was carried out using SPSS 22.0 (SPSS Inc, Chicago, IL, USA) and Stata/MP 14 (StataCorp LLC, Texas, USA) together with the Statistical Assessment Service from IMIM (Hospital del Mar). To analyze associations between categorical variables we used the Chi-square test or the Fisher's exact test as appropriate. Overall survival was plotted by Kaplan–Meier method and curves were compared by the log-rank test. Multivariate analysis was performed with the Cox regression method. All tests were conducted at the two-sided 0.05 level of significance.
Abbreviations
| CTLA-4 | = | Cytotoxic T-lymphocyte antigen 4 |
| EDTA | = | Ethylenediaminetetraacetic acid |
| ICE | = | Ipilimumab-Carboplatin-Etoposide |
| NAA | = | Neuronal auto-antibody |
| ORR | = | Objective response rate |
| OS | = | Overall survival |
| PCD | = | Paraneoplastic cerebellar degeneration |
| PD-1 | = | Programmed cell death protein 1 |
| PFS | = | Progression free survival |
| PNS | = | Paraneoplastic neurological syndrome |
| pRB | = | Retinoblastoma protein |
| PS | = | Performance status |
| SCLC | = | Small cell lung cancer |
Funding details
This work was supported in part by a grant from (1) Fundació La Marató de TV3 (666/C/2013); (2) ISCiii/FEDER (CIBERONC CB16/12/00241, RD12/0036/0051, PIE15/00008, PI15/00146, PI16/00591, PI13/00140); (3) Xarxa de Bancs de Tumors (XBTC); (4) Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR), Generalitat de Catalunya (2014SGR740), (5) Fundació Cellex and (6) Cancer Research UK grant: C491/A12135
supp_data.zip
Download Zip (261.5 KB)Disclosure statement
E.A. and C.O. have received honoraria for consultancy and lectures from Bristol-Myers Squibb outside of the current work. The rest of authors report no conflict of interest.
References
- Oze I, Hotta K, Kiura K, Ochi N, Takigawa N, Fujiwara Y, Tabata M, Tanimoto M. Twenty-seven years of phase III trials for patients with extensive disease small-cell lung cancer: disappointing results. PLoS One. 2009;4:e7835. doi:10.1371/journal.pone.0007835. PMID:19915681.
- Byers LA, Rudin CM. Small cell lung cancer: Where do we go from here? Cancer. 2014;121:664–72.
- Hanna N, Bunn PA, Langer C, Einhorn L, Guthrie T, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43.
- Puglisi M, Dolly S, Faria A, Myerson JS, Popat S, O'Brien MER. Treatment options for small cell lung cancer – do we have more choice? Br J Cancer. 2010;102:629–38. doi:10.1038/sj.bjc.6605527.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi:10.1056/NEJMoa1003466.
- Brahmer J, Reckamp K, Baas P, Crinò L, Eberhardt W, Poddubskaya E, Antonia S, Pluzanski A, Vokes E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. doi:10.1056/NEJMoa1504627.
- Darnell RB, Posner JB. Paraneoplastic syndromes and the nervous system. N Engl J Med. 2003;3:287–8.
- Maddison P, Newsom-Davis J, Mills K, Souhami R. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet. 1999;353:117–8. doi:10.1016/S0140-6736(05)76153-5. PMID:10209997.
- Wirtz PW, Lang B, Graus F, Van Den Maagdenberg AMJM, Saiz A, De Koning Gans PA, Twijnstra A, Verschuuren JJGM. P/Q-type calcium channel antibodies, Lambert-Eaton myasthenic syndrome and survival in small cell lung cancer. J Neuroimmunol. 2005;164:161–5. doi:10.1016/j.jneuroim.2005.04.001.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi:10.1126/science.aaa1348. PMID:26068851.
- Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, Yung R, Parmigiani G, Dorsch M, Peacock CD, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–73. doi:10.1158/0008-5472.CAN-08-4210.
- Miller CW, Simon K, Aslo A, Kok K, Yokota J, Buys CHCM, Terada M, Koeffler HP. p53 Mutations in Human Lung Tumors. Cancer Res. 1992;143:1695–8.
- Ku GY, Yuan J, Page DB, Schroeder SEA, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi:10.1002/cncr.24951.
- Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi. F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensivedisease-small-cell lungcancer: Results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi:10.1093/annonc/mds213.
- Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34:3740–8. doi:10.1200/JCO.2016.67.6601.
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi:10.1016/S1470-2045(16)30098-5.
- Ott PA, Elez E, Hiret S, Kim D-W, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;:JCO. 2017.72.506.
- Arriola E, Wheater M, Galea I, Cross N, Maishman T, Hamid D, Stanton L, Cave J, Geldart T, Mulatero C, et al. Outcome and Biomarker Analysis from a Multicenter Phase 2 Study of Ipilimumab in Combination with Carboplatin and Etoposide as First-Line Therapy for Extensive-Stage SCLC. J Thorac Oncol. 2016;11:1511–21. doi:10.1016/j.jtho.2016.05.028.
- Travis WD. Pathology and Diagnosis of Neuroendocrine Tumors: Lung Neuroendocrine. Thorac Surg Clin. 2014;24:257–66. doi:10.1016/j.thorsurg.2014.04.001.
- Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33:270–98. doi:10.1053/j.seminoncol.2006.03.008.
- Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56:715–9. doi:10.1002/ana.20269.
- Titulaer MJ, Klooster R, Potman M, Sabater L, Graus F, Hegeman IM, Thijssen PE, Wirtz PW, Twijnstra A, Smitt PAES, et al. SOX antibodies in small-cell lung cancer and Lambert-Eaton myasthenic syndrome: frequency and relation with survival. J Clin Oncol. 2009;27:4260–7. doi:10.1200/JCO.2008.20.6169.
- Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Pnas. 1983;80:7636–40. doi:10.1073/pnas.80.24.7636.
- Bach JF, Koutouzov S, Van Endert PM. Are there unique autoantigens triggering autoimmune diseases? Immunol Rev. 1998;164:139–55. doi:10.1111/j.1600-065X.1998.tb01216.x.
- Kai W. Wucherpfennig. Auroimmunity in the Central Nervous System: Mechanisms of Antigen Presentation and Recognition. Clin. Immunol. Immunopathol. 1994;72:293–306.
- De Beukelaar JW, Smitt PAS, Hop WC, Kraan J, Hooijkaas H, Verjans GMGM, Gratama JW. Imbalances in circulating lymphocyte subsets in Hu antibody associated paraneoplastic neurological syndromes. Eur J Neurol. 2007;14:1383–91. doi:10.1111/j.1468-1331.2007.01986.x.
- Albert ML, Darnell JC, Bender a, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–4. doi:10.1038/3315.
- Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer–a quantitative western blot analysis. Ann Neurol. 1990;27:544–52. doi:10.1002/ana.410270515.
- Graus F, Dalmou J, Reñé R, Tora M, Malats N, Verschuuren JJ, Cardenal F, Viñolas N, Garcia del Muro J, Vadell C, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi:10.1200/JCO.1997.15.8.2866.
- Gozzard P, Woodhall M, Chapman C, Nibber A, Waters P, Vincent A, Lang B, Maddison P. Paraneoplastic neurologic disorders in small cell lung carcinoma: A prospective study. Neurology. 2015;85:235–9. doi:10.1212/WNL.0000000000001721.
- Sodeyama N, Ishida K, Jaeckle KA, Zhang L, Azuma A, Yamada M, Mizusawa H, Wada Y. Pattern of epitopic reactivity of the anti-Hu antibody on HuD with and without paraneoplastic syndrome. J Neurol Neurosurg Psychiatry. 1999;66:97–9. doi:10.1136/jnnp.66.1.97.
- Atakan S, Bayiz H, Sak S, Poyraz A, Vural B, Yildirim AS, Demirag F, Gure AO. Autologous anti-SOX2 antibody responses reflect intensity but not frequency of antigen expression in small cell lung cancer. BMC Clin Pathol. 2014;14:24. doi:10.1186/1472-6890-14-24. PMID:24940116.
- Tsou JA, Kazarian M, Ankur Patel M, Janice S., Galler M., Laird-Offringa A, Carpenter CL, J. SL. Low level anti-Hu reactivity: a risk marker for small cell lung cancer? Cancer Detect Prev. 2010;32:292–9. doi:10.1016/j.cdp.2008.06.006.
- Hasadsri L, Lee J, Wang BH, Yekkirala L, Wang M. Anti-yo associated paraneoplastic cerebellar degeneration in a man with large cell cancer of the lung. Case Rep Neurol Med. 2013;2013:725936. PMID:24167748.
- Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42:1931–7.
- Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem. 2011;57:168–75. doi:10.1373/clinchem.2010.148205.
- Gozzard P, Chapman C, Vincent A, Lang B, Maddison P. Novel Humoral Prognostic Markers in Small- Cell Lung Carcinoma : A Prospective Study. 2015;430:1–14.
- Berger B, Bischler P, Dersch R, Hottenrott T, Rauer S, Stich O. “Non-classical” paraneoplastic neurological syndromes associated with well-characterized antineuronal antibodies as compared to “classical” syndromes – More frequent than expected. J Neurol Sci. 2015;352:58–61. doi:10.1016/j.jns.2015.03.027.
- Verschuuren JJ, Perquin M, ten Velde G, De Baets M, Vriesman PB, Twijnstra A. Anti-Hu antibody titre and brain metastases before and after treatment for small cell lung cancer. J Neurol Neurosurg Psychiatry. 1999;67:353–7. doi:10.1136/jnnp.67.3.353.
- Carpentier AF, Rosenfeld MR, Delattre JY, Whalen RG, Posner JB, Dalmau J. DNA vaccination with HuD inhibits growth of a neuroblastoma in mice. Clin Cancer Res. 1998;4:2819–24.
- Musunuru K, Darnell RB. Paraneoplastic neurologic disease antigens: RNA-Binding Proteins and Signaling Proteins in Neuronal Degeneration. Annu Rev Neurosci. 2001;24:239–62. doi:10.1146/annurev.neuro.24.1.239.
- Tanaka M, Maruyama Y, Sugie M, Motizuki H, Kamakura K, Tanaka K. Cytotoxic T cell activity against peptides of Hu protein in anti-Hu syndrome. J Neurol Sci. 2002;201:9–12. doi:10.1016/S0022-510X(02)00157-0.
- Dalmau J, Graus F, Cheung N-K V., Rosenblum MK, Ho A, Cañete A, Delattre J-Y, Thompson SJ, Posner JB. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75:99–109. doi:10.1002/1097-0142(19950101)75.:1%3c99::AID-CNCR2820750117%3e3.0.CO;2-I.
- DeLuca I, Blachère NE, Santomasso B, Darnell RB. Tolerance to the neuron-specific paraneoplastic HuD antigen. PLoS One. 2009;4:e5739. doi:10.1371/journal.pone.0005739. PMID:19492067.
- Posner JB, Dalmau J. Paraneoplastic syndromes. Curr Opin Immunol. 1997;9:723–9. doi:10.1016/S0952-7915(97)80055-6.
- Winter SF, Sekido Y, Minna JD, McIntire D, Johnson BE, Gazdar AF, Carbone DP. Antibodies against autologous tumor cell proteins in patients with small-cell lung cancer: association with improved survival. J Natl Cancer Inst. 1993;85:2012–8. doi:10.1093/jnci/85.24.2012.
- Maddison P, Thorpe A, Silcocks P, Robertson JFR, Chapman CJ. Autoimmunity to SOX2, clinical phenotype and survival in patients with small-cell lung cancer. Lung Cancer. 2010;70:335–9. doi:10.1016/j.lungcan.2010.03.002.
- McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi:10.1126/science.aaf1490.
- Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab Increases Activated T Cells and Enhances Humoral Immunity in Patients With Advanced Melanoma. J Immunother. 2012;35:89–97. doi:10.1097/CJI.0b013e31823aa41c.
- Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJP, van der Burg S, Kapiteijn E, Michielin O, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi:10.1126/scitranslmed.3008918. PMID:25232180.
- Morita M, Fukuhara T, Takahashi H, Maemondo M. Small cell lung cancer and progressive retinopathy. BMJ Case Rep. 2014;2014:2–5. doi:10.1136/bcr-2014-205888.
- Hiasa Y, Kunishige M, Mitsui T, Kondo S, Kuriwaka R, Shigekiyo S, Kanematsu T, Satake N, Bando Y, Kondo A, et al. Complicated paraneoplastic neurological syndromes: A report of two patients with small cell or non-small cell lung cancer. Clin Neurol Neurosurg. 2003;106:47–9. doi:10.1016/S0303-8467(03)00059-3.
- Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, van der Holt B, Vecht C, Sillevis Smitt P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409–18. doi:10.1093/brain/awg133.
- Greenlee JE, Dalmau J, Lyons T, Clawson S, Smith RH, Pirch HR. Association of anti-Yo (type I) antibody with paraneoplastic cerebellar degeneration in the setting of transitional cell carcinoma of the bladder: detection of Yo antigen in tumor tissue and fall in antibody titers following tumor removal. Ann Neurol. 1999;45:805–9. doi:10.1002/1531-8249(199906)45.:6%3c805::AID-ANA18%3e3.0.CO;2-G.
- Selby KJ, Warner J, Klempner S, Konstantinopoulos PA, Hecht JL, Ph D. Case Report Anti-Yo Antibody Associated With Occult Fallopian Tube Carcinoma. 2011;:536–8.
- Nagashima T, Mizutani Y, Kawahara H, Maguchi S, Terayama Y, Shinohara T, Orba Y, Chuma T, Mano Y, Itoh T, et al. Anti-Hu paraneoplastic syndrome presenting with brainstem-cerebellar symptoms and Lambert–Eaton myasthenic syndrome. Neuropathology. 2003;23:230–8. doi:10.1046/j.1440-1789.2003.00501.x.
- Hanna GJ, Sridharan V, Margalit DN, La Follette SK, Chau NG., Rabinowits G, Lorch JH, Haddad RI, Tishler RB, Anderson KS, et al. Salivary and serum HPV antibody levels before and after definitive treatment in patients with oropharyngeal squamous cell carcinoma. Cancer Biomark. 2017;19:129–36. doi:10.3233/CBM-160071.
- Wood O, Woo J, Seumois G, Savelyeva N, McCann KJ, Singh D, Jones T, Peel L, Breen MS, Ward M, et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget. 2016;7:56781–97. doi:10.18632/oncotarget.10788.
- Pagès F, Galon J, Dieu-Nosjean M-C, Tartour E, Sautès-Fridman C, Fridman W-H. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi:10.1038/onc.2009.416.
- Fridman WH, Galon J, Dieu-Nosjean M-C, Cremer I, Fisson S, Damotte D, Pagès F, Tartour E, Sautès-Fridman C. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1–24.
- Poschke I, Faryna M, Bergmann F, Flossdorf M, Lauenstein C, Hermes J, Hinz U, Hank T, Ehrenberg R, Volkmar M, et al. Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:e1240859. doi:10.1080/2162402X.2016.1240859. PMID:28123878.
- Eerola AK, Soini Y, Pääkkö P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6:1875–81.
- Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Pnas. 2011;108. doi:10.1073/pnas.1110814108.
- Cañadas I, Taus Á, Villanueva X, Arpí O, Pijuan L, Rodríguez Y, Menéndez S, Mojal S, Rojo F, Albanell J, et al. Angiopoietin-2 is a negative prognostic marker in small cell lung cancer. Lung Cancer. 2015;90:302–6. doi:10.1016/j.lungcan.2015.09.023.
- Cañadas I, Taus A, González I, Villanueva X, Gimeno J, Pijuan L, Dómine M, Sánchez-Font A, Vollmer I, Menéndez S, et al. High circulating hepatocyte growth factor levels associate with epithelial to mesenchymal transition and poor outcome in small cell lung cancer patients. Oncotarget. 2014;5:5246–56. doi:10.18632/oncotarget.2124.
- Cañadas I, Rojo F, Taus Á, Arpí O, Arumí-Uría M, Pijuan L, Menéndez S, Zazo S, Dómine M, Salido M, et al. Targeting epithelial-to-mesenchymal transition with Met inhibitors reverts chemoresistance in small cell lung cancer. Clin Cancer Res. 2014;20:938–50. doi:10.1158/1078-0432.CCR-13-1330.
