ABSTRACT
This study investigated the anti-tumor effects of stereotactic body radiation therapy (SBRT) with thymosin alpha-1 (Tα1) in heavily pretreated, metastatic esophageal squamous cell carcinoma (mESCC) patients. Thirty-one patients with at least 2 metastatic sites were enrolled. SBRT was delivered with a daily fraction of 5.0 Gy for a total dosage of 25 Gy over one week to one metastatic lesion. Concurrent Tα1 (1.6mg subcutaneously) was administered twice a week with an interval of 3-4 days until tumor progression of other documented metastatic lesions. Anti-tumor effects (the primary endpoint) were evaluated by assessing the CT/MRI response of other distinct measurable lesions. Secondary endpoints included treatment safety, survival outcomes and immune-related blood parameters. This study was registered at ClinicalTrials.gov (NCT 02545751). Partial response occurred in three (9.7%) patients, and 11 (35.5%) patients had stable metastatic disease, which yielded a metastatic-lesion control rate of 45.2%. Seventeen (54.8%) patients were documented to have progressive disease in other metastatic lesions. The median overall survival and abscopal progression free survival (APFS) times were 5.2 and 2.9 months, respectively. Significant differences in survival outcomes were observed between the abscopal control group (without progression in the abscopal lesions at 12 weeks) and the non-control group (P = 0.035 and 0.044, respectively). Treatment-related toxicity was acceptable, and no grade 4 acute toxicity occurred. Immunomonitoring of lymphocytes showed that the proportion of CD8+ T cells after treatment was significantly different between the abscopal control group and the non-control group (P=0.047). In conclusion, the combination of SBRT with Tα1 produced encouraging effects in heavily pretreated, mESCC patients and further research on radiation enhanced immunotherapy is warranted.
Introduction
Carcinoma of the esophagus remains one of the most virulent cancers worldwide, and esophageal squamous cell carcinoma (ESCC) accounts for nearly 90% of these cases in China.Citation1 The management of esophageal cancer commonly includes surgery, radiotherapy and chemotherapy. However, even with the combination of these treatment approaches, the majority of patients will ultimately develop recurrence and/or distant metastases.Citation2,Citation3
Recently, insights regarding the synergistic effects between radiotherapy and immunotherapy have elicited renewed interest in the ability of radiotherapy to induce tumor regression outside of the radiation fields leading to meaningful clinical benefit.Citation4-Citation6 In a proof-of-principle study published in the Lancet, 41 patients with metastatic lesions were treated with radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF). Abscopal responses were observed in 11 patients. This study demonstrated that immune-mediated abscopal responses were not just anecdotal clinical cases but produced valuable benefit in some metastatic patients. Furthermore, in-situ anti-tumor vaccines triggered by focal high-dose radiotherapy (vaccination effect) were considered as the underlying mechanism responsible for such effects.Citation7,Citation8
Thymosin alpha-1 (Tα1) is a synthetic 28-amino acid peptide.Citation9 Preclinical data indicate that Tα1 can upregulate major histocompatibility complex (MHC) class I antigen expression in normal and transformed cells. Clinical studies have further suggested that Tα1 can stimulate innate as well as adaptive immune responses in cancer patients.Citation10,Citation11 Based on these findings, we postulated that stereotactic body radiation therapy (SBRT) plus Tα1-mediated immune effects could contribute to better disease control of non-irradiated metastatic lesions in heavily pretreated, metastatic ESCC (mESCC) patients and provide additional clues to the potential mechanisms responsible.
Materials and methods
Ethics statement
This study was conducted as a prospective trial in Hangzhou Cancer Hospital from January 2016. Subsequently, the Central Hospital of Lishui City joined this trial in May 2016. This trial was approved by the institutional review board (IRB) of each hospital, and all patients provided written informed consent before enrollment. We confirm that the present study was performed in accordance with good clinical practice guidelines and the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (NCT 02545751).
Eligibility criteria
Patients who fulfilled the following criteria were eligible for the present study: I). Patients with cytopathologically confirmed ESCC with persistent and metastatic or recurrent and metastatic disease, despite the primary treatment strategies. II). Patients with at least 2 measurable metastatic lesions/sites with a largest diameter of at least 10 mm or larger on a CT/MRI scan. Malignant lymph nodes had to meet the criterion of a short axis ≥ 15 mm by CT scan. Primary tumors of the esophagus were not considered a measurable lesion. In addition, metastatic lesions within a prior radiation field were acceptable as long as the disease had progressed in the radiation field according to the RECIST 1.1 criteria. Out of filed regional metastatic lymph nodes were also candidates for tumor response. III). ECOG performance status of 0 to 1. VI). Life expectancy ≥3 months. V). Patients with adequate baseline organ and marrow function as defined by an absolute neutrophil count ≥1,500/mm3, white blood cell count ≥3,000/mm3, platelet concentration ≥50,000/μL, total bilirubin less than 1.5 times the upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase less than 2.5 times the ULN, and serum creatinine less than 1.5 times the ULN. The minimum patient age was 18 years.
Treatment plan
All patients were given subcutaneous injections of Tα1 × 1.6mg (=1 amp) twice per week with an interval of 3–4 days each week until the documented progression of other non-irradiated metastatic lesions. SBRT was concurrently delivered on the first day of the initiation of Tα1 using a high-energy linear accelerator. The prescribed dosage was 25 Gy in 5 fractions administered over one week. The gross tumor volume (GTV) was defined as gross disease based on the CT/MRI scan. The clinical target volume (CTV) was defined as the outside expansion of GTV and a margin to encompass any microscopic disease. The planning target volume (PTV) was formed by the isotropic expansion of the CTV by 5mm. Furthermore, 99% of the PTV was required to receive 95% of the prescription dose.
Evaluation and follow-up
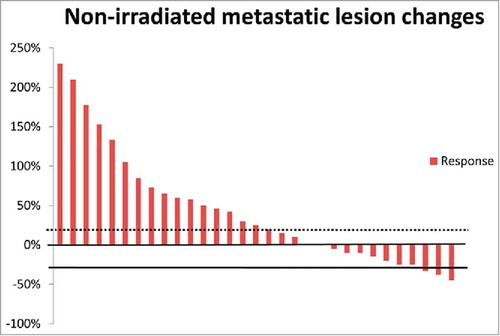
All the patients were hospitalized during the treatment course. Physical examinations and routine blood tests were conducted every week. Metastatic lesions were monitored biweekly by CT/MRI scans. To better explore the anti-cancer effects, which were purely mediated by the Tα1-induced immune response, the proportion of patients with stable disease in non-irradiated metastatic sites at 12 weeks was set as the primary endpoint. Secondary endpoints included the treatment safety and survival outcomes. A complete abscopal response was defined as the complete disappearance of all measurable non-irradiated lesions. A partial metastatic lesion response was defined as a ≥30% decrease in the longest diameter of all the other measureable lesions. Progressive disease was defined as a ≥20% increase in the longest diameter of all the other measureable lesions, whereas stable disease was defined as insufficient shrinkage or growth to qualify for a partial response or progressive disease. For mESCC patients with two measureable metastatic lesions before enrollment, the best response of the non-irradiated lesion was documented, and in the case of more non-irradiated lesions, the overall response of the non-irradiated lesions was documented. Toxicities were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). To better investigate the mechanisms responsible for the observed responses, immunomonitoring of lymphocytes at baseline and the end of the treatment was also required after consultation between the two hospitals and was approved by the IRBs. To label lymphocyte subsets, peripheral blood samples were incubated with either the combination of a FITC-conjugated monoclonal antibody against human CD3 and a PE-conjugated monoclonal antibody against human CD56 or the combination of a FITC-conjugated monoclonal antibody against human CD4 and a PE-conjugated monoclonal antibody against human CD8. Then, labelled lymphocytes were analyzed by flow cytometry. All antibodies were purchased from BD Biosciences (CA, USA).
Statistical analysis
This trial used an optimal two-stage design as proposed by Simon: an additional 19 patients could be enrolled in stage two only if at least one patient among the first 10 patients had at least stable disease in non-irradiated lesions.Citation12 A sample size of 29 was required to accept the hypothesis that the metastatic-lesion control rate in heavily pretreated, mESCC patients was greater than 20% with 80% power and to reject the hypothesis that the metastatic-lesion control rate was less than 5% with an α error of 0.05. Considering some deviant cases, the preplanned accrual number was set to 31 patients. Overall survival (OS) was determined as the time that elapsed from the date of treatment initiation to the date of death. In case of missing data, patients were censored at the last follow-up. Abscopal progression free survival (APFS) was defined as the interval between the first day of treatment and the date of documented failure of any abscopal lesion. Survival curves were calculated using the Kaplan-Meier method and compared with the log-rank test. Flow cytometry data were examined by paired Student's t test (continuous variable) or the Mann-Whitney U test (abnormal distribution variable) as appropriate. All the statistical analyses were performed using SPSS version 22.0 (SPSS, Armonk, New York, USA) with intention-to-treat analyses.
Results
Patient characteristics
Patients were recruited from January 2016 to August 2016. In stage one, six patients were evaluated as having at least stable metastatic lesions based on CT/MRI scans. Thus, the trial proceeded to stage two, as planned, and an additional 21 mESCC patients were enrolled. The characteristics of the 31 patients are summarized in . The median age was 59 years (range, 46–71 years), with 3 women and 28 men. Most patients (54.8%) received primary therapy with concurrent chemoradiotherapy. Eighteen (58.1%) patients had two metastatic sites and 10 patients had three sites at the time of treatment initiation. The abdomen was the most common site of metastasis, and an additional 5 patients had at least 2 sites of metastasis. All non-irradiated metastatic lesions were separated from the PTV and did not receive any X-rays.
Table 1. Baseline patient characteristics.
Anti-tumor effects in non-irradiated lesions
During the trial, fourteen (45.2%) patients were observed to have at least stable metastatic lesions at 12 weeks based on the evaluation criterion. Of these patients, nine patients received first-line therapy of definitive concurrent chemoradiotherapy, 3 patients received postoperative radiotherapy/chemoradiotherapy, and 2 patients had neoadjuvant chemoradiotherapy and surgery, respectively. Ten of the 14 metastatic-lesion controllers presented with 2 metastatic lesions, and no patients with more than 4 metastatic lesions at baseline achieved an anti-tumor response. Of the 14 SBRT fields, seven were located in the liver and five were in the lung. More interestingly, 3 of the 14 patients were recorded as having a partial metastatic-lesion response. One patient was evaluated as having a clinically complete response, and the other had a partial response after concurrent chemoradiotherapy. Another patient who had a partial metastatic-lesion response received neoadjuvant chemoradiotherapy followed by esophagogastrectomy. This patient was approved as having a pathologically complete response to neoadjuvant chemoradiotherapy. The percent changes for non-irradiated metastatic lesions in all the enrolled patients are shown in .
Acute toxicity
Acute toxic reactions were assessed in all 31 patients. In general, the combination of SBRT and Tα1 yielded acceptable toxicity. No grade 4 adverse events were observed during the treatment. The most common hematologic toxicity was leukocytopenia, with 2 (6.5%) patients and 1 (3.2%) patient showing grade 2 and 3 leukocytopenia, respectively. Grade 3 neutropenia was reported in 1 patient. In terms of non-hematologic toxicity, three patients experienced grade 3 fatigue. Other grade 3 non-hematologic toxicities included pain (6.5%) and abnormal liver function (6.5%). The detailed acute toxicity profile is listed in .
Table 2. Acute treatment-related adverse events.
Survival outcome
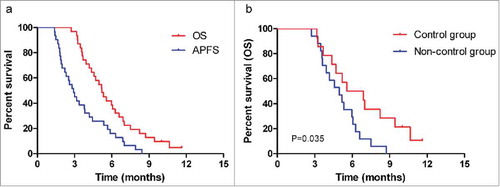
The median follow-up period was 6.1 months (range: 2.7–11.7 months). At the last follow-up in June 2017, 2 patients were still alive, and both of these patients showed a partial metastatic-lesion response. The median OS time was 5.2 months (95% CI: 4.3–6.1), and the 6-month OS rate was 37.8% (95% CI: 0.209–0.547). The median APFS time was 2.9 months (95% CI: 2.2–3.6), and the 6-month APFS rate was 14.5% (95% CI: 0.020–0.270; ). In the abscopal control group, the median OS and APFS times were 5.6 months (95% CI: 2.4–8.7) and 3.0 months (95% CI: 1.3–4.7), respectively. In the non-controllers, the median OS and APFS times were 5.0 months (95% CI: 3.6–6.3) and 2.2 months (95% CI: 1.4–3.0), respectively. Significant differences in OS and APFS were seen in the subgroup analysis (P=0.035 and 0.044, respectively; and Fig. S1).
Changes in immune-related blood parameters
Routine blood tests were performed before and at the end of treatment (). Only the lymphocyte-monocyte ratio (LMR) at the end of the treatment was significantly higher than that at baseline (6.88 ± 6.30 vs 4.37 ± 2.66, P=0.043). Other blood parameters showed no significant changes at the two time points. Subgroup analysis between the abscopal control group and non-control group revealed that the platelet-lymphocyte ratio (PLR) at the end of treatment showed marginal significance between the two subgroups (104.24 ± 54.43 vs 178.56 ± 135.80, P=0.051).
Table 3. Comparison of blood parameters in abscopal control group and non-control group.
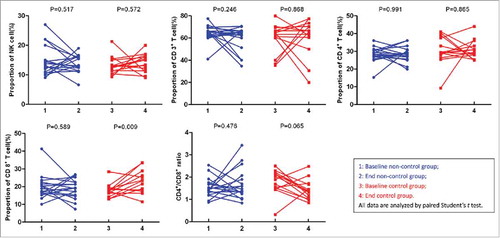
Additionally, lymphocyte subtypes were also recorded based on the treatment protocol. There were no significant changes in the proportions of natural killer (NK) cells, CD3+, CD4+, or CD8+ cells and the CD4+/CD8+ cell ratio before and after treatment for the whole cohort (Table S1). Subgroup analysis found that the proportion of CD8+ T cells at the end of treatment was significantly higher in metastatic-lesion controllers than that in non-controllers (23.18 ± 6.41 vs 18.76 ± 5.43, P = 0.047). Furthermore, the proportion of CD8+ T cells after treatment in the abscopal control group exhibited significant differences from that at baseline (23.18 ± 6.41 vs 17.65 ± 3.78, P = 0.009; ).
Discussion
To date, little evidence is available for the management of heavily pretreated, mESCC patients. Our trial first demonstrated that for these patients, the combinatorial therapy of SBRT and Tα1 achieved its prespecified endpoint with more than 20% of patients having stable metastatic lesions.
Ideally, the optimal immune response modifier is one that will dampen many levels of tumor-associated immunosuppression.Citation13 One such candidate is Tα1. Tα1 has been shown to increase NK-cell activity, shift T helper cells to the Th1 cell subset, promote levels of cytotoxic T (CD8+) cells, and increases the expression of Th1-type cytokines, such as Interleukin(IL)-2 and interferon-α (IFN-α).Citation14 In the late 1970s, Wara and co-workers showed for the first time the immune-enhancing capabilities of thymosin fraction 5 therapy in patients with squamous cell carcinoma patients of the esophagus (ESCC) and head and neck (HNSCC) undergoing radiotherapy.Citation15 Subsequently, a randomized, double-blind trial was conducted to evaluate the immunorestorative properties of Tα1 in non-small cell lung cancer (NSCLC) patients. The delivery of Tα1 after radiotherapy led to a statistically significant improvement in relapse-free survival and OS (P=0.04 and 0.009, respectively) and immune cell function and numbers, which had been depleted by radiation treatment (P=0.04).Citation16 In the present study, our results also showed significant survival advantages in the abscopal control group. Such survival differences in our study might be attributable partly to the significant improvement in the proportion of CD8+ T cells. In addition to Tα1, other types of immune modifiers include the aforementioned GM-CSF, ILs and IFN-α. As described above, preclinical data have shown that in murine melanoma model, GM-CSF is significantly expressed in irradiated melanoma cells and it stimulates potent, long-lasting and specific anti-tumor immunity.Citation17 In the first proof-of-principle trial, the combination of GM-CSF with irradiation yielded exciting results, as approximately 25% of patients were documented to have at least a partial abscopal response.Citation7 For ILs, a randomized controlled study conducted in a murine HNSCC model showed that the combination of mouse IL-2 and mouse IL-12 with irradiation led to a significant increase in the anti-tumor effects when compared with single therapy or controls. In particular, the combinatorial therapy significantly promoted the infiltration of CD4+ and CD8+ T-lymphocytes.Citation18
However, accumulating preclinical evidence has demonstrated that the effectiveness of radiotherapy depends not only on the “quantity” of radiation dose to the target area, but also on the “quality” of the RT-induced immunogenic cell death (ICD) of malignant cells and the release of danger-associated molecular patterns (DAMPs) in a dose-dependent manner.Citation19,Citation20 In mouse models of either breast or colon cancer, Dewan et al. showed that fractionated (8 Gy × 3 or 6 Gy × 5), but not single-dose (20 Gy × 1), radiotherapy induced an immune-mediated abscopal response when in combination with an anti-CTLA-4 antibody.Citation21 In another study using B16-OVA (ovalbumin) tumor cells in a mouse model, fractionated irradiation with medium-sized radiation doses of 7.5 Gy per fraction gave the best tumor control and tumor immunity while maintaining lower regulatory T cell numbers when compared with single-dose radiotherapy.Citation22 However, there are only anecdotal clinical case reports that support at least some of the findings made in preclinical models regarding the ability of SBRT to evoke an immune-mediated anti-tumor response.Citation8 Considering the metastatic sites in ESCC patients and the feasibility of treatment plan, 25 Gy delivered by 5 Gy per fraction was chosen as the ideal dose to metastatic lesions in our trial. A comparable fractionation schedule was able to evoke an improved response to immunotherapy in a widely metastatic chemo-refractory NSCLC patient earlier. This patient received 6 Gy × 5 fractions to a single liver metastatic lesion with the combination of ipilimumab, and follow-up PET/CT imaging showed a dramatic decrease of metabolic activity in multiple metastatic lesions within the liver and bone.Citation23
Moreover, we only recorded 3 mESCC patients as having a partial metastatic-lesion response and no patients with a complete response in the current study. This response rate was obviously lower than that using GM-CSF and other immunotherapy.Citation7,Citation24 The intratumoral characteristics of diseases with a high-frequency of mutations, such as melanoma, NSCLC and breast cancer, may help to explain this result. Additionally, our results also support that an increased platelet-lymphocyte ratio might be associated with tumor progression in esophageal cancer patients.Citation25
In conclusion, combining SBRT with Tα1 yielded promising treatment results in heavily pretreated mESCC patients. Future preclinical studies are warranted to elucidate the enormous potential of this combined treatment modality.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
DxD, TS, HD and ZJ performed the trial. DxD, HD and PC provided the study material and analyzed and interpreted the results. DxD and SxW designed the trial and wrote the manuscript. All the authors critically reviewed the report and approved the final version of the report for submission. The corresponding author (SxW) had access to the primary data, took responsibility for the accuracy and completeness of the data reporting, and had the final responsibility for the decision to submit the manuscript for publication.
supp_data.zip
Download Zip (46.5 KB)Funding
There was no funding support for this study.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016.
- Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi:10.1016/S0140-6736(17)31462-9. PMID:28648400.
- Smyth E, Lagergren J, Fitzgerald R, Lordick F, Shah M, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. doi:10.1038/nrdp.2017.48. PMID:28748917.
- Van Limbergen EJ, De Ruysscher DK, Olivo PV, Marcus D, Berbee M, Hoeben A, Rekers N, Theys J, Yaromina A, Dubois LJ. Combining radiotherapy with immunotherapy: the past, the present and the future. Br J Radiol. 2017;90(1076):20170157. doi:10.1259/bjr.20170157. PMID:28541096.
- Chajon E, Castelli J, Marsiglia H, De CR. The synergistic effect of radiotherapy and immunotherapy: A promising but not simple partnership. Critical Reviews in Oncology. 2017;111:124. doi:10.1016/j.critrevonc.2017.01.017.
- Dan I, Pop L, Takeshima T, Iyengar P, Hannan R. Rationale and evidence to combine radiation therapy and immunotherapy for cancer treatment. Cancer Immunology Immunotherapy Cii. 2017;66(3):281. doi:10.1007/s00262-016-1914-6. PMID:27743027.
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795. doi:10.1016/S1470-2045(15)00054-6. PMID:26095785.
- Ngwa W, Ouyang Z. Following the Preclinical Data: Leveraging the Abscopal Effect More Efficaciously. Front Oncol. 2017;7:66. doi:10.3389/fonc.2017.00066. PMID:28447024.
- Goldstein AL, Guha A, Zatz MM, Hardy MA, White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972;69(7):1800−3. doi:10.1073/pnas.69.7.1800. PMID:4505657.
- Garaci E, Pica F, Serafino A, Balestrieri E, Matteucci C, Moroni G, Sorrentino R, Zonfrillo M, Pierimarchi P, Sinibaldi-Vallebona P. Thymosin α1 and cancer: Action on immune effector and tumor target cells. Ann N Y Acad Sci. 2012;1269(1):26−33. doi:10.1111/j.1749-6632.2012.06697.x. PMID:23045967.
- Garaci E, Pica F, Rasi G, Favalli C. Thymosin alpha 1 in the treatment of cancer: from basic research to clinical application. Int J Immunopharmacol. 2000;22(12):1067–76. doi:10.1016/S0192-0561(00)00075-8. PMID:11137613.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi:10.1016/0197-2456(89)90015-9. PMID:2702835.
- Deplanque G, Shabafrouz K, Obeid M. Can local radiotherapy and IL-12 synergise to overcome the immunosuppressive tumor microenvironment and allow “in situ tumor vaccination”? Cancer Immunol Immunother Cii. 2017;66(7):833–40. doi:10.1007/s00262-017-2000-4. PMID:28409192.
- King CWTRS. Thymosin Apha 1–A Peptide Immune Modulator with a Broad Range of Clinical Applications. Clinical & Experimental Pharmacology. 2013;3(4):133–149.
- Wara WM, Ammann AJ, Wara DW. Effect of thymosin and irradiation on immune modulation in head and neck and esophageal cancer patients. Cancer Treat Rep. 1978;62(11):1775–8. PMID:728896.
- Scher HI, Shank B, Chapman R, Geller N, Pinsky C, Gralla R, Kelsen D, Bosl G, Golbey R, Petroni G. Randomized trial of combined modality therapy with and without thymosin fraction V in the treatment of small cell lung cancer. Cancer Res. 1988;48(6):1663. PMID:2830968.
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–43. doi:10.1073/pnas.90.8.3539. PMID:8097319.
- Xian J, Yang H, Lin Y, Liu S. Combination nonviral murine interleukin 2 and interleukin 12 gene therapy and radiotherapy for head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131(131):1079–85. doi:10.1001/archotol.131.12.1079. PMID:16365221.
- Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett. 2015;368(2):185. doi:10.1016/j.canlet.2015.03.024. PMID:25799953.
- Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831. doi:10.1158/2326-6066.CIR-14-0069. PMID:25187273.
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2009;15(17):5379. doi:10.1158/1078-0432.CCR-09-0265. PMID:19706802.
- Schaue D, Ratikan JA, Iwamoto KS, Mcbride WH. Maximizing Tumor Immunity With Fractionated Radiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1306. doi:10.1016/j.ijrobp.2011.09.049.
- Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Cancer Immunol Res. 2013;1(6):365–72. doi:10.1158/2326-6066.CIR-13-0115. PMID:24563870.
- Levy A, Chargari C, Marabelle A, Perfettini JL, Magné N, Deutsch E. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur J Cancer. 2016;62:36. doi:10.1016/j.ejca.2016.03.067. PMID:27200491.
- Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2016;23(2):646–54. doi:10.1245/s10434-015-4869-5. PMID:26416715.