ABSTRACT
Despite successful introduction of NK-based cellular therapy in the treatment of myeloid leukemia, the potential use of NK alloreactivity in solid malignancies is still elusive. We performed a phase I clinical trial to assess the safety and efficacy of in situ delivery of allogeneic NK cells combined with cetuximab in liver metastasis of gastrointestinal origin. The conditioning chemotherapy was administrated before the allogeneic NK cells injection via hepatic artery. Three escalating doses were tested (3.106, 8.106 and 12.106 NK cells/kg) following by a high-dose interleukin-2 (IL-2). Cetuximab was administered intravenously every week for 7 weeks. Nine patients with liver metastases of colorectal or pancreatic cancers were included, three per dose level. Hepatic artery injection was successfully performed in all patients with no report of dose-limiting toxicity. Two patients had febrile aplasia requiring a short-term antibiotherapy. Grade 3/4 anemia and thrombopenia were also observed related to the chemotherapy. Objective clinical responses were documented in 3 patients and among them 2 occurred in patients injected with cell products harboring two KIR ligand mismatches and one in a patient with one KIR ligand mismatch. Immune monitoring revealed that most patients presented an increase but transient of IL-15 and IL-7 cytokines levels one week after chemotherapy. Furthermore, a high expansion of FoxP3+regulatory T cells and PD-1+ T cells was observed in all patients, related to IL-2 administration. Our results demonstrated that combining allogeneic NK cells transfer via intra-hepatic artery, cetuximab and a high-dose IL-2 is feasible, well tolerated and may result in clinical responses.
Introduction
NK cells are components of innate immunity recognizing and killing tumor- or virus-infected cells and producing various cytokines that play an important role in shaping the immune response.Citation1 Particularly activated NK cells produce large amounts of IFN-γ promoting dendritic cell maturation and Th1 polarization of adaptive immune responses.Citation2,Citation3 NK cells recognize target cells through a variety of activating and inhibitory receptors. Activating receptors, such as NKG2D and NKG2 C, bind MHC-related molecules, which are upregulated during times of cellular stress and/or viral infection. Alternatively they can be activated by the engagement of Natural Cytotoxicity Receptors (NCR) or CD16, which is a receptor for the Fc portion of some immunoglobulins G (IgG). Many receptors that inhibit NK cell function belong to the KIR superfamily. To date, 4 different inhibitory KIRs have been identified that bind to different allelic groups of HLA-A, HLA-B, or HLA-C. KIR2DL1 recognizes HLA-C alleles with a Lys80 residue (group C2), KIR2DL2 and KIR2DL3 recognize HLA-C with an Asn80 residue (group C1), KIR3DL1 is the receptor for HLA-B alleles sharing the Bw4 specificity and KIR3DL2 recognizes HLA-A3/A11 alleles.Citation4
The use of NK cells as an anti-cancer treatment has been reported by a number of studies over the past decade.Citation5,Citation6 Velardi et al. have first reported that the presence of such HLA-C/KIR mismatches promote graft-versus-leukemia effects without increasing the risk to develop graft-versus-host disease in leukemic patients treated by T cell depleted haploidentical stem cell transplantation.Citation7 Passweg JRCitation8 and Miller JSCitation9 then demonstrated that allogeneic NK cell adoptive transfer is feasible, safe and does not induce significant toxicity. Treatment of myeloid acute leukemia patients by adoptive transfer of alloreactive NK cells was shown as a potential effective strategy leading in some major haematological responses in patients treated in the context of a HLA/KIR mismatch.Citation9,Citation10
Furthermore, Miller J and colleagues also established the interest of lymphodepleting chemotherapy prior to the adoptive transfer in order to potentiate NK cell expansion. Indeed, these authors established that high doses of fludarabine and cyclophosphamide enhance the availability of interleukin-15 (IL-15) in the recipient serum.Citation9 Beyond the absence of significant toxicity, Miller et al. confirmed that NK cell alloreactivity (ie the absence on tumor cells of a cognate HLA ligand recognized by donor-derived NK cells) provided complete responses in heavily pre-treated leukemic patients. Other clinical studies have also reported the safety and efficacy of such haploidentical NK cell adoptive transfer in acute myeloid leukemia.Citation10-12
One particular interest of NK cell therapy is the ability of these lymphocytes to recognize a broad range of cancer cells, independently of specific tumor antigens. Expression of the NK cell activating ligands (MICA, MICB, ULBPs) was documented in many solid tumors including gastrointestinal cancers.Citation13 However, the clinical efficacy of NK cell based therapy in solid malignancies remains elusive.Citation9,Citation14,Citation15 NK express high affinity receptors (CD16) recognizing IgG constant fraction. Interactions of NK cells with tumor cells stained with monoclonal antibodies lead to a sustained NK cells activation through CD16 and enhanced their cytotoxic functions (ADCC: Antibody-dependent Cellular Cytotoxicity). Cetuximab is a chimeric human-murine IgG1 antibody targeting the extracellular domain of EGFR, thereby inhibiting the binding of activating ligands to the receptor.Citation16,Citation17 Cetuximab and has been shown to mediate ADCC activity and Cetuximab-mediated ADCC predicted responsiveness.Citation18,Citation19
Pioneering clinical trials have suggested the potential interest of activated cytotoxic lymphocyte injections as an adjuvant treatment of liver carcinoma when these lymphocytes were delivered directly in the liver artery.Citation18,Citation19
We have evaluated, in a phase I clinical trial, the feasibility and safety of adoptive cell transfer of allogeneic NK cells via hepatic artery infusion, combining with IV Cetuximab, to promote ADCC in EGFR positive liver metastases of gastrointestinal cancers.
Results
Patient's characteristics
Nine patients (3 women and 6 men) were enrolled and completed the protocol between 2009 and 2012. Median age was 60 years old (range 50–66). Six patients had a metastatic colorectal carcinoma and 3 patients a pancreatic adenocarcinoma. Patient's characteristics are summarized in . Patients treated for a pancreatic adenocarcinoma were exposed to gemcitabine, 5-fluorouracil and oxaliplatin before inclusion in the present clinical trial. Before inclusion, colorectal carcinoma patients were all exposed to 5-fluorouracil, oxaliplatin, irinotecan, bevacizumab and anti-EGFR in the case of wild type KRAS status. Six patients presented an EGFR positive-tumor and this was not performed in three patients ( and Fig. S1). All patients had measurable liver metastases according to RECIST criteria v1.1.
Phenotypic and functional characteristics of expanded allogeneic NK cells
Volunteer's allogeneic donors were selected in patient's family or among platelet's donors of the French blood bank. A leukapheresis was performed, after having controlled the absence of infectious disease in the donors by measuring serology for hepatitis, HIV, HTLV. The mean number of peripheral blood leukocytes recovered was 14.43 109 cells (range 8.18-19.3 109). The leukapheresis product from each donor was analyzed by flow cytometry. The composition of the final cell therapy products was depicted in . It included 2.31 109 monocytes (range 0.43-2.38), 0.73 109 B cells (range 0.44-1.19) and 0.88 109 NK cells (range 0.328-1.53). All patient received the selected dose excepted patient 9. The percentage of CD3+ T lymphocytes in the final product was 0.49% (range 0.001-1) and the mean T lymphocyte number adoptively transferred was 0.1 106 T cells/kg (range 0-0.22).
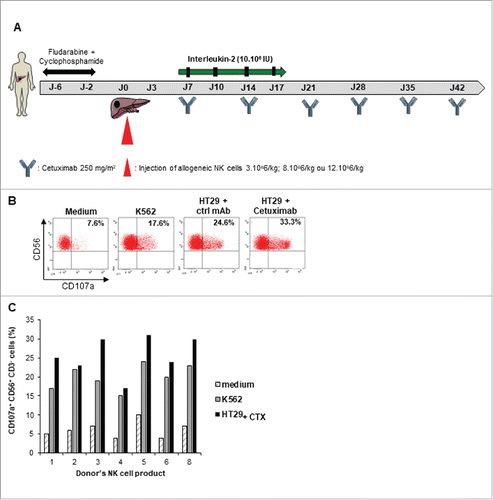
To evaluate natural cytotoxic function of allogeneic NK cells, the reactivity of each patient's final product was assayed against the standard NK-sensitive target K-562 cells and the colorectal cancer cell line HT29. The NK cells degranulation was measured using CD107 a expression by flow cytometry. For seven available final NK cells products, we found that the NK cells exhibited effective cytotoxic activity against the two target tumor cells. As expected, the NK cell cytotoxicity against EGFR-expressing HT29 cell line was increased in presence of Cetuximab ( and ).
Figure 1. Cytotoxic activity of expanded NK cells. A. Adoptive NK cell transfer schedule. B-C, Expanded NK cell products from 7 donors were stimulated by K562 or Cetuximab coated-HT29 cell line for 4 h at effector to target ratio 2:1 before CD107 a staining. Expression of CD107 a was assessed by flow cytometry on CD3- CD56+ NK cells. B. Representative dot plot of one donor's NK cell product is shown (patient 2). Percentages refer to the percentage of CD107 a+ CD56+ cells among CD3- NK cells. Rituximab coated-HT29 cell line was used as control mAb (ctrl mAb). C. Each column indicates the percentage of CD107 a+ CD56+ CD3- NK cells from a single donor.
Toxicity aspects
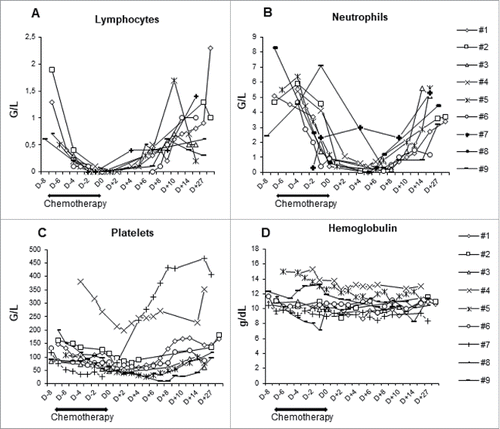
After administration of fludarabine/cyclophosphamide chemotherapy and infusion of purified NK cells the complete lymphopenia occurred 5 to 7 days after chemotherapy (), and the mean time for lymphocyte recovery (ie an absolute lymphocyte count above 500/mm3) was 10 days (range 8–13 days). Altogether, the addition of only one dose of cyclophosphamide (60 mg/kg) to 5 days of fludarabine (25 mg/m2/day) is manageable but induces only a short-term lymphopenic period. Neutropenia was also observed in all patients, with a mean duration of 6 days (1-9 days) (). Two patients had febrile aplasia requiring a short-term antibiotherapy. Grade 3/4 anemia and thrombopenia were also described () but had only little impact since red blood cell transfusion was only prescribed in one patient ( and ). Acute graft versus host disease was recently reported in patients receiving IL-15/4-1BBL activated NK cells following T-cell depleted stem cell transplantation,Citation20 but we did not observe any adverse event related to the low residual allogeneic T lymphocytes. As NK cell infusion was intra-arterially infused, liver toxicities were also monitored. No grade 3/4 toxicities were reported based on ASAT, ALAT or Alkaline phosphatase concentrations. Two patients (1 and 9) presented an increase of GGT after cell infusion however their GGT values were subnormal prior enrolment in the trial (). Furthermore, any severe adverse effects associated to high dose of interleukin-2 (IL-2) and Cetuximab treatments following the NK cell transfer was reported in these patients. Altogether, in situ allogeneic NK cells infusion in liver metastasis plus systemic Cetuximab was well tolerated and no dose limiting toxicity was reported.
Clinical outcome
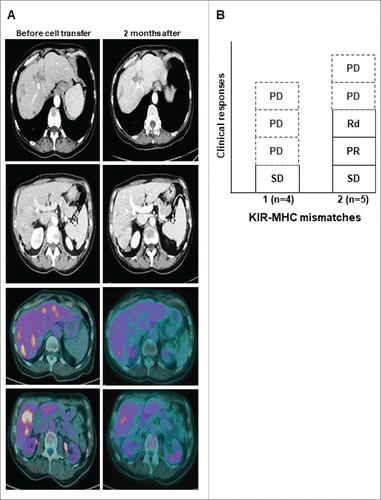
Tumor responses were assessed four weeks after the adoptive transfer by CT-scan (). One partial response was observed in a colorectal cancer patient who was injected with 8.106/kg NK (). This patient had only a liver metastatic disease, a KRAS exon 2 wild type colorectal cancer and never responded to previous therapies including anti-EGFR. FDG-PET scan assessment confirmed a decrease of liver metastases metabolic activity in most lesions after NK cells transfer. Another patient with 3 liver metastases of a pancreatic adenocarcinoma experienced a dissociated response with the progression of two lesions and the complete regression of the smallest lesion in the right liver (). Two additional patients with colorectal cancer experienced stable disease. However, all patients had progressive diseases 4 months following study enrollment. Treatment efficacy was also analyzed according to the number of KIR ligand mismatches. Four NK cell products displayed one KIR ligand mismatch while 2 such mismatches were observed in the 5 other patients. A stable disease was observed in patients receiving a cell therapy product with one KIR ligand mismatch. One partial response, a dissociated response and a stable disease occurred in the 5 patients treated with NK cell products harboring 2 KIR ligand mismatches (). Altogether, this escalating dose phase I trial provides evidence that the clinical efficacy of liver artery allogeneic NK cell adoptive transfer might be increased by using KIR ligand poly mismatched products.
Figure 3. Clinical activity. A Representative tumor responses evaluation by CT-scan and PET-scan before (left) and after (right) 2 months of NK cell transfer in patient 6 (PR). B, Objective responses according to the number of KIR ligand mismatch. PD, progressive disease; PR, partial response; Rd, dissociated response; SD, stable disease.
Assessment of peripheral blood cytokines levels
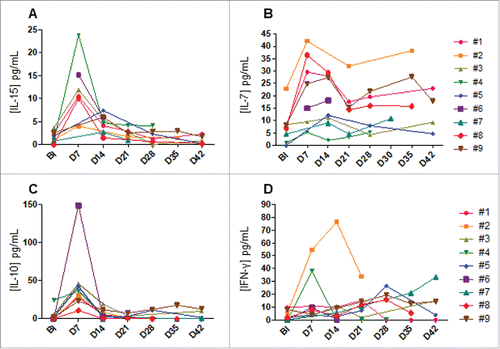
IL-15 has been shown to drive in-vivo NK-cell homeostatic expansion,Citation21 thus we measured the level of endogenous IL-15 weekly throughout the treatment (). All the patients had increasing IL-15 levels from baseline to day 7 after the NK cells infusion (1.7 ± 1.2 pg/mL versus 12.6 ± 6.6 pg/mL) and dropped at day 14 (4.7 ± 1.9 pg/mL). Similarly, the plasmatic concentration of the IL-7 was picked also at day 7 but remained elevated throughout the study (). Unique RT-PCR primers for donor-specific MHC class I alleles that did not cross-react with recipient MHC allele sequences could be generated for 7 patients. In most patients (5 out of 7), donor cells persisted in the blood for 7 days but could not be detected at later time points, correlating to the decrease in plasmatic IL-15 concentration ( and Fig. S2). These data suggested that intra-hepatic delivery of NK cells could transiently traffic in peripheral blood upon homeostatic IL-15 production. Furthermore, IL-10 and IFN-γ concentrations were also monitored to assess the activation/suppression of the immune system. The IL-10 concentration picked at day 7 and then dropped early at day 14 (). A similar kinetic of IFN-γ level was observed for two patients ().
Expansion of FoxP3+ regulatory T cells and PD-1+ T cells in peripheral blood following high-dose IL-2 treatment
Patients received high-dose of IL-2 (10.106 IU) from day 0 to day 17 following the allogeneic NK cells transfer. We then investigated the influence of this treatment on regulatory CD4+ T lymphocytes (Tregs) and exhausted T cells. For this purpose, FoxP3+ Tregs were monitored by flow cytometry at the indicated times. Tregs levels are similar in all patients at baseline (prior chemotherapy). We found a significantly high increase of Tregs starting at day 7 after NK cell transfer, with a peack at day 14 (4.41% to 53.39% baseline versus day 14 respectively P < 0.001), before gradually decreasing five days after the end of IL-2 injections. However the circulating level of these cells were still high through the treatment, as compared to baseline (16.10% versus 4.41% respectively (P = 0.014) (-). The inhibitory receptors PD-1 and Tim3 are expressed by T cells upon a chronic activation, and characterized exhausted T cells that loss most of T cell functions.Citation22,Citation23 Moreover, PD-1 expression has also been reported as a marker of circulating tumor-specific T cells in cancer patients.Citation24,Citation25 Like Tregs, we found similar kinetic of circulating PD-1+ T cells in most patients concurrently to the IL-2 therapy. These cells highly expanded at day 14 before dropped from day 28 when IL-2 treatment was stopped ( and ). However, no obvious change of Tim3+ T cells was found in most patients (data not shown). Thus, high expansion of Tregs and PD-1+ T cells occurred in patients receiving high-dose IL-2 therapy after adoptive cell transfer.
Figure 5. Monitoring of FoxP3+Tregs and PD-1+T through treatment. Flow cytometry analysis was performed from PBMC collected in each patient at the indicated time points. A. Tregs representative plot from one patient (#5). The kinetic of percentage (B) and absolute count of FoxP3+ Tregs are shown. The percentage of PD-1+ CD4 (D) and CD8 (E) T cells are shown. Baseline (BI)
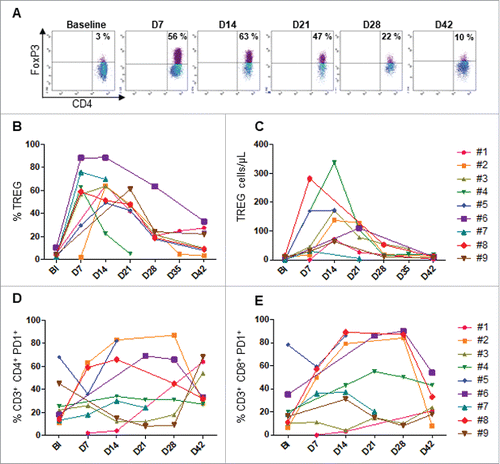
Table 1. Patient's main clinical characteristics and doses
Table 2. Main toxicities.
Discussion
This phase I study determined that hepatic artery allogeneic NK cell therapy, combined to an anti-EGFR monoclonal antibody and high dose IL-2 treatment, is well tolerated. All patients received standard lymphodepleting chemotherapy regimen using cyclophosphamide (60 mg/kg) and fludarabine (25 mg/m2/day). The addition of only one dose of cyclophosphamide to 5 days of fludarabine is manageable but induces only a short-term lymphopenic period. A limited antitumor activity was observed in early clinical trials assessing the clinical interest of autologous NK cell adoptive transfer.Citation15,Citation26,Citation27 Even if one partial response, one dissociated response and two disease stabilizations were observed in these highly pretreated patients, the antitumor activity of allogeneic NK cell therapy appeared also dismal here. No obvious correlation was found between treatment efficacy and main patients’ clinical characteristics.
One limiting factor here might be the lymphodepleting chemotherapy containing fludarabine and unique infusion of cyclophosphamide. This regimen only induced a short-term lymphopenia and low levels of IL-15. In the pivotal study reported by Miller JS et al for the treatment of myeloid leukemia, dosage of IL-15 was identified as a potential biomarker of efficacy.Citation9 These authors observed an increase of IL-15 above 40 pg/mL 7 days after the adoptive transfer in most patients that correlated with the in vivo amplification of infused NK cells. In our study, IL-15 levels increased above the baseline and were higher than those of Wallen H and colleaguesCitation28 but were lower than those reported by Miller JS,Citation9 suggesting that two infusions of cyclophosphamide could be required during the lympho-depleting chemotherapy to ensure an effective plasmatic IL-15 availability. Nonetheless, a chimerism could be observed in 5 evaluated patients despite the low level of IL-15 measured in this cohort (). The in situ injection through hepatic artery may counterbalance this limitation by promptly increasing the tumor infiltration by NK cells, an approach previously used in hepatocarcinoma with objective clinical responses.Citation29
Table 3. Persistence of donor circulating NK cells post injection
In order to promote in vivo NK cell activation, patients received 6 injections of high-dose IL-2 treatment from the 6th hour post NK cell transfer until day 17. This therapy was associated with a high expansion of CD4+CD25+Foxp3+Tregs and PD-1+ T cells in patient's peripheral blood. Thus this IL-2 regimen generates an immunosuppressive environment for the transferred cells. Expansion of host Tregs has been associated with lack of NK cells in vivo expansion.Citation30-32 Furthermore clinical responses to high-dose IL-2 therapy are limited due to selective expansion of Foxp3+ICOS+ Tregs, rather than natural killer cells and effector T cells.Citation33,Citation34 To improve the treatment efficacy, Miller's group proposed that depletion of Treg would improve anti-AML NK cell function.Citation35
Direct adoptive transfer of immune cells by the liver artery is an attractive option to enhance homing of lymphocytes in liver metastases. The potential interest of lymphocytes liver artery infusion was reported in a study using Indium-111 (111-In)-oxinate-labeled lymphocytes as a source of donor lymphocyte infusion. In this clinical trial, allogeneic lymphocytes homed in liver metastases, and the liver to sternum ratio of radioactivity was higher in patients treated with direct hepatic arterial injections compared with those receiving IV infusions.Citation36 These results supported a phase I clinical trial investigating the safety of T lymphocytes genetically modified with a chimeric antigen receptor (CAR T cells) targeting the carcinoembryonic antigen (CEA) and delivered by percutaneous hepatic artery infusions. Six patients were treated with anti-CEA CAR T cells by intrahepatic artery ± IL-2 support.Citation37 No grade 3–4 adverse event was observed in these trials. Of note, none of the 9 patients treated in the present phase I clinical trial experienced grade 3 or 4 hepatic cytolysis adverse event related to intra-hepatic artery allogeneic NK cell infusions.
Typing of HLA and KIR is important for donor selection because their polymorphisms affect NK cell function and thereby the clinical outcomes of NK cell therapy.Citation38 Considering the inhibitory KIR, the preferable donors are those which possess a KIR for which the cognate ligand is absent in the recipient, in particular when the donors also possess the corresponding ligand themselves.Citation39 In this study, we used HLA typing to determine KIRL mismatches and found that most clinical responses occurred in patients having at least two mismatches suggesting the importance of NK cells licensing.
ADCC is a key mechanism of action of several antibodies and it can be mediated by the Fc receptor CD16 expressed by NK cells. As previously reported, we show that Cetuximab is able to mediate ADCC in vitroCitation40 and this NK-mediated cytotoxicity is prevented when inhibitory KIRCitation41 or ILT2 are overexpressed.Citation42 Then, it was reasonable to hypothesize that combination of NK alloreactivity and Cetuximab-mediated ADCC would promote NK cytotoxic functions. The observation that KIR-ligand mismatches seem to be correlated with the clinical outcomes of alloreactive NK adoptive transfer supports this assumption. Anti-KIR monoclonal antibodies might be another attractive therapeutic option to promote ADCC and NK cell activities. The observation that KIR-mediated inhibition can dampen ADCC activity supports the development of KIR neutralizing monoclonal antibodies. Fully humanized anti-KIR antibodies blocking inhibitory KIR interactions with their cognate MHC class I ligands have been currently developed.Citation43,Citation44 Although CARs strategies have focused on T cells, CAR-modified NK cells have gained considerable interest recently. The development of CAR NK cells technology may provide a method to direct NK cells more specifically to cancer cells, with less functional heterogeneity and less risk of adverse effects than CAR T cells.Citation45
In conclusion, these results prompt us to further develop targeted adoptive NK cell transfer in solid tumor using tumor-specific monoclonal antibodies and in situ delivery.
Material and method
Patients
Six patients treated for a colorectal cancer and three pancreatic cancer patients were enrolled in this trial between October 2009 and December 2012 (NCT 02845999) at university hospital of Besançon. The main objective of this study will be to demonstrate the safety of NK hepatic intraarterial infusion in association with cetuximab. Secondary objectives will include the assessment of the clinical efficacy of this strategy. The median age of the patients was 60 years (range, 51–66) with a majority of males (67%). Eight patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 and one patient had an ECOG PS 1. All patients had liver metastases, 5 patients (56%) presented only a liver-limited metastatic disease and 4 patients had 2 or more visceral sites involved by metastases. All patients had been exposed to at least two lines of chemotherapy before inclusion. Epidermal Growth Factor Receptor (EGFR) expression status was assessed by immunohistochemistry (mouse, clone 3C6, Roche-Ventana) on a Ventana BenchMark ULTRA XT staining platform. Patients having a membrane expression higher than 1% of the tumor cells were considered as eligible. The KRAS mutation status was routinely performed in 4 patients. Among them, two patients had a KRAS mutation (data not shown). The clinical trial was approved by the university hospital of Besançon ethic committee and by the national drug agency, Agence Nationale de Sécurité du Médicament, ANSM, France, N° Eudra-CT 2008-A01134-51). All patients gave their written consent.
Donor selection
Donor identification was performed among patients’ relative in a first step. In the absence of familial donor, selection was performed among healthy blood donors from the French Blood Bank (EFS, Bourgogne Franche-Comté). At least one KIRL (Killer Immunoglobulin Receptor Ligand) mismatch was required. MHC class I alleles considered for donor selection were HLA A3-A11, HLA-Cw or HLA Bw4. A two digits HLA genotyping assay was used to determine donor and patient HLA A3-A11, Cw or Bw4 status. High-resolution genotyping was performed to confirm HLA allelic expression.
Allogeneic NK cells production
Peripheral blood mononuclear cells (PBMC) from selected healthy volunteer's donors were isolated by leukapheresis. CD3+ T cells were depleted using anti-CD3 magnetic microbeads and the CliniMACS program Depletion 2.1 according to manufacturer instructions (Miltenyi Biotech GmBH). Meanwhile, plasma was produced from a 100 mL blood sample taken from the donor. T cell depleted mononuclear cells were then washed and cultured overnight with 10% autologous sera and 1000 IU/mL of IL-2 (Novartis) in X-VIVO 15 medium (Lonza). Before adoptive transfer, cells were washed two times and suspended at 5.106/mL in albumin 4% (LFB, France). The composition of the cell product was analyzed by flow cytometry using CD19 (BD Pharmingen), CD3, CD56, CD14 and Annexin V (Beckman Coulter) staining. Liberation criteria included: cell viability>75%, percentage of CD3−CD56+ lymphocytes >20% and percentage of residual CD3+ T lymphocytes < 1% of in the final product.
Treatment procedure
The treatment schedule is summarized in . Patients were treated with lymphodepleting chemotherapy (fludarabine 25 mg/m2 and cyclophosphamide 60 mg/kg) before NK cells injection. After overnight interleukin-2 activation, the cell therapy product was immediately infused through the hepatic artery under radiological guidance by the interventional radiologist. Allogeneic NK cells were adoptively transferred according to a dose escalation protocol, classical 3+3 design. The three escalating dose levels were 3.106, 8.106 and 12.106 NK cells/kg of recipient body weight. IL-2 (10.106 IU) was administered subcutaneously, starting 6 hours after the adoptive transfer and every 3 days for 6 injections. The anti-EGFR monoclonal antibody Cetuximab (250 mg/m2) was administered intravenously every week for 7 weeks, starting from day 1. The patients were kept under observation for 24 h post procedure and discharged on the subsequent day. During the hospital stay, all clinical parameters and adverse events were recorded.
Safety and efficacy assessments
Adverst events (AEs) were collected at baseline and during the study, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The DLT was defined as any of the following AEs considered related to NK cells injection that occur in at least 3 out of 6 patients at a given dose level, with severity grade 3 or 4 according to CTCAE version 4.0. The objective responses included complete response, partial response and stable disease based on investigator-assessed tumor response according to RECIST criteria v1.1.
Immunomonitoring
Plasma and PBMC were collected at day 7, 14, 21, 28, 35 and 42 after NK cell adoptive transfer. The following cytokines IL-15, IL-7, IL-10 and IFN-γ were measured in the plasma using ELISA kit from Diaclone biotech (IL-7, IL-10 and IFN-γ) and R&D Systems (IL-15) according to the manufacturer instructions. Regulatory T cells (Tregs) and PD-1 and TIM3 expression on T lymphocytes were monitored using flow cytometry. Briefly, Tregs analysis was performed by multicolor staining using the following surface monoclonal antibodies against: CD3 (VM53, BD Biosciences), CD4 (RPA-T4, BD Biosciences), CD127 (A7R34, eBioscience), CD25 (MOPC-21, Biolegend, Ozyme), and the intracellular antibody FoxP3 (259D, Biolegend, Ozyme, France) according to the manufacturer's intracellular protocol (00-5523-00, eBioscience, France). For PD-1 and TIM-3 expression on T cells, the following surface monoclonal antibodies against CD3, CD4, CD8 (SK-1, Biolegend), PD-1(MIH-4, BD Biosciences) and TIM-3(R&D Systems, UK) were used and incubated with PBMC for 30 min at 4°C. Cells were analyzed on a FACS Canto II using DIVA software (BD Biosciences).
NK cells degranulation assay
NK cells were cocultured in the presence of target cells (K562 and HT29) for 4 h at a 2:1 Effector:Target ratio with GolgiStop (BD Biosciences) according to the manufacturer's protocol. HT-29 was coated with Cetuximab to induce ADCC activity. CD20-specific human IgG Rituximab was used as a negative control. Degranulation capacity of NK cells was monitored by flow cytometric analysis of CD107 a (BD Biosciences) expression. Cells were analyzed on a FACS Canto II using DIVA software (BD Biosciences).
Circulating donor NK cells detection
Peripheral blood donor NK circulating cells were analyzed using a chimerism multiplex STR profiling method. Briefly, Donor (D) and recipient (R) profiles were obtained after PCR amplification of 10 ng of DNA extracted from D and R before NK cell infusion using the PowerPlex® 18D System (Promega, France) according to the manufacturer instructions. Mixed chimerism was considered positive if D profile was recovered in at least 2 out 17 informative loci. R and D Peak areas were used to calculate the ratio between D- and R- derived DNA in a chimeric locus, expressed as mean of all informative loci.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2017ONCOIMM0765R-f07-z-4c.pptx
Download MS Power Point (429 KB)2017ONCOIMM0765R-f06-z-4c.pptx
Download MS Power Point (210.5 KB)Acknowledgments
The authors thank all patients and healthy volunteers who participated in this study. The authors also thank all contributors in oncology and, radiology departments of university hospital and in Etablissement Français du Sang de Bourgogne Franche-Comté. We also thank the Biomonitoring platform of CIC-1431 and the cell therapy unit AICT for their technical supports and the biobank BB-0033-00024 “Tumorothèque régionale de Franche-Comté” for biobanking.
Additional information
Funding
References
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi:10.1038/ni1582.
- Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–75. doi:10.1182/blood-2004-01-0380.
- Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi:10.1038/ni1138.
- Falco M, Moretta L, Moretta A, Bottino C. KIR and KIR ligand polymorphism: a new area for clinical applications? Tissue Antigens. 2013;82:363–73. doi:10.1111/tan.12262.
- Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncog. 2014;19:133–41. doi:10.1615/CritRevOncog.2014011091.
- Berraondo P, Labiano S, Minute L, Etxeberria I, Vasquez M, Sanchez-Arraez A, Teijeira A, Melero I. Cellular immunotherapies for cancer. Oncoimmunology. 2017;6:e1306619. doi:10.1080/2162402X.2017.1306619.
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi:10.1126/science.1068440.
- Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, Favre G, Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8. doi:10.1038/sj.leu.2403524.
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi:10.1182/blood-2004-07-2974.
- Curti A, Ruggeri L, Parisi S, Bontadini A, Dan E, Motta MR, Rizzi S, Trabanelli S, Ocadlikova D, Lecciso M, et al. Larger size of donor alloreactive NK Cell repertoire correlates with better response to NK cell immunotherapy in elderly acute myeloid leukemia patients. Clin Cancer Res. 2016;22:1914–21. doi:10.1158/1078-0432.CCR-15-1604.
- Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–9. doi:10.1182/blood-2011-01-329508.
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui C-H, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi:10.1200/JCO.2009.24.4590.
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–84. doi:10.1073/pnas.96.12.6879.
- Frohn C, Doehn C, Durek C, Böhle A, Schlenke P, Jocham D, Kirchner H. Feasibility of the adoptive transfusion of allogenic human leukocyte antigen-matched natural killer cells in patients with renal cell carcinoma. J Immunother. 2000;23:499–504. doi:10.1097/00002371-200007000-00014.
- Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–97. doi:10.1158/1078-0432.CCR-11-1347.
- Naramura M, Gillies SD, Mendelsohn J, Reisfeld RA, Mueller BM. Therapeutic potential of chimeric and murine anti-(epidermal growth factor receptor) antibodies in a metastasis model for human melanoma. Cancer Immunol Immunother. 37:343–9. doi:10.1007/BF01518458.
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–8. doi:10.1126/science.1241145.
- Srivastava AK, Jaganathan S, Stephen L, Hollingshead MG, Layhee A, Damour E, Govindharajulu JP, Donohue J, Esposito D, Mapes JP, et al. Effect of a Smac Mimetic (TL32711, Birinapant) on the apoptotic program and apoptosis biomarkers examined with validated multiplex immunoassays fit for clinical use. Clin Cancer Res. 2016;22:1000–10. doi:10.1158/1078-0432.CCR-14-3156.
- Trotta AM, Ottaiano A, Romano C, Nasti G, Nappi A, De Divitiis C, Napolitano M, Zanotta S, Casaretti R, D'Alterio C, et al. Prospective evaluation of cetuximab-mediated antibody-dependent cell cytotoxicity in metastatic colorectal cancer patients predicts treatment efficacy. Cancer Immunol Res. 2016;4:366–74. doi:10.1158/2326-6066.CIR-15-0184.
- Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, Lemberg K, Hurley CK, Kleiner DE, Merchant MS, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125:784–92. doi:10.1182/blood-2014-07-592881.
- Marçais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, Rabilloud J, Mayol K, Tavares A, Bienvenu J, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–57. doi:10.1038/ni.2936.
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi:10.1038/ni.2035.
- Lin S-J, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–33. doi:10.1084/jem.20062150.
- Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–8. doi:10.1038/nm.4051.
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi:10.1084/jem.20100637.
- Benyunes MC, Higuchi C, York A, Lindgren C, Thompson JA, Buckner CD, Fefer A. Immunotherapy with interleukin 2 with or without lymphokine-activated killer cells after autologous bone marrow transplantation for malignant lymphoma: a feasibility trial. Bone Marrow Transplant. 1995;16:283–8.
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. doi:10.1056/NEJM198512053132327.
- Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8T cells in patients with metastatic melanoma. PloS One. 2009;4:e4749. doi:10.1371/journal.pone.0004749.
- Qin Z, Chen J, Zeng J, Niu L, Xie S, Wang X, Liang Y, Wu Z, Zhang M. Effect of NK cell immunotherapy on immune function in patients with hepatic carcinoma: A preliminary clinical study. Cancer Biol Ther. 2017;18:323–30.
- Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother CII. 2010;59:1739–44. doi:10.1007/s00262-010-0896-z.
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi:10.3109/14653249.2010.515582.
- Miller JS. Therapeutic applications: natural killer cells in the clinic. Hematol Am Soc Hematol Educ Program. 2013;2013:247–53.
- Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124:99–110. doi:10.1172/JCI46266.
- Yao X, Ahmadzadeh M, Lu Y-C, Liewehr DJ, Dudley ME, Liu F, Schrump DS, Steinberg SM, Rosenberg SA, Robbins PF. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–96. doi:10.1182/blood-2011-10-386482.
- Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–63. doi:10.1182/blood-2013-10-532531.
- Barkholt L, Danielsson R, Calissendorff B, Svensson L, Malihi R, Remberger M, Uzunel M, Thörne A, Ringdén O. Indium-111-labelled donor-lymphocyte infusion by way of hepatic artery and radio-frequency ablation against liver metastases of renal and colon carcinoma after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78:697–703. doi:10.1097/01.TP.0000129807.53523.97.
- Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res. 2015;21:3149–59. doi:10.1158/1078-0432.CCR-14-1421.
- Benson DM, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res. 2014;2:99–104. doi:10.1158/2326-6066.CIR-13-0219.
- Koehl U, Kalberer C, Spanholtz J, Lee DA, Miller JS, Cooley S, Lowdell M, Uharek L, Klingemann H, Curti A, et al. Advances in clinical NK cell studies: Donor selection, manufacturing and quality control. Oncoimmunology. 2016;5:e1115178. doi:10.1080/2162402X.2015.1115178.
- Taylor RJ, Saloura V, Jain A, Goloubeva O, Wong S, Kronsberg S, Nagilla M, Silpino L, de Souza J, Seiwert T, et al. Ex vivo antibody-dependent cellular cytotoxicity inducibility predicts efficacy of cetuximab. Cancer Immunol Res. 2015;3:567–74. doi:10.1158/2326-6066.CIR-14-0188.
- Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–401. doi:10.4049/jimmunol.180.9.6392.
- Roberti MP, Juliá EP, Rocca YS, Amat M, Bravo AI, Loza J, Coló F, Loza CM, Fabiano V, Maino M, et al. Overexpression of CD85j in TNBC patients inhibits Cetuximab-mediated NK-cell ADCC but can be restored with CD85j functional blockade. Eur J Immunol. 2015;45:1560–9. doi:10.1002/eji.201445353.
- Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–86. doi:10.1182/blood-2013-08-519199.
- Vey N, Bourhis J-H, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–23. doi:10.1182/blood-2012-06-437558.
- Oberschmidt O, Kloess S, Koehl U. Redirected primary human chimeric antigen receptor natural killer cells as an “Off-the-Shelf Immunotherapy” for improvement in cancer treatment. Front Immunol. 2017;8:654. doi:10.3389/fimmu.2017.00654.