ABSTRACT
Multiple myeloma (MM) results from expansion of abnormal plasma cells in the bone marrow (BM). Previous studies have shown that monocytes play a crucial role in MM pathophysiology. A 6-sulfo LacNAc-expressing population of dendritic cells (Slan-DCs) that overlaps with intermediate and non-classical monocytes in terms of phenotype has been described. Slan-DCs represent a circulating and tissue proinflammatory myeloid population which has been shown to play a role in different cancer contexts, and which exhibits a remarkable plasticity. Herein, we studied Slan-DCs from the BM and blood of MM patients. We performed quantitative and functional analyses of these cells from 54 patients with newly diagnosed, symptomatic MM, 21 patients with MGUS and 24 responding MM patients. We found that circulating Slan-DCs were significantly decreased in MM patients as compared to those of healthy donors or patients with MGUS, while CD14+CD16+ intermediate monocytes accumulate in the BM. Moreover, after activation with TLR7/8 ligand R848, IL-12-producing Slan-DCs from the BM or peripheral blood from MM patients were decreased as compared with healthy donors. We show that MM cell lines or MM cells isolated from patients at diagnosis were able to inhibit the production of IL-12 by Slan-DCs, as well as to shift the phenotype of Slan-DCs towards an intermediate monocyte-like phenotype. Finally, Slan-DCs that have been cultured with MM cells reduced their capacity to induce T cell proliferation and Th1 polarization. We conclude that Slan-DCs represent previously unrecognized players in MM development and may represent a therapeutic target.
Introduction
Multiple myeloma (MM) is characterized by expansion of abnormal plasma cells in the bone marrow (BM). Survival and proliferation of myeloma cells is critically dependent on the BM microenvironment.Citation1 Monocytes and macrophages can produce inflammatory cytokines such as IL-6, which promote growth and increase survival of myeloma cells. In addition, macrophages secrete tumor necrosis factor alpha (TNF-α) which can indirectly promote tumor growth.Citation1, Citation2
The BM microenvironment is modified by the malignant plasma cells. For instance, these can modulate the number and composition of immune cells. Monocytes and macrophages are central in inflammatory responses, and increased numbers of macrophage/monocytes have been found in the BM of MM patients compared with normal controls.Citation2 Tumor-associated macrophages (TAMs) are mainly divided into two phenotypically and functionally distinct subtypes, M1 TAMs (tumoricidal) and M2 TAMs (immunosuppressive) which are extremes of a dynamic continuum rather than mutually exclusive cell states. M2 TAMs participate to the induction of immunosuppressive microenvironment both by versican proteolysis and angiogenesis through VEGF production.Citation3 These M2 TAMs have been shown to accumulate in MM patients bone marrow and this accumulation is associated with poorer survival.Citation4 Human peripheral blood (PB) monocytes can be classified into three distinct populations: classical CD16- CD14+, intermediate CD16+ CD14+ and non-classical CD16+ CD14- monocytes.Citation5 Recent reports showed that the proportion of non-classical monocytes increases with tumor cell load in the BM of MM patients,Citation4 and that these monocytes can be a potential cytological marker of osteoclast precursors.Citation6 Dendritic cells also play a major role in MM pathophysiology. In the blood, they are originally classified into different subtypes that include CD11c+ conventional DCs (cDCs) consisting in either CD141+ or CD1c+ cells, and BDCA2+ plasmacytoid DCs (pDCs). Both cDCs and pDC have been shown to accumulate in the bone marrow of MM patients and participate to tumor progression.Citation7, Citation8
An additional population of PB myeloid cells expressing MDC8 antigen containing a 6-sulfo LacNAc structure and called 6-sulfo LacNac dendritic cells (Slan-DCs), has also been described.Citation9 Slan-DCs in part overlap with non-classical or intermediate monocytes in terms of phenotype, and share a number of functional characteristics with cDCs. Functionally, PB Slan-DCs have been described as potent pro-inflammatory cells based on their capacity to produce large amount of TNF-α and IL-12p70 upon stimulation with Toll-like receptor (TLR) ligands.Citation10,Citation11 Circulating Slan-DCs also promote proliferation, IFN-γ and IL-17A production by T cells.Citation12 These cells have also been shown to play a role in different cancer contexts. For instance, in renal cancer, their phenotype is impacted by the neoplastic cells which make them tolerogenic.Citation13 In contrast, in colon carcinoma, the contingent of circulating Slan-DCs remains normal as well as their inflammatory properties.Citation14 Moreover, Slan-DCs exhibit a remarkable plasticity in terms of phenotype which may depend on the local tissue they infiltrate.Citation15 Recently, it has been shown that circulating Slan-DCs express functional CXCR410. The receptor's respective ligand, CXCL12, is an abundant component of the BM milieu, and is constitutively expressed by stromal cells,Citation16 suggesting that circulating Slan DCs can potentially migrate to the BM.
To gain novel insights on the role of Slan-DCs in MM pathophysiology and their potential impact on clinical outcome, we evaluated their phenotype and functions in the BM and the PB of patients with newly diagnosed monoclonal gammopathies (monoclonal gammopathy of undetermined significance (MGUS) and symptomatic MM). Also, we explored whether MM cells influence the ability of Slan-DCs to promote T-cell activation and proliferation.
Results
Slan-DCs are decreased in MM patients' PB while intermediate monocytes accumulate in the BM
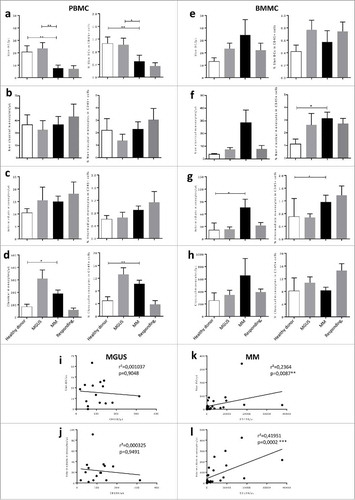
To understand the role of Slan-DCs in MM pathophysiology, we performed quantitative and functional analyses of these cells from 54 patients with newly diagnosed, symptomatic MM, 21 patients with MGUS and 24 responding MM patients. For comparisons, we used BM and PB from 6 healthy controls. Slan-DCs were identified by the expression of M-DC8 and HLA-DR. We also evaluated the different monocyte subsets according to the expression of CD14 and CD16, defined as CD14+CD16- classical monocytes, CD14+CD16+ intermediate and CD14-CD16+ non-classical monocytes (). We observed that percentages and absolute numbers of circulating HLA-DR+ M-DC8+ Slan-DCs were significantly decreased in the PB of MM patients as compared to those of healthy donors or patients with MGUS (). No difference was found in non-classical and intermediate monocytes absolute numbers between the groups whereas classical monocytes were increased in the PB of MM patients (, , ). In contrast, in the BM, no significant difference in the frequencies of Slan-DCs was observed between the different groups (), whereas the frequencies of intermediate and non-classical monocytes significantly increased in MM patients at diagnosis as compared to healthy donors or responding MM patients (, , ). Moreover, the numbers of BM Slan-DCs were positively correlated with CD138+ BM plasma cells in MM patients but not in MGUS patients (, ). The numbers of BM intermediate monocytes were also positively correlated with CD138+ BM plasma cells in MM patients but not in MGUS patients (, ) which is in line with a previous report.Citation4 We also observed a significant correlation between the absolute number of Slan-DCs (Spearman r2 = 0.37, p = 0.0058) but not between Intermediate Monocytes and CD138+ cells (Spearman r2 = 0.04, p = 0.3893) in the BM of responding patients (data not shown). Altogether, these results suggest that circulating Slan-DCs decrease in the PB while intermediate monocytes accumulate in the BM during the MGUS-to-MM progression, and that this accumulation is proportional to the extent of tumor burden.
Figure 1. Gating strategy for analysis of Slan-DC and monocyte subpopulations. Gating was sequentially done on FVD− (live cells) and CD138− after excluding fragments and doublets. Next, CD45+ HLA-DR+ cells were selected, and Slan-DCs were identified as M-DC8+ (a). Monocyte subtypes were discriminated according to the expression of CD16 and CD14 in HLA-DR+ MDC8- cells. Non-classical monocytes are CD16+ CD14− (b), intermediate monocytes CD16+ CD14+ (c) and classical monocytes CD16− CD14+ (d).
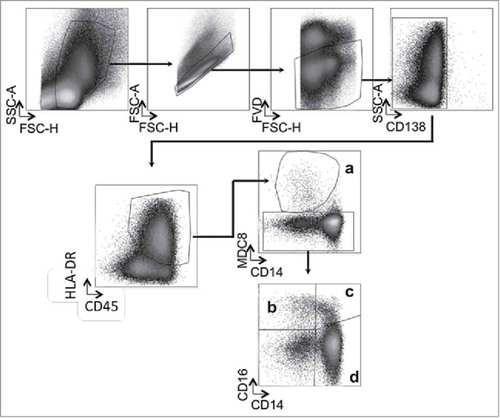
Figure 2. Circulating Slan-DCs are decreased in MM patients while intermediate monocytes accumulate in the bone marrow. The percentage in CD45+ cells and the absolute numbers of Slan-DCs and monocyte populations were compared in the blood and bone marrow from healthy donors, MGUS, MM patients at diagnosis and responding patients. a,b,c,d Number or percentage of indicated cell population from the blood of 11 healthy donors, 10 MGUS, 22 MM and 9 responding patients. e,f,g,h, Number or percentage of indicated cell population from the bone marrow of 6 healthy donors, 15 MGUS, 28 MM and 18 responding patients. i,j,k,l Correlation between the absolute numbers of BM plasma cells and intermediate CD14+ CD16+ monocytes and Slan-DCs in MGUS and MM patients. The Mann-Whitney test was used for comparisons of groups and Spearman's rank test for correlations. p values < 0.05 are represented by *, p values < 0.01 by ** and p values < 0.001 by ***.
Slan-DC secretion of IL-12p70 is inhibited in MM patients
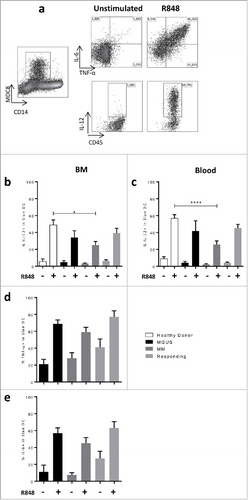
Circulating Slan-DCs in healthy subjects are able to secrete proinflammatory cytokines such as IL-1β, IL-6, TNF-α and IL-12p70 in response to different TLR ligands.Citation10-Citation12 Since the frequencies of these cells were modified in MM patients, we next addressed their functional properties, in particular cytokine production. We performed intracellular analysis of cytokines by flow cytometry after activation with TLR7/8 ligand R848. We observed a significant decrease in IL-12-producing Slan-DCs from the BM or PB from MM patients as compared with healthy donors (PB: Mean ± sem 29.53 ± 5.8% vs 56.86 ±4.32%; BM: 28.38 ± 5.55% vs 48.83 ± 6%, respectively). This decrease was partially restored in responding patients (PB: 45.40 ± 4.34% and BM 39.33 ± 5.62%)(, , ). We also measured the frequency of Slan-DCs in the BM from MM patients to secrete IL-6 and TNF-α, which are known to support myeloma growth. As shown on and , IL-6 and TNF-α positive Slan-DCs were not different in MM patients as compared to MGUS or responding patients.
Figure 3. Secretion of IL-12 but not TNF-α or IL-6 is inhibited in Slan-DCs from MM patients. BM or blood Slan-DCs were cultured in the absence or presence of the TLR7/8 ligand R848 for 24 h and compared in terms of cytokine secretion by flow cytometry. For IL-12p40 secretion, a 6 h pre-incubation was performed. a. A representative experiment is shown. b,d,e. Slan-DCs isolated from BM were stimulated or not with R848 and the production of IL12p40 (b), TNF-α (d) and IL-6 (e) were analyzed by intracellular staining and flow cytometry. c. Slan-DCs isolated from PB were stimulated or not with R848 and the secretion of IL-12p40 was analyzed by intracellular staining and flow cytometry. P values < 0.05 are represented by *. p values < 0.01 by **, p values < 0.001 by *** and p values < 0.0001 by ****.
MM cell lines inhibit IL-12 secretion by Slan-DCs
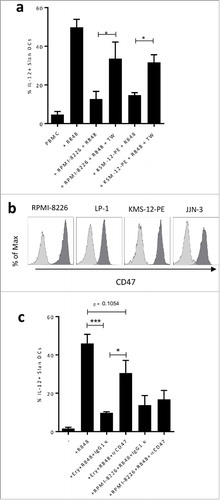
In order to investigate whether the decrease in IL-12 secretion was due to the malignant plasma cells, freshly sorted healthy circulating Slan-DCs were stimulated with R848 in the presence of different MM cell lines (RPMI-8226, JJN-3, LP-1, and KMS-12-PE) and their capacity to secrete IL-12 under this stimulation was measured by ELISA in the supernatant after culture. As expected after R848 stimulation, Slan-DCs produced IL-12p70 (Mean ± sem: 7.73 ± 2.15 pg/mL for medium vs 147.2 ± 97.47 pg/mL in the presence of R848). We could observe that some of the MM cell lines inhibited R848-induced IL-12 secretion by Slan-DCs (). The strongest inhibition was observed with RPMI-8226 and KMS-12-PE while this inhibition was limited with JJN-3. In order to confirm these results, healthy PBMC were cultured in the presence of the MM cell lines and R848, and the production of IL-12p40 was measured by intracellular staining after 24 h of co-culture. We observed that the percentage of IL-12p40 positive Slan-DCs strongly decreased after stimulation in the presence of RPMI-8226 or KMS-12-PE (). In contrast, secretion of TNF-α and IL-6 by PBMCs or sorted Slan-DCs was not inhibited by MM cell lines (). Of note, no inhibition was observed for IL-1β mRNA expression or cytokine production in culture supernatant (data not shown).
Figure 4. MM cells inhibit IL-12 production by Slan-DCs. a, c, e. Sorted Slan-DCs were cultured for 48 h in the absence or presence of R848 and the indicated MM cell lines for 24 h and then compared in terms of cytokine secretion in the culture supernatant by ELISA. For IL-12p70 secretion, a 6 h pre-incubation was performed. b,d, f. Total PBMC were cultured for 18 h in the absence or presence of R848, Golgi Plug and the indicated MM cell line. Then the cytokine secretion of Slan-DCs was analyzed by flow cytometry after intracellular staining. For IL-12p40 detection, a 6 h pre-incubation was performed. g. Sorted Slan-DCs were cultured for 48 h in the absence or presence of R848 or R848 and IFN-γ and the indicated MM cell line, and then analyzed for IL-12p70 secretion in the culture supernatant by ELISA or by flow cytometry after intracellular staining. h. Sorted Slan-DCs were cultured for 48 h in the presence of R848 and sorted CD138+ cells from 4 different MM patients at diagnosis for 24 h and then analysed for IL-12 secretion in the culture supernatant by ELISA. p values < 0.05 are represented by *, p values < 0.01 by ** and p values < 0.001 by ***.
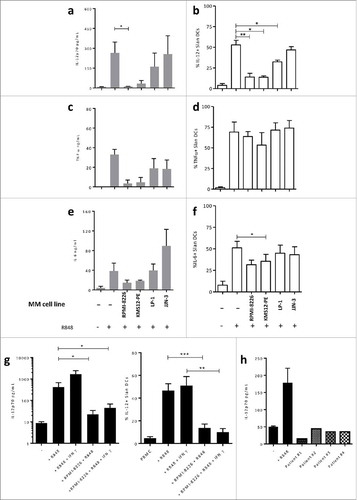
Given that the IL-12 secretion by SlanDCs has been shown to increase when the cells were primed with IFN-γ followed by LPS or R848 simulation,Citation11 we assessed the inhibition of IL-12 by MM cells under both R848 and IFN-γ stimulation. We observed that even with priming with IFN-γ, MM cells were able to decrease IL-12 production by Slan-DCS ().
In order to investigate whether primary MM cells from patients at diagnosis were also able to inhibit the production of IL-12 by Slan-DCs, we sorted CD138+ malignant cells from BMMCs and cocultured these cells with Slan-DCs in the presence of R848. As shown in , the production of IL-12p70 was drastically decreased in the presence of CD138+ cells isolated from 4 different patients. These results suggest that MM cells are able to strongly modulate the function of Slan-DCs.
In order to determine whether IL-12 production by Slan-DCs is inhibited by a contact-dependent mechanism, experiments were performed in which healthy PBMC were incubated or not with MM cell lines (RPMI 8226 and KMS-12-PE). As shown on , when Slan-DCs and MM cell lines were separated by a Transwell, the IL-12 production was restored, suggesting that IL-12 inhibition by MM cells was contact-dependent. Schäkel and colleagues previously reported that Slan-DCs' IL-12 secretion is blocked in the presence of erythrocytes in the culture by a mechanism dependent on their CD47 expression, an effect that can be reversed with an anti-CD47 mAb.Citation11 As CD47 can be overexpressed by malignant plasma cells,Citation17 we explored whether IL-12 inhibition was dependent on CD47 expressed by myeloma cells. As depicted on , CD47 is highly expressed by MM cell lines. Thus, we co-cultured RPMI-8226 with healthy PBMC in the presence of a blocking anti-CD47 monoclonal antibody in order to block CD47/SIRPα interaction. As control, we used freshly isolated erythrocytes to block IL-12 production, as previously demonstrated.Citation11 As shown in , IL-12 production by Slan-DCs was significantly inhibited by erythrocytes (50% vs 15% of IL-12 positive cells respectively) and in the presence of anti-CD47 in the culture, IL-12 production was partially restored as compared with isotype treatment. However, anti-CD47 failed to restore IL-12 production when Slan-DCs were co-cultured with RPMI-8226. These results suggest that IL-12 inhibition by MM cells is not dependent on CD47/SIRPα interaction.
Figure 5. a. Total PBMC were cultured for 48 h in the absence or presence of R848, Golgi Plug and the indicated MM cell line separated or not by a 0.4 µm transwell (TW). IL-12p40 production of Slan-DCs was analyzed by flow cytometry after intracellular staining. b. CD47 expression on myeloma cell lines. c. Total PBMC were cultured for 12 h in the absence or presence of human erythrocytes, then subjected to Ficoll gradient centrifugation to deplete erythrocytes followed by stimulation with R848 and Golgi Plug. The IL-12p40 production of Slan-DCs was analyzed by flow cytometry after intracellular staining after 24 hours. P values < 0.05 are represented by *. p values < 0.01 by ** and p values < 0.001 by ***.
MM cells impact Slan-DCs phenotype
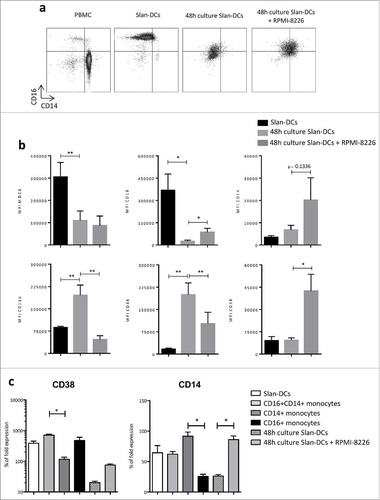
Plasma cells have been shown to affect the phenotype of monocytes and macrophages.Citation18 Moreover, Slan-DCs have been reported to exhibit a remarkable plasticity with a tissue-dependent phenotype.Citation15 Given that MM cells can inhibit IL-12 production, we hypothesized that the Slan-DCs' phenotype could be also impacted by plasma cells. To assess this, freshly-sorted Slan-DCs were cultured with or without the MM cell line RPMI-8226 for 48 h and their phenotype was analyzed by flow cytometry. In the latter setting, we observed that the expression of M-DC8 and CD16 strongly decreased, while CD11b and CD86 were induced, as compared to that of freshly sorted Slan-DCs (). Moreover, in the presence of MM cells, CD86 was significantly less induced on Slan-DCs (). In contrast, when Slan-DCs were cultured in the presence of MM cells, CD14, but not CD11b, was induced. Interestingly, CD38 staining was also significantly increased in the presence of MM cell line. CD38 upregulation was also confirmed by qPCR analysis (), suggesting that CD38 upregulation on Slan-DCs exposed to MM cells was not due to transfer of cell membrane fragments from MM cells via trogocytosis or vesicular transfer.
Figure 6. MM cell line impacts Slan-DCs phenotype. Slan-DCs were sorted from total PBMC and then maintained in the presence or absence of a MM cell line for 48 h before phenotyping. a. Dot plot representing the expression of CD14 and CD16 on total PBMC, freshly-sorted Slan-DCs or Slan-DCs after culture in the presence or absence of RPMI-8226. b. Graphs show the level of expression of indicated antigens in freshly-sorted Slan-DCs or Slan-DCs after culture with or without RPMI-8226. (n = 4). c. Slan-DCs were cultured in the presence or absence of RPMI-8226 for 48 h and then FACS sorted before RT-qPCR analysis of CD14 and CD38 expression (n = 3).*p<0.05, **p<0.01.
MM cells inhibit the Slan-DCs capacity to induce T cell proliferation and Th1 polarization
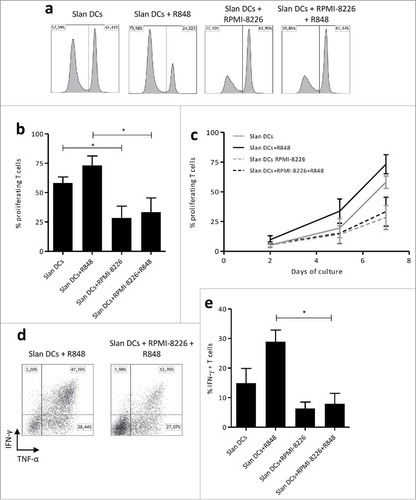
Since MM cells inhibit Slan-DCs secretion of IL-12, we explored whether MM cells could modulate their capacity to induce T cell proliferation and Th1 differentiation, given the pivotal role of IL-12 in Th1 polarization of CD4+ T cells. To this aim, we performed coculture experiments of T cells and Slan-DC. As demonstrated on , Slan-DCs efficiently induced the proliferation of T cells. Notably, their ability to promote T cell proliferation was even more pronounced after R848 priming. However, after pre-culture with RPMI-8226, the fraction of proliferating T cells at day 7 was significantly decreased (). Moreover, RPMI-8226 markedly impaired the capacity of Slan-DCs to induce the differentiation of naïve T cells into IFNγ-producing Th1 cells (,). These results provide evidence that MM cells are able to markedly impair Slan-DCs-mediated T-cell proliferation and polarization.
Figure 7. MM cell line impacts Slan-DCs-mediated T-cell proliferation and Th1 programming. a-c. Slan-DCs were cultured in the presence or absence of RPMI-8226 and/or R848 for 48 h, washed and subsequently co-incubated with allogeneic Cell Fluorescence Proliferation Dye 450-labelled CD3+ T cells. At days 2, 5 and 7, T cells were harvested and analyzed by flow cytometry. Values represent the percentage of proliferating cells stained with Cell Fluorescence Proliferation Dye 450. a. Histograms representing the fluorescence intensity of proliferating T cells at day 7 of culture. b, Percentage of proliferating T cells at day 7c, Percentage of proliferating T cells at days 2, 5 and 7 of culture. d-e Slan-DCs were cultured in the presence or absence of RPMI-8226 and/or R848 for 48 h, washed and subsequently co-incubated with naïve CD45RA+ CD4+ T lymphocytes. After 7 days, the percentage of IFN-γ producing CD4+ T cells was determined by flow cytometry. d. A representative experiment is shown. *p<0.05, by Student t test.
Discussion
The role of DCs in MM pathophysiology has been investigated by several groups. Some, but not all of them, showed that the frequency, phenotype and function of the different DC subsets are altered.Citation7,Citation19-Citation21 Whereas all these studies focused on the distinct myeloid or plasmacytoid DC subsets, little is known on the newly described human myeloid DC subset called Slan-DCs, characterized by M-DC8 expression.Citation9 Recently, the potential role of these particular DCs has been assessed in patients with solid tumors. Thus, it has been demonstrated that Slan-DCs are numerous in metastatic tumor-draining lymph nodes from carcinoma patients, and that Slan-DCs accumulate in renal carcinoma.Citation13,Citation14 Here, we show that the number of Slan-DCs is decreased in the PB of MM patients as compared to healthy donors and to MGUS patients. Moreover, we observed that the number of Slan-DCs increases with tumor load, suggesting that these cells can play a role in MM pathophysiology. These results are consistent with a recent report showing that Slan-DCs express functional CXCR4, indicating that they are able to be recruited to the BM environment in response to CXCL12Citation10.
Accumulating evidence suggest that DCs recruited to the tumor microenvironment undergo changes that endow them with regulatory functions promoting tumor growth.Citation22 In the MM context, Leone and colleagues recently demonstrated that pDCs and cDCs accumulate in the BM environment. Moreover, it has been shown that pDCs confer growth and survival advantage to MM cells.Citation8 In that study, they clearly demonstrated that MM cells abolish cDC and pDC antitumoral function through the decrease of cytotoxic CD38T cell activation.Citation7 It has been previously shown that circulating Slan-DCs secrete a large amount of IL-12 and TNF-α, leading to CD4 Th1 activation, and also CD8 and NK cells' cytotoxic activity, making them a DC population that can enhance antitumoral response.Citation9,Citation23 Therefore, we investigated the function of PB and BM-derived Slan-DCs in MGUS and MM patients. In our study and in line with the literature, Slan-DCs represent about 1% of total PBMC and are the main producer of IL-12 in healthy individuals.Citation12 However, we found that the capacity of R848-activated Slan-DCs to secrete IL-12 is profoundly impaired in both BM and PB Slan-DCs from MM patients. We also found that MM cells exhibit a strong capacity to suppress IL-12 secretion by Slan-DCs, a feature that was dependent on cell-to cell contact. Among surface antigens that can block IL-12 secretion, it was previously reported, that Slan-DCs' IL-12 secretion is blocked in the presence of CD47-expressing erythrocytes in the culture, which can be reversed with an anti-CD47 mAb.Citation11 In our study, anti-CD47 mAb failed to restore IL-12 secretion by Slan-DCs, suggesting that this cytokine's inhibition relies on other contact-dependent mechanisms. It has been recently shown that the IL-12 secreting capacity of Slan-DCs can be inhibited by mesenchymal stem cells through the secretion of prostaglandin E2 via a p38 mitogen-activated protein kinase inhibition.Citation24 Thus, it is possible that this pathway is involved in IL-12 inhibition by MM cells.
We also found that MM cells impair the capacity of Slan-DCs to stimulate CD3+ T cell proliferation and to polarize naïve CD4T cell into Th1 cells. Importantly, Wehner and colleagues showed that Slan-DCs improve NK cells cytotoxic activity. Thus, DC-NK cell interaction induces the maturation of Slan-DCs that, in fine, become more susceptible to promote differentiation of naïve CD4T cells into Th1 cells. In their report, the authors also pointed out that DC-NK cell interaction is critically dependent on IL-1223. Since MM cells inhibit IL-12 production by Slan-DCs, we can speculate that antitumoral cytotoxic activity of NK cells may be drastically decreased in the microenvironment. Altogether, these results provide evidence that MM cells are able to strongly suppress the proinflammatory function of Slan-DCs, leading them towards a more tolerogenic profile. Nevertheless, further studies are required to determine the underlying mechanisms of these alterations.
The extraordinary plasticity of Slan-DCs has been previously described in tonsils, where circulating Slan-DCs can acquire a different phenotype and morphology once they transmigrate to target tissues.Citation15 In the present study, we also observed that the decrease in IL-12 production was associated with important modifications of the phenotype of Slan-DCs by MM cells, as represented by their acquisition of markers of intermediate monocyte-like such as CD14 and CD16. Thus, we can speculate that Slan-DCs from MM patients acquire certain intermediate monocyte markers though the impact of plasma cells and may overlap intermediate monocytes which have been shown to accumulate in the BM of MM patients in relation with tumor load.Citation4,Citation6 These observations are in line with the accumulating evidence that circulating Slan-DCs represent a subset of monocytes that fully differentiate into more “DC-like” or “macrophage-like” cells depending on the microenvironnement of the target tissue.Citation25
Taken together, our results suggest that, in MM patients, Slan-DCs can be recruited to the BM and modified by the MM cells to decrease their proinflammatory functions through IL-12 inhibition and change of phenotype, leading to less T cell activation, thus propitiating tumor escape. During this process, Slan-DCs also acquire CD38, a finding already reported in a previous study by Arana et al,Citation26 suggesting that this population can be targeted by the immunotherapy drugs such as Daratumumab (DARA), which has been approved by the U.S Food and Drug Administration for MM patients who are refractory to proteasome inhibitors and immunomodulatory agents. DARA can deplete CD38+ MM cells through different mechanisms, such as complement-dependent cytotoxicity, Ab-dependent cytotoxicity, Ab-dependent phagocytosis and programmed cell death.Citation27-Citation29 Further studies should be aimed at establishing whether such MM cell-modified Slan-DCs can be depleted by DARA, or if their plasticity can be exploited for restoring their proinflammatory functions through immunotherapeutic strategies.
Materials and methods
Patients and biological samples
PB and BM samples were obtained from newly diagnosed monoclonal gammopathies classified as MGUS (n = 21) or symptomatic MM (n = 54), and 24 responding patients according to the International Myeloma Working Group criteriaCitation30,Citation31 (Supplementary Table 1). All patients were enrolled in clinical research protocols approved by local ethics committees. Written informed consent was obtained from each patient in accordance with the Declaration of Helsinki. BM aspirates from healthy individuals (n = 6) were used as controls. PBMC from healthy donors were obtained from the Etablissement Français du Sang Ile-de-France after informed consent. PB mononuclear cells (PBMCs) and BM mononuclear cells (BMMCs) were isolated using Lymphocyte Separation Medium (MP Biomedicals) by density gradient centrifugation.
Cell sorting
Slan-DCs were isolated from PBMC using Slan-(M-DC8)+ Monocyte Isolation Kit, (Miltenyi Biotec, Paris, France). Allogeneic naïve CD4+T cells and CD3+ T cells were isolated from PBMC of healthy donors using MagniSort Human CD4 Naïve Enrichment Kit or MagniSort Human T Cell Enrichment Kit, respectively (eBioscience, Paris, France). Malignant CD138+ cells were isolated from BMMCs of MM patients using CD138 Microbeads (Miltenyi Biotec). All sorted cell populations exhibited high purity (>90%), as revealed by flow cytometry.
Flow cytometry analysis
Absolute numbers of Slan-DCs and monocytes were determined using 50 µL of whole PB and BM samples from healthy donors and patients using Trucount tubes (BD Biosciences). Samples were then incubated for 15 min with 450 µL of BD FACS Lysis Solution (BD Biosciences) and analyzed by flow cytometry. Slan-DCs and monocytes were identified, respectively, as HLA-DR+/CD45+/M-DC8+ or HLA-DR+/M-DC8-/CD45+/CD16+/−/CD14+/− cells.Citation14 For immunophenotyping, cells were first incubated with Fixable Viability Stain 575V (BD Biosciences) to exclude dead cells from the analysis. The following mAb were used for surface staining: CD14 Pacific Blue (clone M5E2), CD16 FITC (clone 3G8), CD45 BV510 (clone H130), CD86 BV421 (clone 2331), HLA-DR BV785 (clone G46-6), all from BD Biosciences, CD11b PC7 (clone Bear1, Beckman Coulter), M-DC8 PE (clone DD1, Miltenyi Biotec). Stained cells were analyzed with a Gallios or Cytoflex (Beckman Coulter) flow cytometer and Kaluza Analysis software version 1.5a (Beckman Coulter).
Intracellular analysis of Slan-DCs cytokine production
PBMCs or BMMCs from patients or healthy donors (2 × 106/mL) were cultured for 24 h in 24-well plates in RPMI 1640 supplemented with 10% heat-inactivated human serum (Sigma-Aldrich), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM Minimum Essential Medium (MEM) Vitamin Solution (all from Life Technologies, Saint-Aubin, France). Cells were activated by 10 µg/mL R848 (InvivoGen, Toulouse, France), in the presence of Golgi Plug (BD Biosciences). For IL-12p40 detection, a 12 h pre-incubation was performed before R848 addition. After 24 h, cells were harvested, stained for cell-surface markers as indicated, fixed and permeabilized using BD Cytofix/Cytoperm solution (BD Biosciences), washed in Perm/Wash solution (BD Biosciences), and stained with mAb specific for IL-12p40/p70-PE (clone C11.5, BD Biosciences), IL-6-APC (clone MQ2-13A5, Miltenyi Biotec) or TNF-α-APC-Vio770 (clone cA2, Miltenyi Biotec).
MM cell lines
Human MM cell lines RPMI-8226, JJN-3, LP-1 and KMS-12-PE were obtained from the American Type Culture Collection (Rockville, MD, USA). All cell lines were cultured in RPMI 1640 supplemented with 10% heat-inactivated bovine serum (Sigma-Aldrich), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM minimum Essential Medium (MEM) Vitamin Solution (Life Technologies). Cells were maintained in log-phase growth in a humidified atmosphere containing 5% CO2 at 37°C.
Co-culture of Slan-DCs and MM cells
Slan-DCs isolated from PBMC were cultured for 12 h in 96-well plates in the presence or absence of MM cell lines or MM cells from patient at a 1/2 ratio before the addition of 10 µg/mL R848 with or without recombinant human IFN-γ (100 ng/mL, Peprotech), after which, they were cultured for 36 additional hours. Supernatants were harvested and frozen at -20°C until ELISA analysis. IL-6, IL-12p70 and TNF-α were measured using commercial kits (Ready-Set-go, eBiosciences). For intracellular cytokine analysis, PBMC (2 × 106/mL) were cultured in 24-well plates exactly as above. When indicated, PBMC were separated from MM cells during culture using Transwell (filter pore size 0.4 µm, Nunc). When indicated, the mAb anti-CD47 (10 µg/mL, clone B6H12, BD Biosciences) or the corresponding isotype at the same concentration was added to the culture.
T cell polarization and proliferation assay
Slan-DCs isolated from healthy PBMC were cultured in the presence or absence of the RPMI-8226 cell line overnight before the addition of 10 µg/mL R848, and then cultured for a total of 48 h. The RPMI-8226 cells were then removed by either magnetic cell sorting with anti-CD138 microbeads (Stemcell Technologies) or FACS using mAb anti-CD138 APC (Miltenyi Biotec). For proliferation and polarization, 1 × 105 T lymphocytes or CD4 naïve T cells were co-cultured with precultured Slan-DCs at a ratio of 1:10 in U-bottomed 96-well plates. T-cell proliferation was assessed by Cell proliferation dye eFluor® 450 (eBioscience) on days 2, 5 and 7. For T cell polarization analysis, CD4 naïve T cells were re-stimulated at day 7 with 25 ng/mL phorbol 12-myristate 13-acetate (PMA) and ionomycine (1μg/mL) (both from Sigma) for 5 h. Golgi Plug (BD Biosciences) was added for the last 4 h. Cells were fixed and permeabilized as described above before intracellular staining with IFN- γ (clone B27, BD Biosciences) specific mAb.
RT-qPCR analysis
Healthy PBMC were stained with CD16-Alexa647 (Biolegend), HLA-DR FITC (Beckman Coulter), CD14 Pacific Blue (Beckman Coulter) and M-DC8 PE (Miltenyi Biotec) and FACS sorted on a MoFloAstrios Cell Sorter before RNA extraction. After 48 h of coculture, Slan-DCs and RPMI-8226 were stained with CD138-APC and Fixable Viability Dye 450 (Thermofisher) and sorted on a MoFloAstrios Cell Sorter (Beckman Coulter) before RNA extraction. All populations exceeded 90% purity. RNA extraction was performed using RNeasy Mini kit (Qiagen, Cergy Pontoise, France). RNA was subjected to reverse transcription (High Capacity RNA-to-cDNA Master Mix, ThermoFisher Scientific) and quantified by real-time quantitative PCR using commercially available primer/probes sets (Assay-On-Demand, ThermoFisher Scientific): GAPDH (Hs99999905_m1), CD38 (Hs01120071_m1), CD14 (Hs02621496_s1). Real-Time PCR were performed on a 7500 Fast Dx Real-Time PCR Instrument (ThermoFisher Scientific). Relative expression for the mRNA transcripts was calculated using the ∆∆Ct method and GAPDH mRNA transcript as reference.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). Mann-Whitney test for comparisons of groups of patients, Student t-test for comparison of cell culture conditions and Spearman's rank test for correlations were used.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
Authors' contribution
All authors listed on the manuscript have contributed substantially to this work: conception and design: M.M., B.L., F.M., B.G.; financial support: M.M.; administrative and logistical support: M.M.; provision of study materials and patient care: R.T., F.V., L.G., Z.M., E.B., F.M., M.M.; experimental work: B.L., F.D.V., N.S.; collection and assembly of clinical data: N.S., I.B., C.M., F.M.; data analysis and interpretation: B.L., F.M., B.G., M.M.; manuscript writing: B.L., B.G., M.M.; final approval of manuscript: all co-authors.
2017ONCOIMM0906R1-file002.docx
Download MS Word (44.8 KB)Acknowledgments
The authors thank Annie Munier from the LUMIC flow cytometry facility. MM thanks Prof. J.V. Melo (Adelaide, Australia) for critical reading of the manuscript. The authors also thank the Association for Training, Education and Research in Hematology, Immunology and Transplantation for the generous and continuous support to the research work. This work was supported by a grant from INCA (Emergence).
Additional information
Funding
References
- Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125:3049–58. doi:10.1182/blood-2014-11-568881. PMID:25838343.
- Roussou M, Tasidou A, Dimopoulos MA, Kastritis E, Migkou M, Christoulas D, Gavriatopoulou M, Zagouri F, Matsouka C, Anagnostou D, et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia. 2009;23:2177–81. doi:10.1038/leu.2009.130. PMID:19587705.
- Chen H, Campbell RA, Chang Y, Li M, Wang CS, Li J, Sanchez E, Share M, Steinberg J, Berenson A, et al. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood. 2009;113:1992–2002. doi:10.1182/blood-2008-02-133751. PMID:19060246.
- Sponaas AM, Moen SH, Liabakk NB, Feyzi E, Holien T, Kvam S, Grøseth LA, Størdal B, Buene G, Espevik T, et al. The proportion of CD16(+)CD14(dim) monocytes increases with tumor cell load in bone marrow of patients with multiple myeloma. Immun Inflamm Dis. 2015;3:94–102. doi:10.1002/iid3.53. PMID:26029369.
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi:10.1182/blood-2010-02-258558. PMID:20628149.
- Petitprez V, Royer B, Desoutter J, Guiheneuf E, Rigolle A, Marolleau JP, Kamel S, Guillaume N. CD14+ CD16+ monocytes rather than CD14+ CD51/61+ monocytes are a potential cytological marker of circulating osteoclast precursors in multiple myeloma. A preliminary study. Int J Lab Hematol. 2015;37:29–35. doi:10.1111/ijlh.12216.
- Leone P, Berardi S, Frassanito MA, Ria R, De Re V, Cicco S, Battaglia S, Ditonno P, Dammacco F, Vacca A, et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood. 2015;126:1443–51. doi:10.1182/blood-2015-01-623975. PMID:26185130.
- Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai YT, Mitsiades C, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–23. doi:10.1016/j.ccr.2009.08.019. PMID:19800576.
- Schakel K, Mayer E, Federle C, Schmitz M, Riethmuller G, Rieber EP. A novel dendritic cell population in human blood: one-step immunomagnetic isolation by a specific mAb (M-DC8) and in vitro priming of cytotoxic T lymphocytes. Eur J Immunol. 1998;28:4084–93. doi:10.1002/(SICI)1521-4141(199812)28:12%3c4084::AID-IMMU4084%3e3.0.CO;2-4. PMID:9862344.
- Hansel A, Gunther C, Ingwersen J, Starke J, Schmitz M, Bachmann M, Meurer M, Rieber EP, Schäkel K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1T-cell responses. J Allergy Clin Immunol. 2011;127:787–94 e1-9. doi:10.1016/j.jaci.2010.12.009. PMID:21377044.
- Schakel K, von Kietzell M, Hansel A, Ebling A, Schulze L, Haase M, Semmler C, Sarfati M, Barclay AN, Randolph GJ, et al. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–77. doi:10.1016/j.immuni.2006.03.020. PMID:16782032.
- Schakel K, Kannagi R, Kniep B, Goto Y, Mitsuoka C, Zwirner J, Soruri A, von Kietzell M, Rieber E. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi:10.1016/S1074-7613(02)00393-X. PMID:12354382.
- Toma M, Wehner R, Kloss A, Hubner L, Fodelianaki G, Erdmann K, Füssel S, Zastrow S, Meinhardt M, Seliger B, et al. Accumulation of tolerogenic human 6-sulfo LacNAc dendritic cells in renal cell carcinoma is associated with poor prognosis. Oncoimmunology. 2015;4:e1008342. doi:10.1080/2162402X.2015.1008342. PMID:26155414.
- Vermi W, Micheletti A, Lonardi S, Costantini C, Calzetti F, Nascimbeni R, Bugatti M, Codazzi M, Pinter PC, Schäkel K, et al. slanDCs selectively accumulate in carcinoma-draining lymph nodes and marginate metastatic cells. Nat Commun. 2014;5:3029. doi:10.1038/ncomms4029. PMID:24398631.
- Micheletti A, Finotti G, Calzetti F, Lonardi S, Zoratti E, Bugatti M, Stefini S, Vermi W, Cassatella MA. slan/M-DC8+ cells constitute a distinct subset of dendritic cells in human tonsils. Oncotarget. 2016;7:161–75. PMID:26695549.
- Rankin SM. Chemokines and adult bone marrow stem cells. Immunol Lett. 2012;145:47–54. doi:10.1016/j.imlet.2012.04.009. PMID:22698183.
- Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, Sanderson R, Yang Y, Wilson C, Zangari M, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–57. doi:10.1182/blood.V99.5.1745. PMID:11861292.
- Beider K, Bitner H, Leiba M, Gutwein O, Koren-Michowitz M, Ostrovsky O, Abraham M, Wald H, Galun E, Peled A, et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget. 2014;5:11283–96. doi:10.18632/oncotarget.2207. PMID:25526031.
- Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–8. doi:10.1182/blood.V98.10.2992. PMID:11698282.
- Raje N, Gong J, Chauhan D, Teoh G, Avigan D, Wu Z, Chen D, Treon SP, Webb IJ, Kufe DW, et al. Bone marrow and peripheral blood dendritic cells from patients with multiple myeloma are phenotypically and functionally normal despite the detection of Kaposi's sarcoma herpesvirus gene sequences. Blood. 1999;93:1487–95. PMID:10029575.
- Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–7. doi:10.1182/blood.V100.1.230. PMID:12070032.
- Ma Y, Aymeric L, Locher C, Kroemer G, Zitvogel L. The dendritic cell-tumor cross-talk in cancer. Curr Opin Immunol. 2011;23:146–52. doi:10.1016/j.coi.2010.09.008. PMID:20970973.
- Wehner R, Lobel B, Bornhauser M, Schakel K, Cartellieri M, Bachmann M, Rieber EP, Schmitz M. Reciprocal activating interaction between 6-sulfo LacNAc+ dendritic cells and NK cells. Int J Cancer. 2009;124:358–66. doi:10.1002/ijc.23962. PMID:18942710.
- Langosch S, Wehner R, Malecka A, Franks HA, Schakel K, Bachmann M, Jackson AM, Schmitz M. Impact of p38 mitogen-activated protein kinase inhibition on immunostimulatory properties of human 6-sulfo LacNAc dendritic cells. Immunobiology. 2016;221:166–74. doi:10.1016/j.imbio.2015.09.012. PMID:26391152.
- Ancuta P. A slan-based nomenclature for monocytes? Blood. 2015;126:2536–8. doi:10.1182/blood-2015-10-675470. PMID:26635407.
- Arana P, Zabaleta A, Lasa M, Maiso P, Alignani D, Jelinek T, Segura V, van den Bossche W, Teodosio C, Damasceno D, et al. High-Throughput Characterization and New Insight into the Role of Tumor Associated Macrophages (TAMs) in Multiple Myeloma (MM). Blood. 2016;128:482.
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. doi:10.4049/jimmunol.1003032. PMID:21187443.
- Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P, et al. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcgamma Receptor-Mediated Cross-Linking. J Immunol. 2016;197:807–13. doi:10.4049/jimmunol.1501351. PMID:27316683.
- Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–21. doi:10.1080/19420862.2015.1007813. PMID:25760767.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48. doi:10.1016/S1470-2045(14)70442-5. PMID:25439696.
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5. doi:10.1182/blood-2010-10-299487. PMID:21292775.
