ABSTRACT
Programmed cell death protein 1 (PD-1) immune checkpoint inhibitors have shown activity in patients with advanced renal cell carcinoma (RCC). However, the role of PD-1 expression in tumor-infiltrating lymphocytes (TILs) as a biomarker for poor outcome is not clear. In this study, we evaluated the prognostic value of TIL PD-1 expression in patients with clear cell RCC (ccRCC). 82 patients who underwent nephrectomy for localized or metastatic ccRCC and followed up for at least four years were searched from our database and retrospectively enrolled. Their fixed primary tumor specimens were stained with anti-PD-1 (NAT105). The specimens were classified as negative or positive for PD-1 expression, and the positive specimens were further scored in 10% increments. 37 (45.12%) patients were negative (<1% stained), 26 (31.71%) patients were low (<10 and 10%), and 19 (23.17%) patients were high (20–50%) for PD-1 expression. The prognostic value of TIL PD-1 expression was evaluated by univariate Cox proportional hazards regression on overall and recurrence-free survivals. Higher TIL PD-1 expression was not associated with increased risk of death (P = 0.336) or with increased risk of recurrence (P = 0.572). Higher primary tumor stage was associated with increased risk of recurrence (P = 0.003), and higher Fuhrman nuclear grade was associated with increased risk of death (P <0.001) and with increased risk of recurrence (P <0.001). Our study shows that TIL PD-1 expression by immunohistochemistry (IHC) does not correlate with poor clinical outcome in patients with ccRCC and is inferior to established prognosticating tools.
Introduction
Renal cell carcinoma (RCC) presents as regional or metastatic disease in a third of patients at the time of diagnosis.Citation1 While those with clinically localized disease are surgically treated, the recurrence rate is 20–40%.Citation2 In sum, approximately half of all RCC patients are eligible for systemic treatment at some point of the disease course. Immunotherapy is a promising treatment modality that bolsters host anti-tumor response and may accomplish durable remission. For example, high-dose interleukin-2 (IL-2) is the only agent to have demonstrated durable remission in RCC to date, albeit largely displaced by angiogenesis and mammalian target of rapamycin (mTOR) pathway inhibitors due to its modest efficacy and high toxicity.Citation3
RCC is a promising candidate for immunotherapy due to its immunogenic nature. It is characterized by dense intra-tumor immune infiltrate that is associated with prognosis.Citation4-8 Much research interest exists on these tumor-infiltrating immune cells and their altered phenotype—reduced proliferative capacity and effector function—as therapeutic target. This dysfunctional state of T cells, termed ‘exhaustion’ and reviewed extensively elsewhere, arises from chronic antigen exposure in the setting of chronic viral infection or cancer and has been proposed as a mechanism of immune evasion in RCC.Citation9-13
Exhausted T-cells characteristically overexpress inhibitory co-receptors such as programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).Citation9,Citation14 The latest developments in cancer immunotherapy modulate these inhibitory pathways to reverse T cell exhaustion and reinvigorate immune response.Citation10,Citation11 The best known example is an anti-PD-1 antibody nivolumab that was approved in 2015 for RCC treatment on the basis of a phase III trial that showed improved survival, higher response rate, and less frequent adverse events compared to an mTOR inhibitor everolimus.Citation15,Citation16 An anti-CTLA-4 antibody ipilimumab is also being evaluated as an adjunct therapy with nivolumab in a phase III trial at the time of writing [NCT02231749].
While nivolumab unequivocally demonstrated its efficacy in clinical trials, there is a need for a better tool for patient selection. This is particularly important given modest objective response rates; 1% of the Phase III trial subjects showed complete response and 25% partial response,Citation16 similar to figures reported with high-dose IL-2 treatment.Citation3 This suggests that both IL-2 and nivolumab may be highly effective only in a subset of ‘immune-responsive’ patients who we cannot yet reliably identify.Citation17 Furthermore, its benefit in patients who show any less than complete response remains to be seen, extrapolating from the precedent high-dose IL-2 study in which all partial-responders eventually demonstrated recurrence in contrast to 17% of complete-responders.Citation3 Long-term benefit of nivolumab treatment is under active research.Citation18
A number of studies have associated PD-1 and Programmed death-ligand 1 (PD-L1) expression on immunohistochemistry (IHC) in RCC with clinical outcomeCitation19-30 and/or response to immunotherapeutic agent with conflicting results.Citation16,Citation19-32 While earlier studies reported a significant association between PD-1/PD-L1 expression and survival or recurrence, later studies did not replicate those findings. In addition to prognostication, no study has demonstrated correlation between PD-L1 expression and treatment benefit, highlighting the need to elucidate the pathophysiological role of PD-1 and PD-L1.
In this study, we aimed to add to the current knowledge on prognostic value of tumor-infiltrating lymphocyte (TIL) PD-1 expression by evaluating its impact on overall survival (OS) and recurrence-free survival (RFS) in a single-center cohort with long-term clinical follow-up. We concurrently evaluated association between primary tumor stage (pT stage), Fuhrman nuclear grade (FNG)—two widely used prognostic tools—and clinical outcome to compare their prognostic value with that of TIL PD-1 expression.
Methods
Patient selection
Upon approval by Emory University Institutional Review Board, we reviewed the Emory Nephrectomy Registry to identify patients who underwent nephrectomy for treatment of clear cell renal cell carcinoma between 2005 and 2013. The enrolled patients had made full informed consent that permitted storage and analysis of surgically removed organ specimens and blood as well as access to medical records by authorized researchers. Enrollment into this study and procurement of the samples did not incur any risk to the patient beyond that of the actual surgery done as standard of care, and the results of the study did not affect the patient's medical care in any way. Only patients over age of 18 at the time of surgery were reviewed. Inclusion in the study was limited to patients with 4 years of post-operative follow-up unless they had documented death during that period. 82 patients were identified who met the inclusion criteria and had archived formalin-fixed paraffin-embedded tumor tissue blocks available for study. Their primary tumor tissue blocks were retrieved from our nephrectomy tissue library irrespective of presence or absence of nodal disease or distant metastasis at the time of surgery. Medical records of the patients were accessed on November 2016 to obtain clinical information such as age at intervention, sex, and race as well as tumor pathology.
Tumor pathology and clinical follow-up information
Primary tumor stage and Fuhrman nuclear grade of the specimens obtained from anatomic pathology reports were re-evaluated and confirmed. Baseline presence or absence of metastatic disease was determined from pre-operative clinic notes and cross-sectional imaging studies (MRI or IV-contrasted CT) of the chest, abdomen, and pelvis. Serial post-operative clinic notes and radiology reports were accessed to document disease course. Standard of care post-operative surveillance at our institution consisted of symptom review and cross-sectional imaging studies every 3 months for the first year, spaced out to every 6 months until 5 years after the surgery. Patients without evidence of disease after 5 years were evaluated by renal ultrasound annually. Patient vital status was obtained from chart review, and if not documented in our institution's medical records, from Social Security Death Index. Patients alive at the time of chart review were censored on the date of the most recent presentation to our institution or documented communication by phone. Date of recurrence was defined as the date of the first imaging study that showed tumor growth at the surgical site or at a distant site. Imaging findings that were concerning but not certain to be recurred RCC were ascertained from biopsy reports. Patients with no evidence of recurrence throughout surveillance were censored on the date of the most recent imaging.
Immunohistochemistry and PD-1 quantification
Tumor sections were de-paraffinized and prepared into slides using standard immunohistochemistry techniques. Two slides were made per each tissue sample. One slide was stained with hematoxylin and eosin, and the other slide was stained for PD-1 using a commercially available andi-PD-1 antibody NAT105 (Abcam) at 1:100 dilution. The H&E sections of the tumors were reviewed for RCC subtype, primary tumor stage, and Fuhrman nuclear grade. The specimens were classified as PD-1-negative (<1% stained) or positive (≥ 1% stained), and the positive specimens were further scored in 10% increments. The pathologist was blinded to clinical outcome.
Statistical methods
ANOVA and χ2 tests were performed to assess univariate association between TIL PD-1 expression and clinical and pathological features (age at intervention, sex, race, pT stage, FNG, and baseline metastasis). Kaplan-Meier survival analysis and Cox proportional hazards regression were conducted to evaluate univariate impact of TIL PD-1 expression on overall survival and recurrence-free survival. The overall survival analysis (death from any cause) included the entire cohort, while the recurrence-free survival analysis included only the patients without evidence of metastatic disease at the time of surgery and with documented post-operative radiologic surveillance. For the survival analyses, PD-1 expression was stratified in two different ways: 2-way (negative/positive) and 3-way (negative/low/high). In the 2-way analysis, the tumor was considered negative if less than 1% of the infiltrates stained for PD-1 and positive if 1% or more stained. In the 3-way analysis, the tumor was considered negative if less than 1% of the infiltrates stained, low if between 1% and 10% stained, and high if 20% or more stained. Finally, univariate Cox proportional hazards regression was performed between pathological features (pT stage and FNG) and survival to compare their prognostic power to that of TIL PD-1 expression. Statistical significance was set at two-sided P < 0.05. All statistical analyses were performed using SAS 9.4.
Results
Cohort description
82 clear cell RCC patients who met the inclusion criteria were enrolled. Patient characteristics are summarized in . Median (range) age at intervention was 60 (26–89), and overall median (IQR) follow-up was 70.7 (48.2–83.8) months. 30 patients had died by the time of chart review with median (IQR) follow-up of 40.6 (16.9–66.3) months. 52 surviving patients had median (IQR) follow-up of 74.3 (63.0–91.8) months. Distribution of pT stage from pT1 to pT4 in ascending order was 30, 19, 32, and 1. The single pT4 patient was negative for PD-1 expression and was merged with the pT3 group for ease of analysis. Distribution of FNG from 2 to 4 was 33, 41, and 8. No patient in our cohort was classified as FNG 1. 13 patients had metastatic disease at the time of surgery, and 69 patients had clinically localized disease. Of the 69 patients with localized disease, 67 patients had radiographic surveillance information available for review and were analyzed for recurrence-free survival. 28 patients had recurred by the time of chart review with median (IQR) follow-up of 12.4 (5.9–23.7) months. 39 recurrence-free patients had median (IQR) follow-up of 70.9 (59.4–84.7) months.
Table 1. Patient characteristics and tumor pathology by TIL PD-1 positivity (N = 82).
TIL PD-1 expression
Representative photomicrographs of clear cell RCC tumor-infiltrating lymphocytes stained for PD-1 are shown in . The distribution of TIL PD-1 expression of the overall survival cohort (N = 82), measured in 10% increments, is shown in . By the 2-way stratification, 37 (45.12%) patients were negative and 45 (54.88%) patients were positive for PD-1 expression. Most covariates (age at surgery, race, pT stage, FNG, and presence of baseline metastasis) were not significantly associated with TIL PD-1 expression by ANOVA and χ2 tests except for patient sex (). By the 3-way stratification, 37 (45.12%) patients were negative, 26 (31.71%) patients were low, and 19 (23.17%) patients were high for PD-1 expression. The distribution of TIL PD-1 expression of the recurrence-free survival cohort (N = 67) is shown in . By the 2-way stratification, 32 (47.76%) patients were negative and 35 (52.24%) patients were positive. By the 3-way stratification, 32 (47.76%) patients were negative, 21 (31.34%) patients were low, and 14 (20.90%) patients were high for PD-1 expression. The specimens from female patients were likely to express less PD-1 than those from male patients in 3-way stratification (χ2 P = 0.018): they expressed 20, 9, and 3 in negative, low, and high PD-1 levels, in comparison to 17, 17, and 16 in those from male patients, respectively.
Figure 1. Photomicrograph (20x magnified) of lymphocytes infiltrating clear cell RCC tumor, stained for PD-1. 1 A (left): H&E stained primary clear cell renal cell carcinoma. 1B (right): PD-1 stained primary clear cell renal cell carcinoma. TILs expressing PD-1 are stained brown on cell membrane. The estimated value of PD-1 positivity of this slide is 40%. Black arrow: PD-1 positive lymphocyte; Red arrow: PD-1 negative RCC tumor cell.
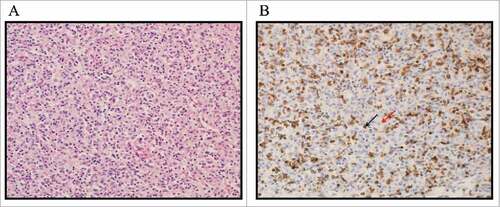
Table 2. Distribution of TIL PD-1 expression and its impact on risk of death.
Table 3. Distribution of TIL PD-1 expression and its impact on risk of recurrence.
Since PD-1 is known to be expressed in macrophagesCitation36 as well as in TILs, a separate experiment was done to verify that the PD-1 expression we scored was contributed by mostly TILs and not macrophages. Three tissue slides were cut from 19 randomly selected primary ccRCC samples. One set was stained with H&E, the other with CD68, an immune marker of macrophages, and the last with PD-1. All 19 samples demonstrated scattered patterns of CD68. 17 samples stained positive for PD-1, and 2 were negative. Comparing the areas of heavy PD-1 positivity with the same areas on the CD68 stained slide showed that the staining pattern differed between the two stains, showing that lymphocytes, not macrophages, are preferentially stained by PD-1 (data not shown).
Impact of TIL PD-1 expression on overall survival
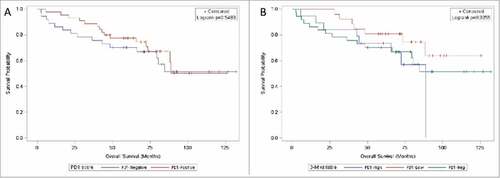
Univariate Cox proportional hazards regression on overall survival (N = 82) was performed by TIL PD-1 expression level (). In the 2-way analysis, PD-1-positive patients did not have a significantly increased risk of death compared to PD-1-negative patients (HR = 1.25; 95% CI 0.61–2.55; HR P-value = 0.548). Subdivision of the PD-1-positive patients into PD-1-low and PD-1-high patients did not reveal a significant association between survival and TIL PD-1 expression on any level: PD-1-low (HR = 0.58; 95% CI 0.24–1.43; HR P-value = 0.241), PD-1-high (HR = 1.21; 95% CI 0.51–2.89; HR P-value = 0.669), and overall (log-rank P = 0.336). Kaplan-Meier survival curves by PD-1 expression levels are shown in .
Figure 2. Kaplan-Meier curves on overall survival by TIL PD-1 expression. 2 A (left): TIL PD-1 expression stratified negative (<1% stained, blue) / positive (≥ 1% stained, red). 2B (right): TIL PD-1 expression stratified negative (<1% stained, green) / low (<10 and 10%, red) / high (20–50%, blue). Vertical tick marks represent censored subjects.
Impact of TIL PD-1 expression on recurrence-free survival
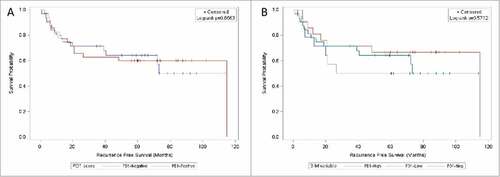
Univariate Cox proportional hazards regression on recurrence-free survival (N = 67) was performed by TIL PD-1 expression level (). In the 2-way analysis, PD-1-positive patients did not have an increased risk of recurrence compared to PD-1-negative patients (HR = 1.07; 95% CI 0.5–2.27; HR P-value = 0.866). Subdivision of the PD-1-positive patients into PD-1-low and PD-1-high patients did not result in a significant association between recurrence and TIL PD-1 expression on any level: PD-1-low (HR = 0.74; 95% CI 0.29–1.85; HR P-value = 0.512), PD-1-high (HR = 1.29; 95% CI 0.51–3.24; HR P-value = 0.588), and overall (log-rank P = 0.572). Kaplan-Meier analysis on recurrence-free survival by PD-1 expression levels are shown in .
Figure 3. Kaplan-Meier curves on recurrence-free survival by TIL PD-1 expression. 3 A (left): TIL PD-1 expression stratified negative (<1% stained, blue) / positive (≥ 1% stained, red). 3B (right): TIL PD-1 expression stratified negative (<1% stained, green) / low (<10 and 10%, red) / high (20–50%, blue). Vertical tick marks represent censored subjects.
Impact of primary tumor stage and Fuhrman nuclear grade on clinical outcome
Univariate Cox proportional hazards regression on overall survival (N = 82) and recurrence-free survival (N = 67) was performed by pT stage and FNG (). Higher pT stage trended with increased risk of death (N = 82): pT3–4 had a 2.28 times increased risk over pT1 (95% CI 0.98–5.3; HR P-value = 0.055; overall log-rank P = 0.053). Higher pT stage significantly correlated with risk of recurrence (N = 67): pT3–4 had 3.84 times increased risk over pT1 (95% CI 1.56–9.47; HR P-value = 0.003; overall log-rank P = 0.003). The correlation between Fuhrman nuclear grade and clinical outcome was more pronounced. Higher FNG significantly correlated with increased risk of death (N = 82) on all grades: FNG 3 (HR over FNG 2 = 3.75; 95% CI 1.4–10.04; HR P-value = 0.009), FNG 4 (HR over FNG 2 = 10.5; 95% CI 3.17–34.79; HR P-value <0.001), and overall (log-rank P <0.001). Similarly, Higher FNG significantly correlated with increased risk of recurrence (N = 67) on all grades: FNG 3 (HR over FNG 2 = 3.99; 95% CI 1.47–10.83; HR P-value = 0.007), FNG 4 (HR over FNG 2 = 16.2; 95% CI 4.58–57.28; HR P-value <0.001), and overall (log-rank P <0.001).
Table 4. Distribution of pT stage and FNG and their impact on risk of death and risk of recurrence.
Discussion
Programmed cell death protein 1 was first identified in 1992 as a transmembrane receptor that induces cell death in murine T-cell hybridoma and lymphoid/myeloid progenitor cell lines.Citation33 PD-1 receptor is expressed in multiple immunocytes such as thymocytes undergoing positive selection, mature T and B cells following activation, and macrophages.Citation34-36 PD-1 pathway is crucial for immune homeostasis and prevention of autoimmunity; PD-1 deficient mice develop various immunopathologies such as glomerulonephritis, arthritis, and cardiomyopathy.Citation37-39 In contrast, high PD-1 expression in T cells has been associated with good clinical outcome in autoimmune and inflammatory diseases and poor outcome/response to therapy in chronic viral infection and vaccination.Citation40
The observation that PD-L1 is expressed in a broad range of human cancers led to a hypothesis that it contributes to tumor immune evasion from tumor-infiltrating lymphocytes.Citation41,Citation42 The hypothesis has been subsequently tested in various cell-lines and murine cancer models and resulted in multiple immune checkpoint inhibitors being developed.Citation11,Citation43 PD-1 blockade in particular has produced positive clinical results in treatment of advanced melanoma, non-small cell lung carcinoma, renal cell carcinoma, and metastatic bladder cancer.Citation11 However, response rate and treatment benefit have been modest. The role of PD-1 and PD-L1 expression as a determinant of responsiveness to therapy or prognostic marker is not established across different cancers, including renal cell carcinoma.
In this study, we evaluated the association between tumor-infiltrating lymphocyte PD-1 expression and clinical outcome in 82 clear cell renal cell carcinoma patients with long clinical follow-up (>4 years). We did not find TIL PD-1 expression on immunohistochemistry to correlate with risk of death or recurrence, whether stratified in a binary (negative/positive) or in a semi-quantitative (negative/low/high) manner. However, we did find primary tumor stage and Fuhrman nuclear grade to correlate with clinical outcome; primary tumor stage significantly correlated with risk of recurrence, and Fuhrman nuclear grade significantly correlated with both risk of death and risk of recurrence. This confirmed the utility of the two established prognostic markers over TIL PD-1 expression.
Several prior studies have correlated PD-1/PD-L1 expression on immunohistochemistry with clinical outcome (). However, substantial inter-study variability exists among the conclusions of the studies even when the comparison is confined to clear cell subtype. Among the studies that quantified PD-1 expression, Thompson, et al., Kang, et al., and Giraldo, et al. reported significant association with clinical outcome, with a caveat that the last study noted inconsistent staining between invasive margin and tumor core. In contrast, Shin, et al. found only a trend between PD-1 positivity and cancer-specific and progression-free survivals. Among the studies that quantified PD-L1 expression, Thompson, et al., Choueiri, et al., Shin, et al., and Abbas, et al. reported significant association. Giraldo, et al. found borderline association, while Leite, et al. found it to correlate with pathological features such as higher FNG (P = 0.021) and microvascular invasion (P = 0.039) but no other prognostic factors.
Table 5. Studies that correlated PD-1 or PD-L1 expression in tumor cells or in immune cells with clinical outcome in the descending order of publication.
The inter-study discrepancies stem from technical challenges of immunohistochemistry as well as intrinsic complexity of tumor biology. Arguably the largest sources of discrepancy are the lack of standards in PD-1/PD-L1 scoring methods and threshold for positivity as well as inter-rater reliability (). Among the listed studies, proportion of samples read as positive ranged from 18.8% to 43.2% for PD-1 positivity, and from 12.6% to 56.5% for PD-L1 positivity. Our study reports 54.88% of the cohort to be PD-1 positive. This value is higher than what others reported because the low threshold of 1% we used. Using a different cutoff for PD-1/PD-L1 dramatically changed the fraction of ‘positive’ patients; applying a threshold of 5–10% resulted in a value in line with other studies. Dramatic variation of percent positivity with different thresholds has been criticized as a design flaw in other studies of this kind.Citation44 Further stratification of PD-1 expression into negative/low/high in this study was intended to partially mitigate this issue.
There are other technical challenges of immunohistochemistry that need to be addressed. Except the earliest studies that used fresh-frozen tissue, most studies used fixed tissue, because it was conducive to large retrospective studies. It has been suggested that fixation process may compromise antigen staining and underestimate the prevalence of PD-L1 expression.Citation20 Furthermore, several different antibody clones with presumably different affinities have been used. The prevalence of PD-L1 expression were shown to vary significantly when using different antibodies.Citation44 Another technical issue is that, while it is known that PD-1 and PD-L1 are expressed in multiple cell types ranging from tumor cells to stromal cell elements and various immune cell types, investigators have not employed methods such as double staining to control which cell type is counted, nor do we know which cell population is clinically relevant for predicting prognosis or treatment benefit.Citation44
Tumor heterogeneity further complicates the matter. A multi-region sequencing study showed that spatially separated samples from the same RCC tumor underwent a number of convergent and divergent mutations, some of which are signatures of good prognosis and some of poor prognosis.Citation45 Supporting that finding, studies on variability of PD-L1 expression within tumor and between primary and metastatic sites have been inconclusive, neither showing dependence or independence.Citation46,Citation47 It remains to be determined whether sampling multiple sites adds value to clinical decision making or where in the tumor spatially (invasive margin versus tumor core) best represents the tumor microenvironment. A way to potentially circumvent the tumor heterogeneity issue is to measure systemic markers circulating in the blood, and to that end, an association between level of soluble PD-L1 circulating in serum and survival has been reported in clear cell RCC patients.Citation48
Lastly, cellular PD-1/PD-L1 expression seems to occur in a spectrum rather than simple on/off. Flow cytometric studies have reported different tiers of cellular PD-1 expression that would not be distinguishable using immunohistochemistry techniques. Blackburn et al. identified two subsets of exhausted CD8 T cells from mice with chronic LCMV infection and demonstrated that the T cells expressing intermediate level of PD-1 could be reinvigorated with PD-1 blockade while the T cells expressing high level of PD-1 could not, representing a more terminally differentiated T cell population.Citation49 It is known that PD-1 is transiently expressed in effector T cells and chronically expressed in exhausted T cells, and these two T cell subsets have opposite prognostic implications.Citation14 To our knowledge, no IHC study has analyzed differential PD-1 expression and considered its clinical impact as it relates to prognosis or response to anti-PD-1 therapy. In this regard, immunohistochemistry may be lacking the quantitative resolution needed to study PD-1 and PD-L1 expression. Recent studies have quantified PD-L1 on cell lines and mouse models using quantitative RT-PCR or flow cytometry and may represent future directions.Citation50,Citation51
This study shares the limitations listed above with other studies; therefore, inter-study comparison must be done with caution. Other limitations of this study include a relatively small cohort of retrospective nature that can introduce selection bias. We only performed univariate analysis with PD-1 expression and not adjust for pT stage and FNG for two reasons: first, we wanted to compare the prognostic value of PD-1 positivity to those of pT stage and FNG, and second, the univariate correlations were already extremely weak that multivariate analysis would not bring forth statistical significance. In regard to the choice of antibody, the anti-PD-1 antibody employed in this study (clone NAT105) was not used in clinical trials but has been shown to produce a clear staining pattern with good effect in a preceding study.Citation22
It is evident much research is needed before PD-1 blockade immunotherapy can be optimally directed to RCC patients. The biological link between PD-1 pathway and immune dysfunction must be better characterized in the tumor microenvironment, and in order to do so, a reliable and repeatable way to quantify tumor PD-1 expression is necessary. We argue that PD-1/PD-L1 immunohistochemistry in its current form is an inadequate tool for studying tumor PD-1 biology and is inferior to established tools such as primary tumor stage or Fuhrman nuclear grade in prognosticating. We suggest that an effort to standardize PD-1 scoring and validate the results using external and/or inter-institutional cohort is necessary before effective stratification of patients and clinical application of PD-1 blockade immunotherapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 66(1):7–30. doi:10.3322/caac.21332.
- Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843–852. http://www.ncbi.nlm.nih.gov/pubmed/14680319. doi:10.1016/S0094-0143(03)00056-9.
- Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, Sherry RM, Royal RE, Steinberg SM, Rosenberg S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113(2):293–301. doi:10.1002/cncr.23552.
- Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27(28):4709–4717. doi:10.1200/JCO.2008.18.9498.
- Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24(13):1997–2005. doi:10.1200/JCO.2005.03.9594.
- Webster WS, Lohse CM, Thompson RH, Dong H, Frigola X, Dicks DL, Sengupta S, Frank I, Leibovich BC, Blute ML, et al. Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer. 2006;107(1):46–53. doi:10.1002/cncr.21951.
- Igarashi T, Takahashi H, Tobe T, Suzuki H, Mizoguchi K, Nakatsu HO, Ito H.. Effect of tumor-infiltrating lymphocyte subsets on prognosis and susceptibility to interferon therapy in patients with renal cell carcinoma. Urol Int. 2002;69(1):51–56. doi:10.1159/000064361.
- Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89(10):1906–1908. doi:10.1038/sj.bjc.6601400.
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi:10.1038/ni.2035.
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi:10.1038/nri3862.
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi:10.1016/j.it.2015.02.008.
- Frankenberger B, Noessner E, Schendel DJ. Immune suppression in renal cell carcinoma. Semin Cancer Biol. 2007;17(4):330–343. doi:10.1016/j.semcancer.2007.06.004.
- Geissler K, Fornara P, Lautenschläger C, Holzhausen H-J, Seliger B, Riemann D. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4(1):e985082. doi:10.4161/2162402X.2014.985082.
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(April):227–242. doi:10.1038/nri3405.
- FDA Approves Nivolumab to Treat Advanced Kidney Cancer. The ASCO Post. http://www.ascopost.com/News/34072. Published 2015. Accessed January 1, 2016.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015:1803–1813. doi:10.1056/NEJMoa1510665.
- Quinn DI, Lara PN, Ph D, Lara PN. Renal-Cell Cancer – Targeting an Immune Checkpoint or Multiple Kinases. N Engl J Med. 2015;373(19):1–3. doi:10.1056/NEJMe1511252.
- McDermott DF, Motzer RJ, Atkins MB, et al. Long-term overall survival (OS) with nivolumab in previously treated patients with advanced renal cell carcinoma (aRCC) from phase I and II studies. J Clin Oncol. 2016;34:suppl:abstr 4507. http://meetinglibrary.asco.org/content/162949-176.
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Chen L, Zincke H, Blute ML, Leibovich BC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104(10):2084–2091. doi:10.1002/cncr.21470.
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi:10.1158/0008-5472.CAN-05-4303.
- Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757–1761. doi:10.1158/1078-0432.CCR-06-2599.
- Kang MJ, Kim KM, Bae JS, Park HS, Lee H, Chung MJ, Moon WS, Lee DG, Jang KY. Tumor-infiltrating PD1-Positive Lymphocytes and FoxP3-Positive Regulatory T Cells Predict Distant Metastatic Relapse and Survival of Clear Cell Renal Cell Carcinoma. Transl Oncol. 2013;6(3):282–289. doi:10.1593/tlo.13256.
- Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, Bellmunt J, Song J, Carvo I, Lampron M, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178–2184. doi:10.1093/annonc/mdu445.
- Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, Deen K, Carpenter C, Benson P, Ho TH, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: Results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015;21(5):1071–1077. doi:10.1158/1078-0432.CCR-14-1993.
- Giraldo NA, Becht E, Pag??s F, Skliris G, Verkarre V, Vano Y, Mejean A, Saint-Aubert N, Lacroix L, Natario I, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21(13):3031–3040. doi:10.1158/1078-0432.CCR-14-2926.
- Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H.. Clinicopathologic Analysis of PD-L1 and PD-L2 Expression in Renal Cell Carcinoma: Association with Oncogenic Proteins Status. Ann Surg Oncol. 2015:694–702. doi:10.1245/s10434-015-4903-7.
- Leite KRM, Reis ST, Junior JP, Zerati M, Gomes Dde O, Camara-Lopes LH, Srougi M.. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol. 2015;10(1):189. doi:10.1186/s13000-015-0414-x.
- Abbas M, Steffens S, Bellut M, Becker JU, Großhennig A, Eggers H, Wegener G, Kuczyk MA, Kreipe HH, Grünwald V, et al. Do programmed death 1 (PD-1) and its ligand (PD-L1) play a role in patients with non-clear cell renal cell carcinoma? Med Oncol. 2016;33(6):1–7. doi:10.1007/s12032-016-0770-8.
- Abbas M, Steffens S, Bellut M, Eggers H, Großhennig A, Becker JU, Wegener G, Schrader AJ, Grünwald V, Ivanyi P. Intratumoral expression of programmed death ligand 1 (PD-L1) in patients with clear cell renal cell carcinoma (ccRCC). Med Oncol. 2016;33(7):1–7. doi:10.1007/s12032-016-0794-0.
- Erlmeier F, Hartmann A, Autenrieth M, Wiedemann M, Ivanyi P, Steffens S, Weichert W. PD-1/PD-L1 expression in chromophobe renal cell carcinoma: An immunological exception? Med Oncol. 2016;33(11):1–6. doi:10.1007/s12032-016-0833-x.
- Crispin H, Agarwal AM, Salama ME, Tantravahi SK, Merriman J, Straubhar AM, Poole A, Nussenzveig R, Stenehjem DD, Agarwal N. Correlation of tumor programmed death ligand-1 (PD-L1) expression and response to treatment with high-dose interleukin-2 (HD IL-2) in clear cell metastatic renal cell carcinoma (ccmRCC). J Clin Oncol. 2014;32: suppl; abstr e15584.
- McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34(8):833–842. doi:10.1200/JCO.2015.63.7421.
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. http://www.ncbi.nlm.nih.gov/pubmed/1396582.
- Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171(9):4574–4581. http://www.ncbi.nlm.nih.gov/pubmed/14568931. doi:10.4049/jimmunol.171.9.4574.
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T.. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–772. http://www.ncbi.nlm.nih.gov/pubmed/8671665. doi:10.1093/intimm/8.5.765.
- Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22(5):265–268. http://www.ncbi.nlm.nih.gov/pubmed/11323285. doi:10.1016/S1471-4906(01)01888-9.
- Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10(10):1563–1572. http://www.ncbi.nlm.nih.gov/pubmed/9796923. doi:10.1093/intimm/10.10.1563.
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. http://www.ncbi.nlm.nih.gov/pubmed/10485649. doi:10.1016/S1074-7613(00)80089-8.
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi:10.1126/science.291.5502.319.
- McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612–616. doi:10.1038/nature14468.
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi:10.1038/nm730.
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi:10.1073/pnas.192461099.
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: Implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307–314. doi:10.1007/s00262-004-0593-x.
- Lee C-H, Motzer RJ. Immune Checkpoint Therapy in Renal Cell Carcinoma. Cancer J. 22(2):92–95. doi:10.1097/PPO.0000000000000177.
- Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. doi:10.1056/NEJMoa1113205.
- Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y, Chen L, Kluger HM, et al. PD-L1 expression in clear cell renal cell carcinoma: An analysis of nephrectomy and sites of metastases. J Cancer. 2014;5(3):166–172. doi:10.7150/jca.8167.
- Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res. 2015;3(10):1158–1164. doi:10.1158/2326-6066.CIR-15-0043.
- Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17(7):1915–1923. doi:10.1158/1078-0432.CCR-10-0250.
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105(39):15016–15021. doi:10.1073/pnas.0801497105.
- Messai Y, Gad S, Noman MZ, Le Teuff G Couve S, Janji B, Kammerer SF, Rioux-Leclerc N, Hasmim M, Ferlicot S, et al. Renal Cell Carcinoma Programmed Death-ligand 1, a New Direct Target of Hypoxia-inducible Factor-2 Alpha, is Regulated by von Hippel-Lindau Gene Mutation Status. Eur Urol. 2016;70(4):623–632. doi:10.1016/j.eururo.2015.11.029.
- Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, Nakatani T, Wanibuchi H. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 2016;107(12):1736–1744. doi:10.1111/cas.13099.
