ABSTRACT
Background: Approximately 50% of melanomas harbor BRAF mutations. Treatment with BRAF +/− MEK inhibition is associated with favorable changes in the tumor microenvironment thus providing the rationale for combining targeted agents with immunotherapy.
Methods: Patients with unresectable Stage III or IV BRAFV600E mutant melanoma were enrolled in a single-center prospective study (n = 6). Patients were eligible to receive two courses of HD-IL-2 and vemurafenib twice daily. The primary endpoint was progression-free survival (PFS) with secondary objectives including overall survival (OS), response rates (RR), and safety of combination therapy as compared to historical controls. Immune profiling was performed in longitudinal tissue samples, when available.
Results: Overall RR was 83.3% (95% CI: 36%–99%) and 66.6% at 12 weeks. All patients eventually progressed, with three progressing on treatment and three progressing after the vemurafenib continuation phase ended. Median PFS was 35.8 weeks (95% CI: 16–57 weeks). Median OS was not reached; however, the time at which 75% of patients were still alive was 104.4 weeks. Change in circulating BRAFV600E levels correlated with response. Though combination therapy was associated with enhanced CD8 T cell infiltrate, an increase in regulatory T cell frequency was seen with HD-IL-2 administration, suggesting a potential limitation in this strategy.
Conclusion: Combination vemurafenib and HD-IL-2 is well tolerated and associated with treatment responses. However, the HD-IL-2 induced increase in Tregs may abrogate potential synergy. Given the efficacy of regimens targeting the PD-1 pathway, strategies combining these regimens with BRAF-targeted therapy are currently underway, and the role of combination vemurafenib and HD-IL-2 is uncertain.
Trial Registration: Clinical trial information: NCT01754376; https://clinicaltrials.gov/show/NCT01754376
Introduction
In 2018, in the United States, an estimated 91,270 new cases are expected and approximately 10,000 people will die of cutaneous melanoma. Fortunately, the treatment of melanoma has rapidly evolved over recent years. One of the first breakthroughs was the discovery that high-dose interleukin-2 (HD-IL-2; aldesleukin; Proleukin®) therapy was associated with durable responses in a subset of patients with metastatic disease. Specifically, an analysis of 270 patients treated with HD-IL-2 at the National Cancer Institute (NCI) and other institutions between 1985 and 1993 demonstrated that this approach led to long-term durable response in a minority (6%) of patients and an overall response rate (ORR) of 16%.Citation1 More contemporary cohorts corroborate an ORR in the 15–20% range and durable benefit (e.g. no need for further systemic therapy) in up to 10% of patients and the 5-year overall survival approaching 25%.Citation2,Citation3
Over the past 15 years, improved understanding of the role of activation of the mitogen activated protein kinase (MAPK) pathway in melanomagenesis has led to the development of a number of small molecule inhibitors targeting this pathway. Activating mutations in BRAF are present in approximately 50% of advanced melanomas, with the vast majority possessing a point mutation at codon 600 (V600E being the most common), resulting in constitutive activation of the MAPK pathway.Citation4,Citation5 The resultant unopposed, constitutive activation of extracellular signal-regulated kinase (ERK) leads to the promotion of cellular growth, opposition of apoptosis, and transformation into melanoma.Citation6 The reliance on this pathway, however, also renders mutated cells susceptible to inhibition.Citation7,Citation8 The potent and selective BRAF inhibitors, vemurafenib and dabrafenib, have proven effective in the treatment of BRAF mutant melanoma, with improved progression-free (PFS) and overall survival (OS) in patients with advanced (unresectable Stage III or IV) disease, compared with chemotherapy. Both vemurafenib and dabrafenib are associated with clinical responses in 50–60% of patients, however most responses are partial and disease progression typically occurs at a median of 5–7 months.Citation9,Citation10 More recently, combined inhibition of BRAF and MEK has been determined to be superior to single-agent BRAF inhibitor therapy in advanced, BRAF mutant melanoma.Citation11,Citation12
An alternative combination strategy is the use BRAF-targeted therapy in concert with immunotherapy. The rationale for combination targeted–immunotherapy is based on evidence that oncogenic BRAF contributes to immune escape and that BRAF inhibition leads to: 1) Increased expression of melanocyte differentiation antigens; 2) Decreased immunosuppressive cytokines; 3) Increased CD8+ T cell tumor infiltration and effector activity.Citation13,Citation14 These findings suggest that BRAF-inhibitor therapy may enhance the tumor immune microenvironment and set up the immune system to better recognize tumor antigens. Additionally, these results suggest that combining BRAF inhibitors with T-cell activators, such as HD-IL-2, may improve patient outcome.
Methods
We conducted a non-randomized, single-center, Phase II trial to examine the efficacy and safety of combined vemurafenib and HD-IL-2 in patients with histologically confirmed metastatic melanoma (unresectable Stage IIIC or Stage IV) harboring the BRAFV600E mutation. At consideration of trial enrollment, all patients met standard eligibility requirements for HD-IL-2 treatment, including adequate cardiopulmonary reserve and ability to receive pressors.Citation1 The study was performed at the Massachusetts General Hospital (Boston, MA). The protocol was written by the investigators and funded through the investigator-initiated trials program at Prometheus Laboratories and Roche-Genentech. The protocol was reviewed and approved by the institutional review board and written informed consent was obtained from each participant.
The primary endpoint was to determine efficacy, as measured by PFS, of patients with BRAFV600E mutant metastatic melanoma treated with the combination of vemurafenib and HD-IL-2 in comparison to an historic control of vemurafenib monotherapy. Secondary end points included determination of complete response (CR), partial response (PR), durable response (DR), and overall survival (OS) as well as the toxicity and safety profile of concurrent administration of HD-IL-2 and vemurafenib.
Patients and eligibility
Eligible patients had histologically confirmed, unresectable stage IIIC, IV, or recurrent malignant melanoma with a somatic mutation in BRAFV600E confirmed using a Roche Cobas® BRAF mutation test. Patients were eligible if they had measurable disease by RECIST 1.1 criteria,Citation15 an Eastern Cooperative Oncology Group performance status score of 0 or 1 and adequate end-organ function.Citation16 Patients could have received prior adjuvant therapy as well as prior immunotherapy (vaccine, anti-CTLA-4, anti-PD-1) for their advanced disease though a washout period of 8 weeks was required prior to enrollment. Prior IL-2 or BRAF targeted therapy was not permitted. Concomitant steroid use was not allowed and an 8-week washout was required prior to enrollment. Patients with known brain metastases were excluded, unless they had undergone definitive therapy and were neurologically stable.
Treatment
Patients received oral vemurafenib (960 mg twice daily) for 2 weeks, and then received HD-IL-2 at 600,000 IU/kg/dose intravenously every eight hours to tolerance (maximum 14 doses) over five days on days 15–19 of cycle 1 and again on days 1–5 of cycle 2. A second course of HD-IL-2 could be given at the discretion of the provider if imaging demonstrated evidence of tumor stability or regression. Patients were hospitalized during HD-IL-2 treatment for monitoring and treatment of adverse effects.Citation1
Patients remained on daily vemurafenib throughout the entirety of the HD-IL-2 course and remained on drug for the scheduled 12-week treatment course. Patients were continued on therapy until time of progression or in the setting of an excellent response and mild toxicity patients were treated until 8 months of therapy was completed. At that time a decision was made between the patient and treating physician to stop therapy with vemurafenib and follow expectantly. Treatment response was assessed every 6 weeks for the first 6 months, then every 12 weeks.
Correlative studies
Longitudinal tumor biopsies from easily accessible lesions were performed before treatment, 1–2 weeks into treatment with vemurafenib, 1 week into treatment with HD-IL-2, and at time of recurrence, when feasible (Supplementary Table 1). For those in whom excess tissue was available, histologic and molecular characterization of the tumor was performed to assess immune response. Circulating blood BRAF levels were followed in evaluable patients as described.Citation17
Circulating BRAF levels
Exploratory biomarkers of response and resistance were also studied including quantification of circulating BRAF pre-treatment, on-treatment and at study conclusion. Evaluable patients had a minimum of three plasma samples evaluated. The mutant allele frequency of BRAF at the given time points were obtained using droplet digital PCR. Cell free DNA (cfDNA) was extracted from plasma using the QIAamp Circulating Nucleic Acid Kit (QIAGEN). Isolated cfDNA was amplified using ddPCR Supermix for Probes (Bio-Rad) and BRAF (PrimePCR ddPCR Mutation Assay, Bio-Rad) ddPCR assay. 8 µl of DNA template was added to 10 µl of ddPCR™ Supermix for Probes (Bio-Rad) and 2 µl of the primer/probe mixture. This reaction mix was added to a DG8 cartridge together with 60 µl of Droplet Generation Oil for Probes (Bio-Rad) and used for droplet generation. Droplets were then transferred to a 96 well plate (Eppendorf) and then thermal cycled. Droplets were analyzed with the QX200™ Droplet Reader (Bio-Rad) for fluorescent measurement of FAM and HEX probes. Gating was performed based on positive and negative controls, and mutant populations were identified. The ddPCR data were analyzed with QuantaSoft analysis software (Bio-Rad) to obtain Fractional Abundance of the mutant DNA alleles in the wild-type/normal background.
Immunohistochemistry
Immunohistochemical (IHC) studies were performed on five-micrometer-thick tissue sections. Slides were stained with a CD8 pre-diluted primary antibody (BioCare Medical PM395) followed by a pre-diluted secondary antibody containing alkaline phosphatase conjugated goat anti-rabbit (BioCare RALP525). CD8 slides were developed using Warp Red chromogen kit (BioCare WR806). All slides were counterstained with hematoxylin (Vector H-3401). Areas with the most abundant inflammatory cells (within the lesion and surrounding the lesion) were selected. Photomicrographs were taken of three 40X high power fields. The CD8 cells were scored by a dedicated and blinded dermatopathologist and reported as counts per high power field (HPF).
Flow cytometric analyses of TILs
A tumor infiltrating lymphocyte (TIL) enrichment protocol was used. Tumors were cut, placed in Collagenase Type I (400 U/ml, Worthington Biochemical) and incubated on a shaker at 37°C for 30 minutes. The dissociated tumor was then smashed through a 70 μm filter to obtain a single cell suspension. A sucrose gradient (40%/70% Percoll, GE Healthcare) was used to enrich for TILs from the single cell suspension. Unstimulated human TILs were stained with the following directly labeled antibodies: anti-CD45 (HI30), anti-CD11b (ICRF44), anti-CD3 (HIT3a), anti-CD4 (OKT4) and anti-CD8a (HIT8a; all from Biolegend). Cells were fixed and permeabilized using the FoxP3 buffer kit (eBioscience) and stained with anti-FoxP3 (PCH101, eBioscience). Flow cytometry data were acquired on the BD LSRII flow cytometer and analyzed using FlowJo software (Tree Star).
Statistical considerations
We hypothesized that combination vemurafenib and HD-IL-2 would lead to improved PFS in comparison to the historical control of vemurafenib alone. Initial power calculations called for a cohort size of 49 patients (calculated to provide 90% power to detect a 50% improvement in median PFS from 7 months to 10.5 months, hazard ratio = 0.67), assuming a one-sided, type I error of 10%, and an exponential MLE test. However, accrual to the trial was poor in light of major changes to the standard-of-care therapy of advanced melanoma, including the use of combination BRAF/MEK inhibitors and the emergence of PD-1 checkpoint blockade, the trial was closed prematurely due to poor accrual.
In this limited cohort, response rate was calculated as the proportion of patients with RECIST partial (PR) or complete response (CR) divided by the total number of patients treated. PFS and OS were calculated using the Kaplan-Meier method. Time-to-progression was defined as the interval from beginning therapy to RECIST progression or death.
Results
Manageable grade 3 adverse events occurred in all patients treated with vemurafenib/HD-IL-2
From February 2013 through November 2013, 6 patients possessing a BRAFV600E mutation were enrolled (). The average age at enrollment was 42 (range: 23–58) with equal numbers of men and women. All patients had undergone surgical resection of their primary lesion and the majority (83%) had received prior adjuvant interferon therapy before developing metastatic disease. No patient had received prior anti-PD-1/PD-L1 therapy.
Treatment with combination therapy was generally well tolerated. All patients tolerated the two-week lead-in of vemurafenib without problem. Patients were eligible to receive two courses of HD-IL-2 (two weeks of therapy per course). Three patients received 2 courses of HD-IL-2, two patients had therapy discontinued after 1 course due to progression and one patient had therapy discontinued during course 2 secondary to intolerance to HD-IL-2, specifically neurotoxicity (). Common toxicities observed included fatigue, arthralgia, rash, nausea, diarrhea and capillary leak syndrome. A total of 45 adverse effects (AE) and 23 grade 3–4 AEs were detected (). All patients experienced grade 3 toxicities` however these were successfully managed with supportive care, dose reductions and delays. There was one documented grade 4 AE of delirium that resulted in discontinuation of HD-IL-2.
Table 1. Patient demographics.
Table 2. HD-IL-2 administration.
Table 3. Adverse effects.
Patients received an average 20 out of 28 doses (70%) of HD-IL-2 during course 1. Two patients progressed after course 1 and did not continue on trial. Of the 4 patients who commenced course 2, the average dose administration declined to 15/28 doses (54%). In total, patients received 33/56 (55%) possible doses of HD-IL-2.
High response rates are observed in patients treated with vemurafenib/HD-IL-2
The confirmed systemic ORR was 83.3% (95% CI: 36% to 99%) with a confirmed systemic ORR at 12 weeks of 66.6%. At 12 weeks, three patients had a PR, one had a CR and two showed PD. A patient with subcutaneous disease, not evaluable on scans, clinically responded with resolution of palpable nodules. Two patients progressed while on the active HD-IL-2 phase of therapy, evident on scans obtained after the second week of the first course of HD-IL-2. One patient developed CNS progression alone whereas another patient progressed both intra- and extra-cranially ().
Figure 1. Clinical Data and Response. A) Waterfall plot showing the % change in tumor size based on CT imaging assessment by RECIST1.1. B) Swimmers plot demonstrating progression-free (PFS, blue) and overall survival (OS, red) in weeks. Kaplan Meier curve showing C) the PFS and D) OS in our patient cohort.
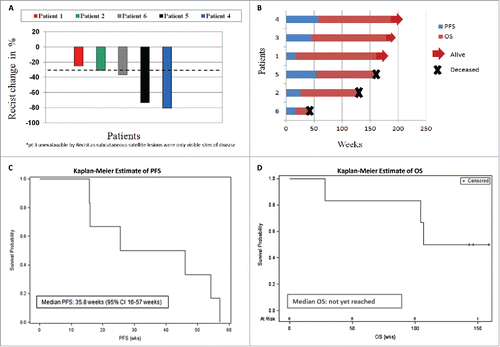
At the time of analysis, the median duration of follow-up was 146.4 weeks (95% CI: 143.1 to 158.7 weeks, ) with a median PFS of 35.8 weeks (95% CI: 16–57 weeks, ) with the death rate in these data being 50% (95% CI: 12% to 88%, ). Median OS was not reached; however, the time at which 75% of patients were still alive was 104.4 weeks ().
Change in BRAFV600E levels in cfDNA correlates with response in patients treated with vemurafenib/HD-IL-2
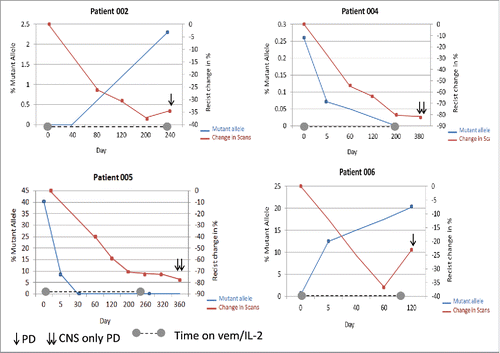
Four patients were evaluable for serial BRAFV600E cfDNA analysis (). Notably, change in BRAFV600Elevels in cfDNA correlated with treatment response. The allelic frequency of the BRAFV600E mutation decreased in two patients (4, 5) who responded to therapy. The most dramatic reduction was seen in patient 5 who at treatment initiation had a mutant allelic frequency of 40% which decreased to 8% after week one of the vemurafenib lead-in with undetectable levels after completion of HD-IL-2 therapy. These values correlated with radiographic response with scans demonstrating a 73% reduction in disease from baseline. Levels of the mutant allele rose in patient 6 shortly after study initiation. Though first follow-up scans demonstrated a PR, subsequent imaging one month later demonstrated interval worsening of thoracic tumor burden. Interestingly, pt 2 had undetectable levels of BRAFV600E at treatment initiation, which was attributed to the low volume of burden of disease. Despite an early response, this patient ultimately progressed with scans demonstrating progressive lymphadenopathy with a concomitant rise in circulating BRAFV600E levels.
Figure 2. BRAFV600E mutant allele frequency in cfDNA following combination vemurafenib and HD-IL-2. Double Y scatter plot comparing the percentage BRAF mutant allele frequency (blue) with % change in scans (per RECIST, red) over time. At day 0 all patient started the 2week lead-in of vemurafenib. Dotted line along X axis indicates the duration of therapy.
Concurrent increase of CD8 T cells, regulatory T cells and myeloid cells is observed in patients treated with vemurafenib and HD-IL-2
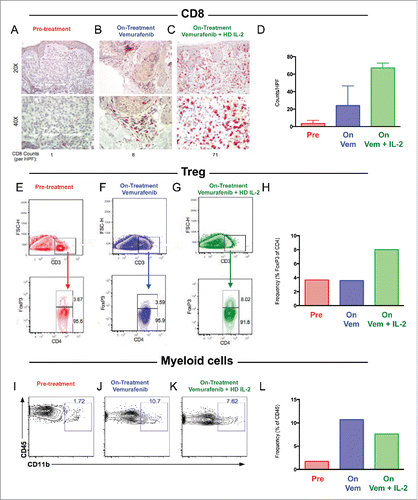
We performed immune profiling in longitudinal tissue samples from a subset of patients on trial with accessible tumor at pre-treatment, early following vemurafenib initiation, as well as early following combination therapy (n = 6 biopsies in 2 patients). We first performed IHC to study the CD8 T cell infiltrate, and observed that CD8 numbers increased slightly when patients were treated with vemurafenib, but significantly when vemurafenib was combined with HD-IL-2 (). In order to better characterize the immune infiltrates in these tumors and considering the effect of IL-2 in inducing regulatory T cells (Treg) in prior works,Citation18,Citation19 we performed flow cytometry to assess the presence of Tregs over the course of therapy. We observed that, although frequency of Tregs was low at baseline and vemurafenib had no effect on these cells, the combination of vemurafenib with HD-IL-2 led to a 2-fold increase in the frequency of these cells ().Citation18 Finally, we investigated the effect of combination therapy on myeloid cells by performing flow cytometry for CD11b. These studies revealed that, although limited in pre-treatment samples, myeloid cells increased 5-fold in presence of vemurafenib, and levels remained high when vemurafenib was combined with HD-IL-2 ().Citation20 In summation, these results suggest that although combination therapy may have beneficial effects on the infiltration of CD8 T cells, these effects may be dampened by concomitant expansion of immunosuppressive cell types such as Tregs and myeloid cells, partially due to HD-IL-2 administration.
Figure 3. Increased CD8 T cells, Regulatory T cells, and Myeloid cells following combination vemurafenib and HD-IL-2. A-D) Immunohistochemical staining for CD8 T cells (red) at 20X and 40X magnification prior to therapy (A), on BRAF inhibitor prior to HD-IL-2 (B) and on combination BRAF inhibitor and HD-IL-2 (C) in two patients with quantification of CD8 per HPF (D). E-H) Flow cytometry showing percentage of Tregs at pre-treatment (E), on vemurafenib prior to HD-IL-2 administration (F), and on combination vemurafenib + HD-IL-2 (G) as well as quantification of Tregs (H). I-L) Flow cytometry showing percentage of CD11b myeloid cells at pre-treatment (I), on vemurafenib prior to HD-IL-2 administration (J), and on combination vemurafenib + HD-IL-2 (K) as well as quantification of CD11b myeloid cells (L).
Discussion
We treated BRAFV600E mutation-positive patients with combination vemurafenib and HD-IL-2. This combination regimen yielded an ORR of of 83.3% at 6 weeks and 66.6% at 12 weeks and a median PFS of 35.8 weeks. Though initial response rates were robust, responses were not durable as all patients eventually experienced disease progression, with three progressing while on treatment (two during HD-IL-2 phase, one during the vemurafenib continuation phase) and the remaining patients progressing following the end of the vemurafenib continuation phase. Median OS was not reached; however, the time at which 75% of patients were still alive was 104.4 weeks. Although toxicity was prevalent with all patients experiencing grade 3 toxicity, it was manageable with most AEs treated successfully with supportive medications and the majority of patients (67%) eligible for the second cycle of HD-IL-2.
A novel aim of this study sought to examine the utility of serial BRAFV600E cfDNA monitoring in patients receiving therapy with vemurafenib and HD-IL-2. In our evaluable patients, a higher burden of disease correlated with increased levels of circulating BRAFV600E cfDNA and changes in BRAFV600E allelic frequency was associated with both treatment response and disease progression. This phenomenon of rapid decrease in the concentration of BRAFV600E cfDNA has since been reported in patients receiving BRAF inhibitor monotherapy as well as combination BRAF/MEK inhibition and has been attributed to rapid tumor cell death and subsequent clearance of cfDNA.Citation21-23 Based on this collective work serial monitoring of BRAFV600E cfDNA holds promise as a monitoring tool for patients with advanced BRAF-mutant melanoma, however further prospective studies are needed to solidify its role in clinical practice.
To our knowledge, this is one of the first clinical trials in unresectable or metastatic melanoma to demonstrate the immunomodulatory effect of the concomitant BRAF inhibitor and cytokine therapy on the tumor microenvironment using longitudinal serial tissue biopsies. Although BRAF inhibitors are not designed to directly activate immune anti-tumor responses, there is evidence to indicate that therapy can enhance anti-tumor immunity by promoting tumor antigen expression, antigen recognition and CD8+ T cell infiltration in tumors.Citation13,Citation14 Our study recapitulates the hypothesis that oncogenic BRAF contributes to immune escape and selective BRAF inhibition can prime an immune-mediated anti-tumor response. Additional translational efforts by our group have confirmed that BRAF inhibition stimulates an immune response via increasing expression of melanocyte differentiation antigens, increased clonality of TILs and expansion of CD8+ T cells as well as increases in T cell cytotoxicity.Citation14,Citation24,Citation25 When analyzing the longitudinal samples from our patients with accessible tumor there was a clear increase in the CD8+ T cell infiltrate via IHC analysis after induction with vemurafenib which recapitulates the immunogenic potential of BRAF inhibition. However, flow cytometry studies demonstrated a significant increase in the frequency of CD11b myeloid cells following BRAF inhibitor treatment suggesting that this treatment may also lead to immune suppressive effects. Furthermore, despite the promising increase in the CD8 infiltrate, flow cytometry results also showed a 2-fold increase in Treg frequency induced by combination with HD-IL-2, suggesting the emergence of a potential resistance mechanism as a result of this treatment strategy. This highlights a key limitation of this specific combination; yet also corroborates prior work and demonstrates positive immunomodulatory effects of BRAF inhibitor monotherapy. These data support the concept of BRAF-targeted therapy in concert with alternative immunotherapies, such as checkpoint inhibitors, and a number of clinical trials evaluating the combination of targeted therapy and checkpoint inhibition are currently underway. Special attention will be focused on the sequencing and timing of these agents as this and other data suggest that BRAF-targeted therapy should be administered first to enhance antigen expression and allow for CD8+ T cell infiltration prior to checkpoint inhibition.Citation13,Citation14,Citation26
The primary limitation of our study was its small sample size. Key reasons for limited accrual were the availability of anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies, as part of clinical trials, shortly following the initiation of this trial, and the emerging data demonstrating the superiority of combined BRAF/MEK inhibitor therapy as the optimal BRAF-targeted therapy. Prior to 2011, when this trial was conceived and written, HD-IL-2 was the treatment of choice in appropriately selected patients with metastatic melanoma because of the potential to produce long-term treatment-free survival,Citation17,Citation27–29 and the ultimate goal of combined vemurafenib plus HD IL-2 was to improve the number of complete and durable responders. However, in this limited sample set, we did not identify any such patients.
In summary, the combination vemurafenib and HD-IL-2 is a reasonably well-tolerated regimen and associated with high response rates. However, with the emergence of effective and more tolerable treatment regimens it is unclear where this combination fits into current standard treatment paradigms.
Two sentence summary
Combination vemurafenib and HD-IL-2 is well tolerated and associated with treatment response in patients with unresectable melanoma however our correlative data demonstrates that HD-IL-2 induced increase in Tregs may abrogate potential synergy. Given the efficacy of regimens targeting the PD-1 pathway, strategies combining these regimens with BRAF-targeted therapy are currently underway, and the role of combination vemurafenib and HD-IL-2 is uncertain.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Table_1.docx
Download MS Word (13.1 KB)Additional information
Funding
References
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi:10.1200/JCO.1999.17.7.2105.
- Joseph RW, Sullivan RJ, Harrell R, Stemke-Hale K, Panka D, Manoukian G, Percy A, Bassett RL, Ng CS, Radvanyi L, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. (Hagerstown, Md: 1997). 2012;35:66–72. doi:10.1097/CJI.0b013e3182372636.
- Payne R, Glenn L, Hoen H, Richards B, Smith JW, 2nd, Lufkin R, Crocenzi TS, Urba WJ, Curti BD. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J Immunother Cancer. 2014;2:13. doi:10.1186/2051-1426-2-13.
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al. High frequency of BRAF mutations in nevi. Nat Genet 2003;33:19–20. doi:10.1038/ng1054.
- Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA, Kefford RF. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–46. doi:10.1200/JCO.2010.32.4327.
- Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi:10.1158/0008-5472.CAN-04-2423.
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi:10.1073/pnas.0711741105.
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi:10.1038/nature04304.
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi:10.1056/NEJMoa1103782.
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi:10.1016/S0140-6736(12)60868-X.
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. doi:10.1056/NEJMoa1406037.
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. doi:10.1056/NEJMoa1412690.
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi:10.1158/0008-5472.CAN-10-0118.
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–31. doi:10.1158/1078-0432.CCR-12-1630.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi:10.1016/j.ejca.2008.10.026.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55. doi:10.1097/00000421-198212000-00014.
- Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013;49:1297–304. doi:10.1016/j.ejca.2012.11.019.
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4(+)CD25(hi) Foxp3(+) regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi:10.1182/blood-2005-06-2399.
- Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–77. doi:10.1200/JCO.2005.03.6830.
- Steinberg SM, Zhang P, Malik BT, Boni A, Shabaneh TB, Byrne KT, Mullins DW, Brinckerhoff CE, Ernstoff MS, Bosenberg MW, et al. BRAF inhibition alleviates immune suppression in murine autochthonous melanoma. Cancer Immunol Res. 2014;2:1044–50. doi:10.1158/2326-6066.CIR-14-0074.
- Panka DJ, Buchbinder E, Giobbie-Hurder A, Schalck AP, Montaser-Kouhsari L, Sepehr A, Lawrence DP, McDermott DF, Cohen R, Carlson A, et al. Clinical utility of a blood-based BRAF(V600E) mutation assay in melanoma. Mol Cancer Ther. 2014;13:3210–8. doi:10.1158/1535-7163.MCT-14-0349.
- Sanmamed MF, Fernandez-Landazuri S, Rodriguez C, Zarate R, Lozano MD, Zubiri L, Perez-Gracia JL, Martín-Algarra S, González A. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015;61:297–304. doi:10.1373/clinchem.2014.230235.
- Schreuer M, Meersseman G, Van Den Herrewegen S, Jansen Y, Chevolet I, Bott A, Wilgenhof S, Seremet T, Jacobs B, Buyl R, et al. Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med. 2016;14:95. doi:10.1186/s12967-016-0852-6.
- Cooper ZA, Frederick DT, Juneja VR, Sullivan RJ, Lawrence DP, Piris A, Sharpe AH, Fisher DE, Flaherty KT, Wargo JA. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunol. 2013;2:e26615. doi:10.4161/onci.26615.
- Bradley SD, Chen Z, Melendez B, Talukder A, Khalili JS, Rodriguez-Cruz T, Liu S, Whittington M, Deng W, Li F, et al. BRAFV600E Co-opts a Conserved MHC Class I internalization pathway to diminish antigen presentation and CD8+ T-cell Recognition of Melanoma. Cancer Immunol Res. 2015;3:602–9. doi:10.1158/2326-6066.CIR-15-0030.
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–94. doi:10.1158/1078-0432.CCR-11-2479.
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4.
- Joseph RW, Eckel-Passow JE, Sharma R, Liu P, Parker A, Jakob J, Buchbinder E, Bassett RL, Davies MA, Hwu P, et al. Characterizing the clinical benefit of ipilimumab in patients who progressed on high-dose IL-2. J Immunother. (Hagerstown, Md : 1997). 2012;35:711–5. doi:10.1097/CJI.0b013e3182742c27.
- Curti BD, Longo DL, Ochoa AC, Conlon KC, Smith JW, 2nd, Alvord WG, Creekmore SP, Fenton RG, Gause BL, Holmlund J, et al. Treatment of cancer patients with ex vivo anti-CD3-activated killer cells and interleukin-2. J Clin Oncol. 1993;11:652–60. doi:10.1200/JCO.1993.11.4.652.
